













实验动物与比较医学 ›› 2023, Vol. 43 ›› Issue (2): 173-179.DOI: 10.12300/j.issn.1674-5817.2022.102
吴昊晟1( ), 苏航1, 朱超1, 王文慧1, 吴生兵2,3, 崔帅2,4, 周美启4,5(
), 苏航1, 朱超1, 王文慧1, 吴生兵2,3, 崔帅2,4, 周美启4,5( )(
)( )
)
收稿日期:2022-07-06
修回日期:2022-09-21
出版日期:2023-04-25
发布日期:2023-05-16
通讯作者:
周美启(1963—),男,博士,教授,博士研究生导师,研究方向:经脉脏腑相关研究。E-mail:meiqizhou@163.com。ORCID: 0000-0002-8798-7121作者简介:吴昊晟(1997—),男,硕士研究生,研究方向:经脉脏腑相关研究。E-mail:811985763@qq.com
基金资助:
Haosheng WU1( ), Hang SU1, Chao ZHU1, Wenhui WANG1, Shengbing WU2,3, Shuai CUI2,4, Meiqi ZHOU4,5(
), Hang SU1, Chao ZHU1, Wenhui WANG1, Shengbing WU2,3, Shuai CUI2,4, Meiqi ZHOU4,5( )(
)( )
)
Received:2022-07-06
Revised:2022-09-21
Published:2023-04-25
Online:2023-05-16
Contact:
ZHOU Meiqi (ORCID: 0000-0002-8798-7121), E-mail: meiqizhou@163.com摘要:
应激性心肌病(stress cardiomyopathy,SC)是一种由强烈精神刺激或躯体应激所致的急性心肌病。目前,在SC的急性或慢性阶段,能够有效降低死亡率、改善预后或预防复发的干预措施极少。因此,通过建立一种可靠、有效的SC动物模型来研究SC的发病机制及其防治方法,尤为重要。本文梳理了国内外现有SC模型的制备方法和评价指标,并展开综述,即论述了儿茶酚胺类激素注射法、束缚法、束缚联合电击法、束缚联合脱水法、迷走神经电刺激等造模方法的操作细节,以及利用旷场实验、心超、心电图、ELISA等方法对相关指标进行评价,以期为研究SC时的模型选择和建立提供有益参考。
中图分类号:
吴昊晟, 苏航, 朱超, 王文慧, 吴生兵, 崔帅, 周美启. 应激性心肌病动物模型的研究进展[J]. 实验动物与比较医学, 2023, 43(2): 173-179.
Haosheng WU, Hang SU, Chao ZHU, Wenhui WANG, Shengbing WU, Shuai CUI, Meiqi ZHOU. Research Progress of Animal Models of Stress Cardiomyopathy[J]. Laboratory Animal and Comparative Medicine, 2023, 43(2): 173-179.
动物 Animal | 品种或品系 Stock or strain | 性别 Sex | 年龄或体质量 Age/weight | 药物 Drug | 剂量 Dose | 给药方案 Dosage regimen | 参考文献 Reference |
|---|---|---|---|---|---|---|---|
| 大鼠 Rat | SD | 雄 | 250~300 g | ISO | 50 mg/kg | 单次腹腔注射 | [ |
| SD | 雌 | 2~4月龄,241±55 g | ISO | 100 mg/kg | 单次腹腔注射 | [ | |
| SD | 雄 | 220~250 g,8周龄 | ISO | 100 mg/kg | 不同部位多点皮下注射,持续3周 | [ | |
| SD | 雌 | 300~350 g | NE | 2~4 mg/kg | 单次腹腔注射 | [ | |
| SD | 雌 | 300~350 g | NE | 4 mg/kg | 单次腹腔注射 | [ | |
| 小鼠Mice | C57BL/6J | 雄/雌 | 8~16周龄 | ISO | 200 mg/kg | 单次腹腔注射 | [ |
| C57BL/6J | / | 16周 | ISO | 400 mg/kg | 单次腹腔注射 | [ | |
| C57BL/6J | 雄性 | 7周龄,20~25 g | ISO | 50 mg/kg | 腹腔注射3周 | [ | |
| 129/Sv | / | 8周 | ISO | 25 mg/kg | 连续皮下注射5 d | [ | |
| C57BL/ 6J | 雄/雌 | 8周 | ISO | 30 mg/kg | 皮下埋置微渗透泵,连续21 d释放ISO | [ |
表1 化学因素建立大鼠、小鼠应激性心肌病模型的方法
Table 1 Methods of constructing rat and mouse model of stress cardiomyopathy by chemical factors
动物 Animal | 品种或品系 Stock or strain | 性别 Sex | 年龄或体质量 Age/weight | 药物 Drug | 剂量 Dose | 给药方案 Dosage regimen | 参考文献 Reference |
|---|---|---|---|---|---|---|---|
| 大鼠 Rat | SD | 雄 | 250~300 g | ISO | 50 mg/kg | 单次腹腔注射 | [ |
| SD | 雌 | 2~4月龄,241±55 g | ISO | 100 mg/kg | 单次腹腔注射 | [ | |
| SD | 雄 | 220~250 g,8周龄 | ISO | 100 mg/kg | 不同部位多点皮下注射,持续3周 | [ | |
| SD | 雌 | 300~350 g | NE | 2~4 mg/kg | 单次腹腔注射 | [ | |
| SD | 雌 | 300~350 g | NE | 4 mg/kg | 单次腹腔注射 | [ | |
| 小鼠Mice | C57BL/6J | 雄/雌 | 8~16周龄 | ISO | 200 mg/kg | 单次腹腔注射 | [ |
| C57BL/6J | / | 16周 | ISO | 400 mg/kg | 单次腹腔注射 | [ | |
| C57BL/6J | 雄性 | 7周龄,20~25 g | ISO | 50 mg/kg | 腹腔注射3周 | [ | |
| 129/Sv | / | 8周 | ISO | 25 mg/kg | 连续皮下注射5 d | [ | |
| C57BL/ 6J | 雄/雌 | 8周 | ISO | 30 mg/kg | 皮下埋置微渗透泵,连续21 d释放ISO | [ |
| 动物Animal | 品种Stock | 性别 Sex | 月龄或体质量 Age/weight | 造模方案 Modeling process | 参考文献Reference |
|---|---|---|---|---|---|
| 大鼠Rat | SD | 雄性 | 3~4月龄,300~350 g | 束缚2 h | [ |
| 大鼠Rat | SD | 雄性 | 300~350 g | 固定30 min,每间隔2 min给予10 s足底电刺激2 mA,每日2次,持续15 d | [ |
| 大鼠Rat | SD | 雄性 | 280~300 g | 限制饮水50 mL/d,每日束缚6~8 h,连续3周 | [ |
表2 物理因素建立大鼠应激性心肌病模型的方法
Table 2 Methods of constructing stress cardiomyopathy model in rats by physical factors
| 动物Animal | 品种Stock | 性别 Sex | 月龄或体质量 Age/weight | 造模方案 Modeling process | 参考文献Reference |
|---|---|---|---|---|---|
| 大鼠Rat | SD | 雄性 | 3~4月龄,300~350 g | 束缚2 h | [ |
| 大鼠Rat | SD | 雄性 | 300~350 g | 固定30 min,每间隔2 min给予10 s足底电刺激2 mA,每日2次,持续15 d | [ |
| 大鼠Rat | SD | 雄性 | 280~300 g | 限制饮水50 mL/d,每日束缚6~8 h,连续3周 | [ |
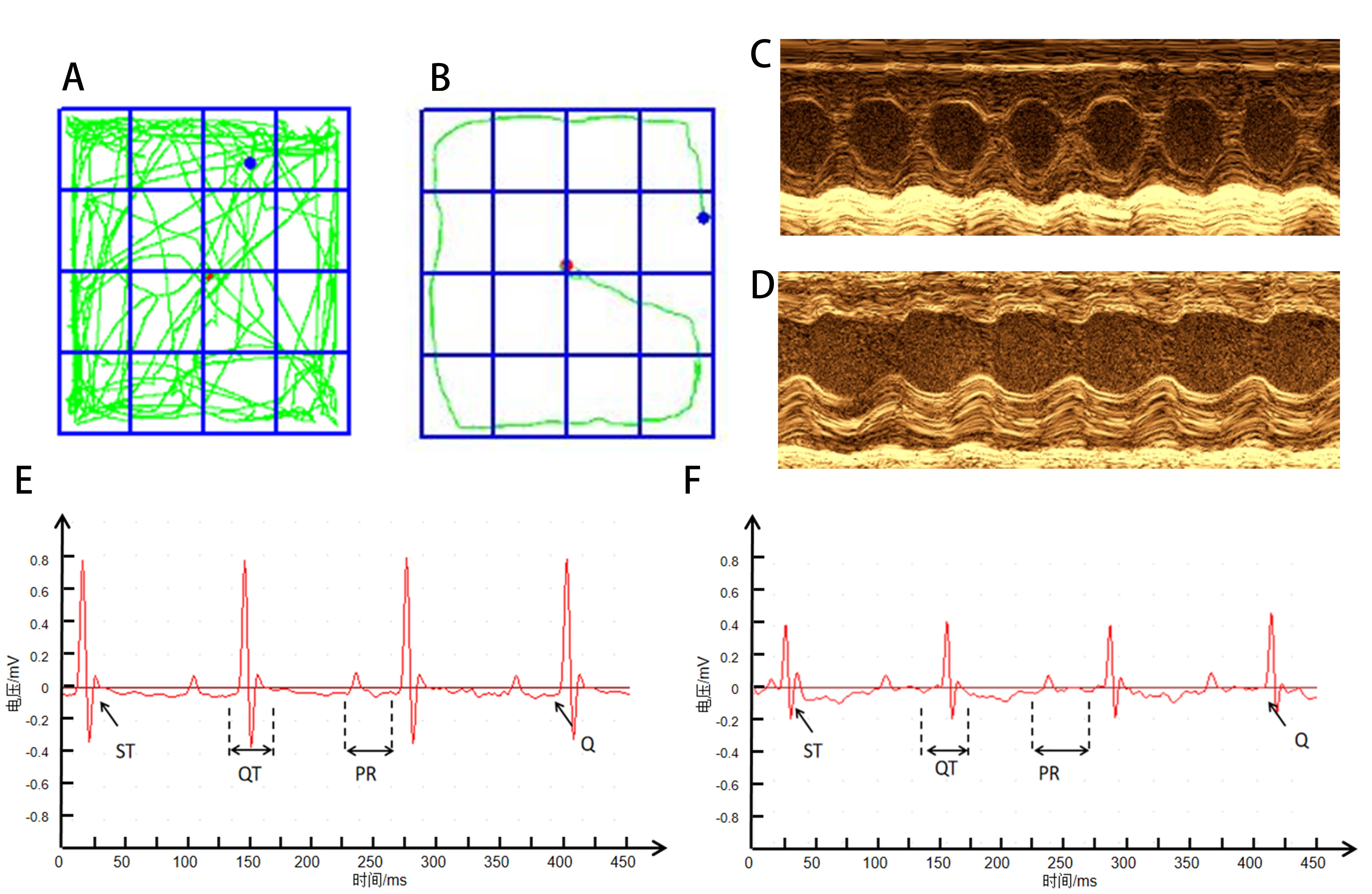
图1 应激性心肌病动物模型的评价指标代表性图像注:A为正常组旷场实验轨迹图;B为SC模型组旷场实验轨迹图;C为正常组心超图;D为SC模型组心超图;E为正常组心电图;F为SC模型组心电图。
Figure 1 Representative images of evaluation index in an animal model of stress cardiomyopathyNote: A is the trajectory diagram of open field experiment in normal group; B is the open field experiment trajectory of SC model group; C is the cardiogram of the normal group; D is the cardiogram of SC model group; E is the electrocardiogram of normal group; F is the electrocardiogram of SC model group.
| 1 | SHAH R M, SHAH M, SHAH S, et al. Takotsubo syndrome and COVID-19: associations and implications[J]. Curr Probl Cardiol, 2021, 46(3):100763. DOI:10.1016/j.cpcardiol.2020. 100763 . |
| 2 | BARBIERI L, GALLI F, CONCONI B, et al. Takotsubo syndrome in COVID-19 era: is psychological distress the key? [J]. J Psychosom Res, 2021, 140:110297. DOI:10.1016/j.jpsychores. 2020.110297 . |
| 3 | CASTILLO RIVERA A M, RUIZ-BAILÉN M, RUCABADO AGUILAR L. Takotsubo cardiomyopathy - a clinical review[J]. Med Sci Monit, 2011, 17(6): RA135-RA147. DOI:10.12659/msm. 881800 . |
| 4 | OKURA H. Update of takotsubo syndrome in the era of COVID-19[J]. J Cardiol, 2021, 77(4):361-369. DOI:10.1016/j.jjcc. 2020.10.004 . |
| 5 | CHHABRA L. Prognostication in takotsubo syndrome[J]. Rev Cardiovasc Med, 2022, 23(3):110. DOI:10.31083/j.rcm2303110 . |
| 6 | TEMPLIN C, GHADRI J R, DIEKMANN J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy[J]. N Engl J Med, 2015, 373(10):929-938. DOI:10.1056/NEJMoa1406761 . |
| 7 | LYON A R, CITRO R, SCHNEIDER B, et al. Pathophysiology of takotsubo syndrome[J]. J Am Coll Cardiol, 2021, 77(7):902-921. DOI:10.1016/j.jacc.2020.10.060 . |
| 8 | LI C M, HUANG D, TANG J, et al. ClC-3 chloride channel is involved in isoprenaline-induced cardiac hypertrophy[J]. Gene, 2018, 642:335-342. DOI:10.1016/j.gene.2017.11.045 . |
| 9 | 张志红. 硫化氢对Takotsubo心肌病的保护作用及其机制探讨[D].石家庄: 河北医科大学, 2017. |
| ZHANG Z H. Protective effect of H2S on takotsubo cardiomyopathy and its mechanism[D]. Shijiazhuang: Hebei Medical University, 2017. | |
| 10 | ALI A, REDFORS B, ALKHOURY J, et al. Sacubitril/valsartan decreases mortality in the rat model of the isoprenaline-induced takotsubo-like syndrome[J]. ESC Heart Fail, 2021, 8(5):4130-4138. DOI:10.1002/ehf2.13530 . |
| 11 | QI C L, SHAO Y Z, LIU X S, et al. The cardioprotective effects of icariin on the isoprenaline-induced takotsubo-like rat model: involvement of reactive oxygen species and the TLR4/NF-κB signaling pathway[J]. Int Immunopharmacol, 2019, 74:105733. DOI:10.1016/j.intimp.2019.105733 . |
| 12 | 杜志君, 蔡伟文, 张文静, 等. 典型与非典型应激性心肌病大鼠模型的制备方法[J]. 广东医学, 2016, 37(7):953-957. DOI:10.13820/j.cnki.gdyx.2016.07.001 . |
| DU Z J, CAI W W, ZHANG W J, et al. Research on the rat model establishment methods of typical and atypical stress cardiomyopathy[J]. Guangdong Med J, 2016, 37(7):953-957. DOI:10.13820/j.cnki.gdyx.2016.07.001 . | |
| 13 | GODSMAN N, KOHLHAAS M, NICKEL A, et al. Metabolic alterations in a rat model of takotsubo syndrome[J]. Cardiovasc Res, 2021, 118(8):1932-1946. DOI:10.1093/cvr/cvab081 . |
| 14 | 尹元立, 高永红. 血清缺血修饰白蛋白与大鼠应激性心肌病相关性分析[J]. 中国循证心血管医学杂志, 2018, 10(5):621-624. DOI:10.3969/j.issn.1674-4055.2018.05.30 . |
| YIN Y L, GAO Y H. Correlation between serum ischemia modified albumin and rat Takotsubo cardiomyopathy[J]. Chin J Evid Based Cardiovasc Med, 2018, 10(5):621-624. DOI:10.3969/j.issn.1674-4055.2018.05.30 . | |
| 15 | 蔡伟文, 杜志君, 查道刚. 大鼠应激性心肌病模型及其复发模型的制备[J]. 岭南心血管病杂志, 2016, 22(3):315-319. DOI:10.3969/j.issn.1007-9688.2016.02.18 . |
| CAI W W, DU Z J, ZHA D G. Preparation for rat model of stress-induced cardiomyopathy and its recurrence model[J]. South China J Cardiovasc Dis, 2016, 22(3):315-319. DOI:10.3969/j.issn.1007-9688.2016.02.18 . | |
| 16 | LIAO X D, CHANG E, TANG X M, et al. Cardiac macrophages regulate isoproterenol-induced takotsubo-like cardiomyo-pathy[J]. JCI Insight, 2022, 7(3): e156236. DOI:10.1172/jci.insight.156236 . |
| 17 | SHAO Y Z, REDFORS B, STÅHLMAN M, et al. A mouse model reveals an important role for catecholamine-induced lipotoxicity in the pathogenesis of stress-induced cardiomyopathy[J]. Eur J Heart Fail, 2013, 15(1):9-22. DOI:10.1093/eurjhf/hfs161 . |
| 18 | 向仕钊, 卞洲艳, 周恒, 等. 比索洛尔联合还原型谷胱甘肽改善应激性心肌病心肌重构[J]. 中国心血管杂志, 2015, 20(5):358-365. DOI:10.3969/j.issn.1007-5410.2015.05.011 . |
| XIANG S Z, BIAN Z Y, ZHOU H, et al. Combined utilization of bisoprolol and glutathione attenuates ventricular remodeling of takotsubo cardiomyopathy in mice[J]. Chin J Cardiovasc Med, 2015, 20(5):358-365. DOI:10.3969/j.issn.1007-5410.2015.05.011 . | |
| 19 | KHURANA I, MAXWELL S, ROYCE S, et al. SAHA attenuates Takotsubo-like myocardial injury by targeting an epigenetic Ac/Dc axis[J]. Signal Transduct Target Ther, 2021, 6:159. DOI:10.1038/s41392-021-00546-y . |
| 20 | WALSH-WILKINSON E, ARSENAULT M, COUET J. Segmental analysis by speckle-tracking echocardiography of the left ventricle response to isoproterenol in male and female mice[J]. PeerJ, 2021, 9: e11085. DOI:10.7717/peerj.11085 . |
| 21 | 万坤铭, 孙帅, 俞超芹. 应激致功能性下丘脑性闭经动物模型研究进展[J]. 南昌大学学报(医学版), 2022, 62(1):99-104. DOI:10.13764/j.cnki.ncdm.2022.01.020 . |
| WAN K M, SUN S, YU C Q. Advances in animal models of stress-induced functional hypothalamic amenorrhea[J]. J Nanchang Univ Med Sci, 2022, 62(1):99-104. DOI:10.13764/j.cnki.ncdm.2022.01.020 . | |
| 22 | 高源, 郭翠娟, 刘仁平, 等. 束缚应激对小鼠卵巢氧化损伤的实验研究[J]. 南昌大学学报(医学版), 2022, 62(2):26-30. DOI:10.13764/j.cnki.ncdm.2022.02.005 . |
| GAO Y, GUO C J, LIU R P, et al. A study on ovarian oxidative damage caused by restraint stress in mice[J]. J Nanchang Univ Med Sci, 2022, 62(2):26-30. DOI:10.13764/j.cnki.ncdm.2022.02.005 . | |
| 23 | 齐跃, 房加雄, 董彦, 等. 束缚诱导大鼠应激性心肌病的有效性评价[J]. 武警后勤学院学报(医学版), 2016, 25(10):791-794. DOI:10.16548/j.2095-3720.2016.10.004 . |
| QI Y, FANG J X, DONG Y, et al. Evaluation of the immobilization effectiveness on inducing stress cardiomyopathy in rats[J]. J Logist Univ PAP Med Sci, 2016, 25(10):791-794. DOI:10.16548/j.2095-3720.2016.10.004 . | |
| 24 | 李永丰, 吴宇蔚, 乔海法. 足底电击应激诱发精神疾病模型的研究进展[J]. 神经解剖学杂志, 2020, 36(4):464-468. DOI:10.16557/j.cnki.1000-7547.2020.04.018 . |
| LI Y F, WU Y W, QIAO H F. Research progress of the mental illness model induced by plantar shock stress[J]. Chin J Neuroanat, 2020, 36(4):464-468. DOI:10.16557/j.cnki.1000-7547.2020.04.018 . | |
| 25 | 李凤, 李玉军, 王凯, 等. 大鼠应激性心肌病模型心肌组织BNP的表达变化[J]. 中国法医学杂志, 2015, 30(5):459-462, 467. DOI:10.13618/j.issn.1001-5728.2015.05.003 . |
| LI F, LI Y J, WANG K, et al. Expression of BNP in a rat model of stress cardiomyopathy[J]. Chin J Forensic Med, 2015, 30(5):459-462, 467. DOI:10.13618/j.issn.1001-5728.2015.05.003 . | |
| 26 | 阿兰达. 大鼠应激性心肌病神经肽Y和PGC-1α表达的实验研究[D]. 武汉: 华中科技大学, 2010. |
| ANANDA S. An Experimental study on the expression of Neuropeptide Y and PGC-1α in a rat model of Stress Cardiomyopathy[D]. Wuhan: Huazhong University of Science and Technology, 2010. | |
| 27 | 石建, 曾安宁, 熊德高. 灯盏花素改善应激性心肌病大鼠心功能异常的机制[J]. 中国老年学杂志, 2016, 36(15):3646-3648. DOI:10.3969/j.issn.1005-9202.2016.15.016 . |
| SHI J, ZENG A N, XIONG D G. Mechanism of breviscapine in improving cardiac dysfunction in rats with stress cardiomyopathy[J]. Chin J Gerontol, 2016, 36(15):3646-3648. DOI:10.3969/j.issn.1005-9202.2016.15.016 . | |
| 28 | 孙艳荣, 富华颖. 制动联合脱水建立应激性心肌病大鼠模型[J]. 泰山医学院学报, 2017, 38(5):497-499. DOI:10. 3969 / j. issn. 1004-7115.2017.05.006 . |
| SUN Y R, FU H Y. Establishment of rat model of stress induced cardiomyopathy by combination of braking and dehydration[J]. J Taishan Med Coll, 2017, 38(5):497-499. DOI:10. 3969 / j. issn. 1004-7115.2017.05.006 . | |
| 29 | 郭瑞威, 杨丽霞, 奚银艳, 等. 电刺激迷走神经建立应激性心肌病家兔模型[J]. 实验动物与比较医学, 2016, 36(3):186-189. DOI:10.3969/j.issn.1674-5817.2016.03.005 . |
| GUO R W, YANG L X, XI Y Y, et al. Establishment of stress induced cardiomyopathy model by stimulation of vagus nerve with electricity signal in rabbit[J]. Lab Anim Comp Med, 2016, 36(3):186-189. DOI:10.3969/j.issn.1674-5817.2016.03.005 . | |
| 30 | GOULD T D, DAO D T, KOVACSICS C E. The open field test[M]//Pietropaolo P. Mood and anxiety related phenotypes in mice. Totowa: Humana Press, 2009:1-20. DOI:10.1007/978-1-60761-303-9_1 . |
| 31 | THIPPESWAMY B S, MISHRA B, VEERAPUR V P, et al. Anxiolytic activity of Nymphaea alba Linn. in mice as experimental models of anxiety[J]. Indian J Pharmacol, 2011, 43(1):50-55. DOI:10.4103/0253-7613.75670 . |
| 32 | LIPKIND D, SAKOV A, KAFKAFI N, et al. New replicable anxiety-related measures of wall vs center behavior of mice in the open field[J]. J Appl Physiol (1985), 2004, 97(1):347-359. DOI:10.1152/japplphysiol.00148.2004 . |
| 33 | YAKUPOGLU H Y, SAEED S, SENIOR R, et al. Reversible exercise-induced left ventricular dysfunction in symptomatic patients with previous Takotsubo syndrome: insights from stress echocardiography[J]. Eur Heart J Cardiovasc Imaging, 2020:jeaa237. DOI:10.1093/ehjci/jeaa237 . |
| 34 | LEE M. Time course of functional recovery in takotsubo (stress) cardiomyopathy: a serial speckle tracking echocardiography and electrocardiography study[J]. J Cardiovasc Imaging, 2020, 28(1):50-60. DOI:10.4250/jcvi.2019. 0083 . |
| 35 | SEMELKA R C, TOMEI E, WAGNER S, et al. Interstudy reproducibility of dimensional and functional measurements between cine magnetic resonance studies in the morphologically abnormal left ventricle[J]. Am Heart J, 1990, 119(6):1367-1373. DOI:10.1016/S0002-8703(05)80187-5 . |
| 36 | MITSUMA W, KODAMA M, ITO M, et al. Serial electrocardiographic findings in women with takotsubo cardiomyopathy[J]. Am J Cardiol, 2007, 100(1):106-109. DOI:10.1016/j.amjcard.2007.02.062 . |
| 37 | ABRAHAM J, MUDD J O, KAPUR N, et al. Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists[J]. J Am Coll Cardiol, 2009, 53(15):1320-1325. DOI:10.1016/j.jacc.2009.02.020 . |
| 38 | GIANNI M, DENTALI F, GRANDI A M, et al. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review[J]. Eur Heart J, 2006, 27(13):1523-1529. DOI:10.1093/eurheartj/ehl032 . |
| 39 | CHHABRA L, BUTT N, AHMAD S A, et al. Electro-cardiographic changes in takotsubo cardiomyopathy[J]. J Electrocardiol, 2021, 65:28-33. DOI:10.1016/j.jelectrocard. 2020.12.006 . |
| 40 | DJURIĆ I, OBRADOVIĆ S, GLIGIĆ B. Dynamics of electrocardiographic changes, brain-natriuretic peptide and cortisol levels in a patient with stress (takotsubo) cardiomyopathy: a case report[J]. Vojnosanit Pregl, 2013, 70(5):511-515. DOI:10.2298/vsp1305511d . |
| 41 | MADHAVAN M, BORLAUG B A, LERMAN A, et al. Stress hormone and circulating biomarker profile of apical ballooning syndrome (Takotsubo cardiomyopathy): insights into the clinical significance of B-type natriuretic peptide and troponin levels[J]. Heart, 2009, 95(17):1436-1441. DOI:10.1136/hrt.2009.170399 . |
| 42 | AHMED K A, MADHAVAN M, PRASAD A. Brain natriuretic peptide in apical ballooning syndrome (Takotsubo/stress cardiomyopathy): comparison with acute myocardial infarction[J]. Coron Artery Dis, 2012, 23(4):259-264. DOI:10.1097/MCA.0b013e3283526a57 . |
| 43 | JOHANSSON G, JONSSON L, LANNEK N, et al. Severe stress-cardiopathy in pigs[J]. Am Heart J, 1974, 87(4):451-457. DOI:10.1016/0002-8703(74)90170-7 . |
| [1] | 梁天薇, 邓亚胜, 黄慧, 荣娜, 刘鑫, 王玉洁, 林江. 围绝经期综合征动物模型的制备方法及评价指标分析[J]. 实验动物与比较医学, 2024, 44(1): 74-84. |
| [2] | 中国研究型医院学会医学动物实验专家委员会. 自发性脑出血动物模型选择及临床前药物试验指南(2024年版)[J]. 实验动物与比较医学, 2024, 44(1): 3-30. |
| [3] | 刘欣, 石少波, 张翠, 杨波, 曲川. 小鼠自体动静脉内瘘端侧吻合模型的建立与评价[J]. 实验动物与比较医学, 2023, 43(6): 595-603. |
| [4] | 万颖寒, 顾也欣, 袁雨浓, 汤忞, 鲁立. FDA现代化法案2.0给疾病动物模型发展带来的启示和思考[J]. 实验动物与比较医学, 2023, 43(5): 472-481. |
| [5] | 谢淑武, 沈如凌, 林金杏, 范春. 雄性不育药物研发相关实验动物模型建立和应用进展[J]. 实验动物与比较医学, 2023, 43(5): 504-511. |
| [6] | 陈艳娟, 沈如凌. 模式动物疾病模型在结直肠癌医学研究中的应用进展[J]. 实验动物与比较医学, 2023, 43(5): 512-523. |
| [7] | 张睿, 吕美豫, 张建军, 刘金莲, 陈彦, 黄志强, 刘尧, 周澜华. 痤疮动物模型建立及评价研究进展[J]. 实验动物与比较医学, 2023, 43(4): 398-405. |
| [8] | 卢今, 王剑, 朱莲, 严国锋, 马政文, 李垚, 戴建军, 朱寅秋, 周晶. 山羊先兆子痫疾病模型的构建及母体生物学特性评价[J]. 实验动物与比较医学, 2023, 43(4): 371-380. |
| [9] | 俞佳慧, 巩倩, 庄乐南. 肺动脉高压动物模型及其在药物研究中的应用进展[J]. 实验动物与比较医学, 2023, 43(4): 381-397. |
| [10] | 邓亚胜, 林江, 甘池伶, 曾官凤, 黄嘉茵, 邓慧芳, 麻颖贤, 韩丝银. 皮肤光老化动物模型制备要素和受试物数据的文献分析[J]. 实验动物与比较医学, 2023, 43(4): 406-414. |
| [11] | 王雪, 呼永河. 糖尿病小鼠模型的常见种类及其构建要素分析[J]. 实验动物与比较医学, 2023, 43(4): 415-421. |
| [12] | 黄慧, 邓亚胜, 梁天薇, 郑艺清, 范燕萍, 荣娜, 林江. 卵巢储备功能减退动物模型的造模方法评价与分析[J]. 实验动物与比较医学, 2023, 43(4): 422-428. |
| [13] | 相磊, 景金珠, 梁震, 阎国强, 郭文峰, 张萌, 张威, 刘亚军. 基于VOSviewer的肌少症动物模型研究可视化分析[J]. 实验动物与比较医学, 2023, 43(4): 429-439. |
| [14] | 谭志刚, 刘锦信, 郑楚雅, 廖文峰, 冯露平, 彭红丽, 严秀, 卓振建. 神经母细胞瘤动物模型研究进展与应用[J]. 实验动物与比较医学, 2023, 43(3): 288-296. |
| [15] | 赖灿, 李乐乐, 胡塔拉, 孟彦. 肾脏间质纤维化动物模型的研究进展[J]. 实验动物与比较医学, 2023, 43(2): 163-172. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||