













实验动物与比较医学 ›› 2023, Vol. 43 ›› Issue (4): 371-380.DOI: 10.12300/j.issn.1674-5817.2023.036
卢今1( ), 王剑1, 朱莲1, 严国锋1, 马政文1, 李垚1, 戴建军2, 朱寅秋1, 周晶1(
), 王剑1, 朱莲1, 严国锋1, 马政文1, 李垚1, 戴建军2, 朱寅秋1, 周晶1( )(
)( )
)
收稿日期:2023-03-21
修回日期:2023-06-06
出版日期:2023-08-25
发布日期:2023-08-25
通讯作者:
周 晶(1985—),女,生物工程硕士,实验师,研究方向:实验动物医学。E-mail:zj2008@shsmu.edu.cn。ORCID:0009-0005-2626-1561作者简介:卢 今(1990—),女,兽医学硕士,实验师,研究方向:实验动物医学。E-mail:ashleelu@shsmu.edu.cn
基金资助:
Jin LU1( ), Jian WANG1, Lian ZHU1, Guofeng YAN1, Zhengwen MA1, Yao LI1, Jianjun DAI2, Yinqiu ZHU1, Jing ZHOU1(
), Jian WANG1, Lian ZHU1, Guofeng YAN1, Zhengwen MA1, Yao LI1, Jianjun DAI2, Yinqiu ZHU1, Jing ZHOU1( )(
)( )
)
Received:2023-03-21
Revised:2023-06-06
Published:2023-08-25
Online:2023-08-25
Contact:
ZHOU Jing (ORCID: 0009-0005-2626-1561), E-mail: zj2008@shsmu.edu.cn摘要:
目的 建立山羊先兆子痫动物模型,并收集该疾病模型各项生理指标和母体的生物学特性数据,与人类该疾病的发生发展特征相对照,为防治人类先兆子痫疾病的发生提供参考。 方法 将23头雌性崇明白山羊分为3组,其中对照组妊娠母羊临产期前不进行任何手术操作;假手术组在母羊妊娠(100±5)d时行先兆子痫假手术造模,仅暴露并游离其子宫体动脉;手术组于母羊妊娠(100±5)d时,在其一侧子宫体动脉上放置银质血管夹,夹闭动脉部分内径,制作先兆子痫模型。对妊娠100~140 d的对照组和手术组母羊连续监测其心率和后肢血压,并利用血流仪结合血流探头、动物生理信号采集仪采集假手术组母羊的子宫动脉侧支内血流数据,以及手术组母羊在放置血管夹前后子宫动脉侧支内的血流变化情况。在预期分娩日期前,取母羊颈静脉血进行血常规、肌酐、尿素氮和血液离子分析;同时采集各组母羊尿液,进行尿蛋白、尿肌酐分析。所有实验组均于母羊妊娠(140±5)d时行剖宫取胎术,然后取各组母羊的肝、肾、子宫和胎盘组织进行苏木精-伊红染色,以进行病理学观察。 结果 手术造模15 min后手术组母羊的子宫体动脉血流量保持稳定,血流流量差相对于对照组和假手术组均降低(P<0.05,P<0.01)。相较于对照组,手术组母羊在产前出现血清渗透压升高、血红蛋白降低、血糖和血清尿素氮值升高,以及钙、钠、氯离子含量增高等变化(均P<0.05),并出现蛋白尿,尿肌酐及尿蛋白肌酐比均明显高于对照组和假手术组(均P<0.05)。手术组动物的手术侧子宫体动脉弹力层较对侧增厚,但结构松散;手术侧胎盘出现细胞间质肿胀、炎性细胞浸润等病理变化。以上生理指标特征均与人类先兆子痫的临床指标较为一致。 结论 通过血流仪验证,以及妊娠后期母羊各项生理指标检测证明,本实验成功构建了山羊先兆子痫模型,并获得了该模型的相关生理指标数据,进一步验证了先兆子痫疾病与子宫动脉病变的相关性。
中图分类号:
卢今,王剑,朱莲,等. 山羊先兆子痫疾病模型的构建及母体生物学特性评价[J]. 实验动物与比较医学, 2023, 43(4): 371-380. DOI: 10.12300/j.issn.1674-5817.2023.036.
Jin LU,Jian WANG,Lian ZHU,et al. Establishment of Preeclampsia Model in Goat and Evaluation on Maternal Biological Characteristics[J]. Laboratory Animal and Comparative Medicine, 2023, 43(4): 371-380. DOI: 10.12300/j.issn.1674-5817.2023.036.
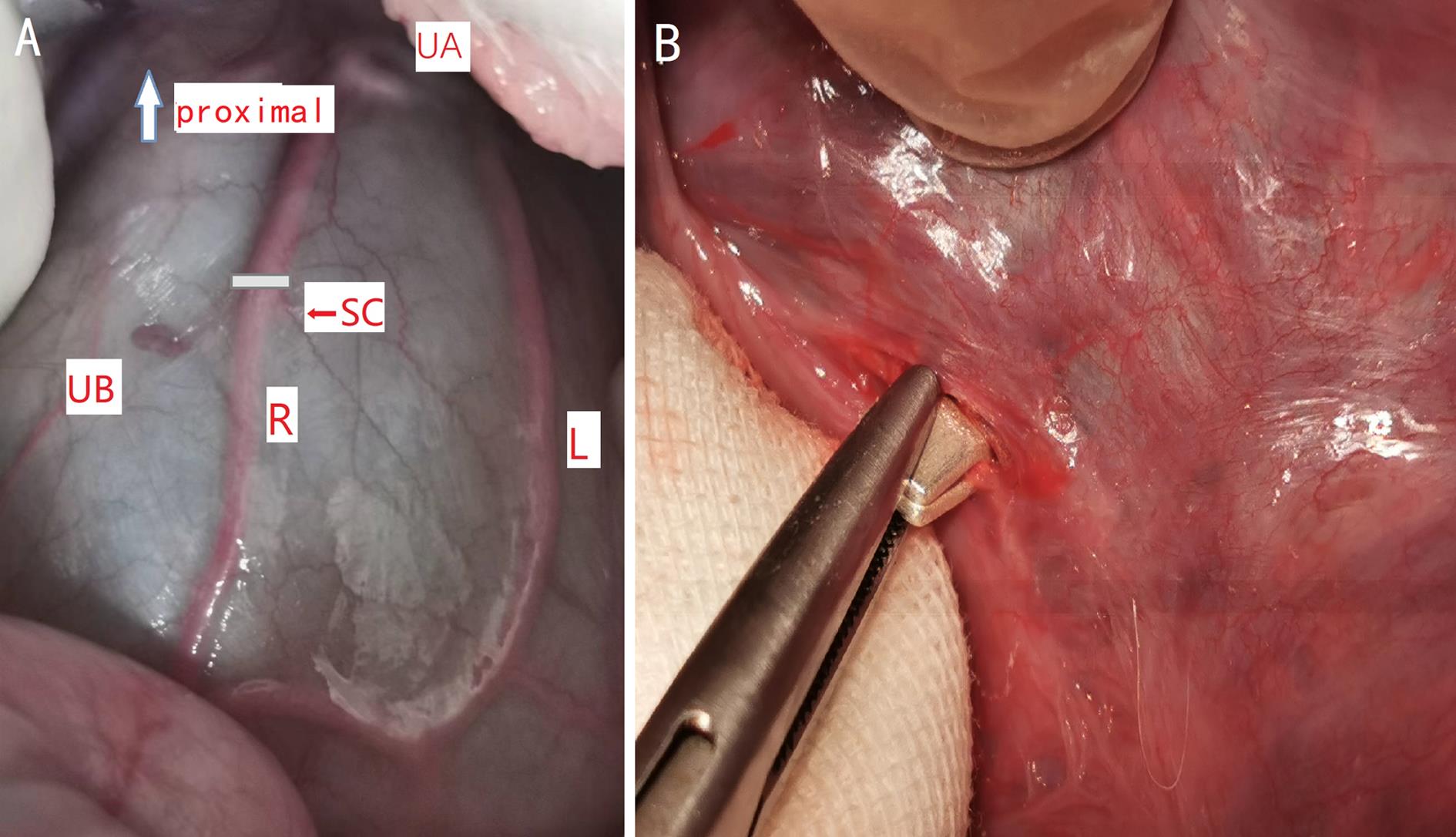
图1 子宫腹侧面血管走向和子宫体动脉上银夹放置位置示意图注:A为子宫腹侧面血管走向示意图(proximal, 近心端;UA,子宫动脉;UB,子宫体;R,右侧子宫体动脉;L,左侧子宫体动脉;SC,银夹位置);B 为银夹部分夹闭子宫体动脉。
Figure 1 Anterior exposure of uterine arteries and the position of the silver clamp placement on uterine body arteryNote:A, Anterior exposure of uterine arteries (UA, uterine artery;UB, uterine body;R, right uterine body artery;L, left uterine body artery;SC, placement of silver clamp); B, Uterine body artery been partially closed by silver clamp.
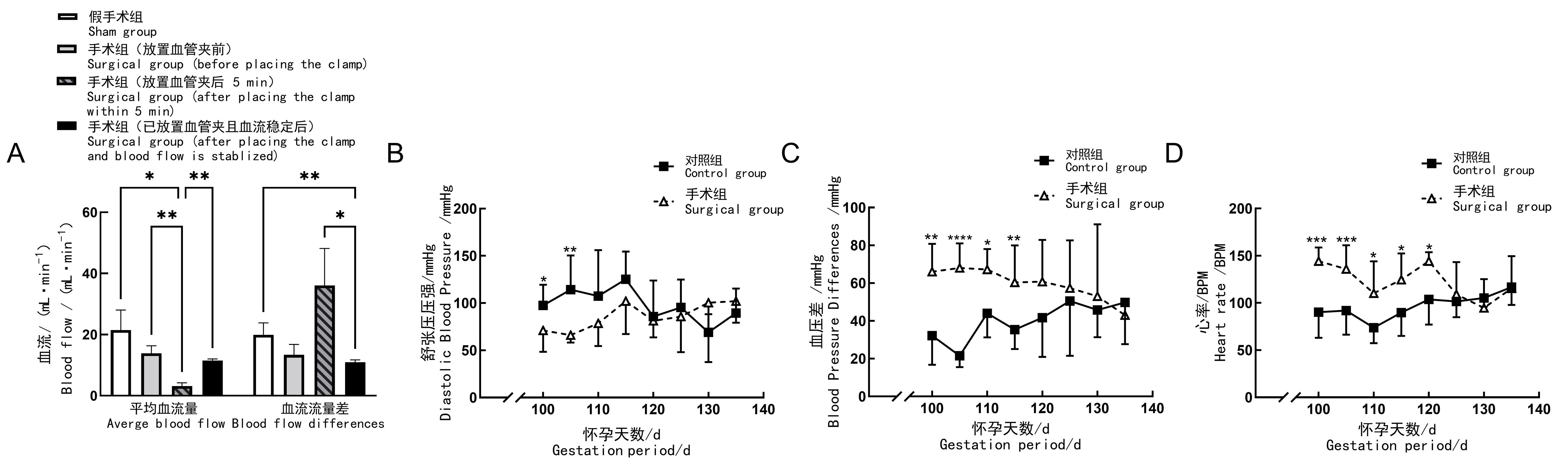
图2 先兆子痫造模后妊娠山羊的血流量、血压及心率情况注:A,子宫体动脉血管内血流变化;B,后肢舒张压变化;C,后肢血压差压变化;D,心率变化(BPM即次/min)。对照组(n=11)妊娠母羊在临产期前不进行任何手术操作;假手术组(n=4)在母羊妊娠100 d时,行先兆子痫假手术造模,即仅暴露并游离其子宫体动脉;手术组(n=8)于母羊妊娠100 d时,在其一侧子宫体动脉上放置银质血管夹,夹闭动脉部分内径,制作先兆子痫模型。组间比较,*P<0.05,**P<0.01,***P<0.001,****P<0.000 1。
Figure 2 Blood flow, blood pressure and heart rate of pregnant goats after preeclampsia modellingNote:A, Blood flow changes of uterine body artery; B, Changes of hind limb diastolic blood pressure;C, Changes of hind limb pulse pressure; D, Change of heart rate (BPM, beat per min). In control group (n=11), no surgical operation was done on animals; in sham group (n=4), sham surgery was performed on the 100th day of gestation, to be specific, only their uterine body arteries were exposed and freed; in surgical group (n=8), preeclampsia modeling was performed by placing silver vascular clamps on one of the uterine body arteries of the animals on the 100th day of gestation to close their internal diameters partially. For comparison between groups, *P<0.05, **P<0.01, ***P<0.001, ****P<0.000 1.
项目 Item | 手术组(n=8) Surgical group (n=8) | 对照组(n=11) Control group (n=11) | |
|---|---|---|---|
术后5 d Postoperation 5 d | 产前 Before parturition | ||
白细胞计数/(103·μL-1) White blood cell count/(103·μL-1) | 38.26±2.81 | 34.00±2.99 | 40.62±1.70 |
淋巴细胞百分比/% Lymphocyte/% | 58.68±2.28 | 58.42±4.22 | 60.94±2.05 |
中间细胞百分比/% Intermediate cell/% | 7.20±1.07 | 7.60±1.09 | 6.61±0.73 |
粒细胞百分比/% Granulocyte/% | 34.12±1.77 | 33.98±3.18 | 32.45±1.63 |
淋巴细胞计数/(103·μL-1) Lymphocyte count/(103·μL-1) | 22.50±1.86 | 20.13±2.85 | 24.81±1.44 |
粒细胞计数/(103·μL-1) Granulocyte count/(103·μL-1) | 12.90±0.81 | 11.30±0.64 | 13.06±0.68 |
红细胞计数/(106·μL-1) Red blood cell count/(106·μL-1) | 1.14±0.14 | 0.65±0.04* | 1.58±0.17 |
血红蛋白计数/(g·L-1) haemoglobin count/(g·L-1) | 101.40±4.24 | 87.75±4.71* | 117.27±5.32 |
血细胞压积/% Packed cell volume/% | 4.52±0.62 | 2.50±0.16* | 6.49±0.76 |
平均红细胞压积/fL Mean corpuscular volume/fL | 40.18±0.31 | 39.53±0.30* | 41.16±0.30 |
平均红细胞血红蛋白量/pg Mean corpuscular hemoglobin/pg | 94.00±10.30 | 135.18±6.02**** | 77.99±4.33 |
平均红细胞血红蛋白浓度/(g·L-1) Mean corpusular hemoglobin concerntration/(g·L-1) | 2 397±286 | 3 526±154**** | 1 924±118 |
红细胞分别宽度变异系数/% coefficient variation of red blood cell volume distribution width/% | 17.26±0.61 | 16.33±0.69 | 17.41±0.43 |
中间细胞计数/(103·μL-1) Intermediate cell count/(103·μL-1) | 2.58±0.44 | 2.40±0.22 | 2.99±0.34 |
表1 先兆子痫山羊模型的血常规组间比较 ()
Table 1 Comparison of blood routine between groups in preeclampsia goat model
项目 Item | 手术组(n=8) Surgical group (n=8) | 对照组(n=11) Control group (n=11) | |
|---|---|---|---|
术后5 d Postoperation 5 d | 产前 Before parturition | ||
白细胞计数/(103·μL-1) White blood cell count/(103·μL-1) | 38.26±2.81 | 34.00±2.99 | 40.62±1.70 |
淋巴细胞百分比/% Lymphocyte/% | 58.68±2.28 | 58.42±4.22 | 60.94±2.05 |
中间细胞百分比/% Intermediate cell/% | 7.20±1.07 | 7.60±1.09 | 6.61±0.73 |
粒细胞百分比/% Granulocyte/% | 34.12±1.77 | 33.98±3.18 | 32.45±1.63 |
淋巴细胞计数/(103·μL-1) Lymphocyte count/(103·μL-1) | 22.50±1.86 | 20.13±2.85 | 24.81±1.44 |
粒细胞计数/(103·μL-1) Granulocyte count/(103·μL-1) | 12.90±0.81 | 11.30±0.64 | 13.06±0.68 |
红细胞计数/(106·μL-1) Red blood cell count/(106·μL-1) | 1.14±0.14 | 0.65±0.04* | 1.58±0.17 |
血红蛋白计数/(g·L-1) haemoglobin count/(g·L-1) | 101.40±4.24 | 87.75±4.71* | 117.27±5.32 |
血细胞压积/% Packed cell volume/% | 4.52±0.62 | 2.50±0.16* | 6.49±0.76 |
平均红细胞压积/fL Mean corpuscular volume/fL | 40.18±0.31 | 39.53±0.30* | 41.16±0.30 |
平均红细胞血红蛋白量/pg Mean corpuscular hemoglobin/pg | 94.00±10.30 | 135.18±6.02**** | 77.99±4.33 |
平均红细胞血红蛋白浓度/(g·L-1) Mean corpusular hemoglobin concerntration/(g·L-1) | 2 397±286 | 3 526±154**** | 1 924±118 |
红细胞分别宽度变异系数/% coefficient variation of red blood cell volume distribution width/% | 17.26±0.61 | 16.33±0.69 | 17.41±0.43 |
中间细胞计数/(103·μL-1) Intermediate cell count/(103·μL-1) | 2.58±0.44 | 2.40±0.22 | 2.99±0.34 |
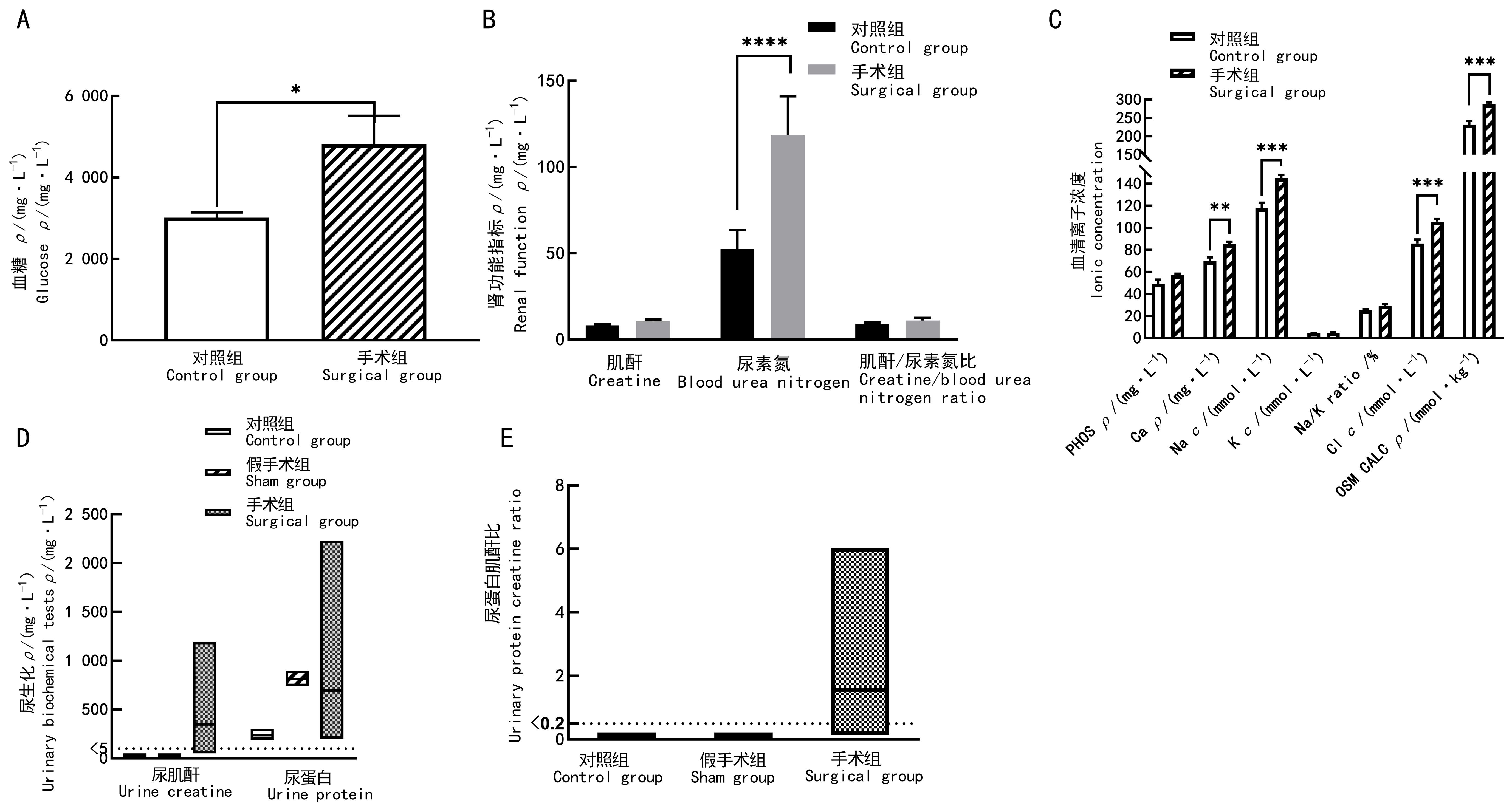
图3 先兆子痫造模后妊娠山羊的血液及尿液生化指标检测结果注:A,血糖;B,肾功能指标;C,血清离子指标(PHOS指磷离子;Ca即钙离子;Na即钠离子;K即钾离子;Na/K指钠钾离子的比值;Cl即氯离子;OSM CALC指血浆渗透压;PHOS和Ca的浓度单位1 mg/L=884 μmol/L);D, 尿肌酐及尿蛋白;E,尿蛋白肌酐比。对照组(n=11)妊娠母羊在临产期前不进行任何手术操作;假手术组(n=4)在母羊妊娠100 d时,行先兆子痫假手术造模,即仅暴露并游离其子宫体动脉;手术组(n=8)于母羊妊娠100 d时,在其一侧子宫体动脉上放置银质血管夹,夹闭动脉部分内径,制作先兆子痫模型。组间比较,*P<0.05,**P<0.01,***P<0.001,****P<0.000 1。
Figure 3 Blood and urinary biochemical results of preeclampsia modeling goat before parturitionNote:A, Blood glucose; B, Renal functional indicators; C, Serum iron indicators (PHOS, phosphate; Ca, calcium; Na, Sodium; K, potassium; Na/K, sodium potassium ratio; Cl, chlorine; OSM CALC, osmotic pressure); D, Urinary creatinine and urinary total protein; E, Creatinine/protein ratio. In the control group (n=11), no surgical operation was done on animals; in sham group (n=4), sham surgery was performed on the 100th day of gestation,to be specific, only their uterine body arteries were exposed and freed;and in surgical group (n=8), preeclampsia modeling was performed by placing silver vascular clamps on one of the uterine body arteries of the animals on the 100th day of gestation to close some of their internal diameters. Comparison between groups, *P<0.05, **P<0.01, ***P<0.001, ****P<0.000 1.
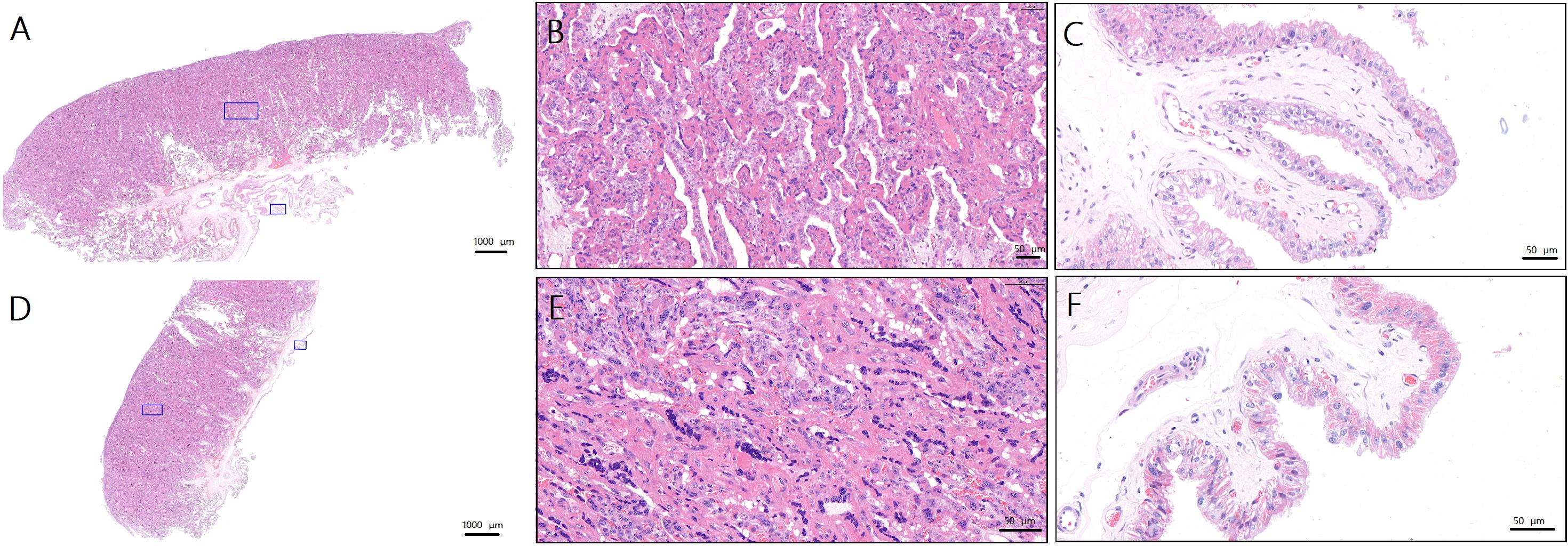
图4 先兆子痫造模后妊娠山羊生产时的手术侧与非手术侧胎盘病理变化(苏木精-伊红染色)注:A,对照组母羊正常胎盘(×1.1;蓝色方框为B、C图的取样位置);B,对照组母羊正常侧胎盘滋养层(×16.9);C,对照组母羊正常侧胎盘绒毛间质(×27.2);D,手术组母羊手术侧胎盘(×1.1;蓝色方框为E、F图的取样位置);E,手术组母羊手术侧胎盘滋养层(×28.7);F,手术组动物手术侧胎盘绒毛间质(×34.3)。A和D图的比例尺大小为1 000 μm;B、C、E和F图的比例尺大小为50 μm。
Figure 4 Pathologic changes in the placenta on the operated and non-operated side during parturition in goats after preeclampsia modeling(HE staining)Note:A, Normal placenta of ewes in control group (×1.1, the blue squares are the sampling sites for figure B and C ); B, Trophoblasts of normal placenta in control group (×16.9); C, Villous stroma of normal placenta in control group (×27.2); D, Surgical side placenta of ewes in surgical group (×1.1, the blue squares are the sampling sites for figure E and F ); E, Trophoblasts of surgical side placenta of ewes in surgical group (×28.7); F, Villous stroma of surgical side placenta of ewes in surgical group (×34.3). Scale bars of figure A and D are 1 000 μm. Proportional scales of figure B, C, E and F are 50 μm.
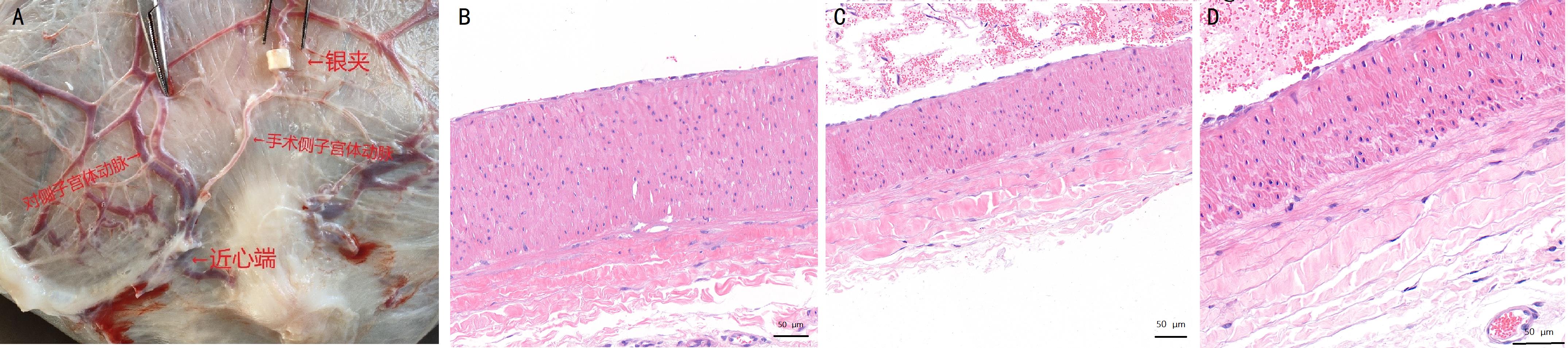
图5 先兆子痫造模后妊娠山羊生产时的两侧子宫体动脉外观及病理比较注:A,妊娠140 d的母羊子宫及动脉示意图(银夹放置在一侧子宫体动脉上);B~D,苏木精-伊红染色观察子宫体动脉病理组织切片(B,非手术侧子宫动脉;C,手术侧造模位置近心端子宫动脉;E,手术侧造模位置远心端子宫动脉)。B~D图的比例尺大小为50 μm。
Figure 5 Comparison of the appearance and pathology of the uterine body arteries on surgical and non-surgical side of the uterus at the time of parturition in pregnant goats after preeclampsia modelingNote:A, Schematic diagram of the uterus and uterine arteries of ewes on the 140th day of gestation (silver clips were placed on one side of the uterine body arteries); B-D, HE-stained histopathologic sections of the uterine body arteries (B, uterine arteries of the nonsurgical side; C, uterine arteries proximal to the surgical side; E, uterine arteries distal from the surgical side). The scale bars figure B-D are 50 μm.
| 1 | FILIPEK A, JUREWICZ E. Preeclampsia - a disease of pregnant women[J]. Postepy Biochem, 2018, 64(4):232-229. DOI: 10.18388/pb.2018_146 . |
| 2 | IVES C W, SINKEY R, RAJAPREYAR I, et al. Preeclampsia—pathophysiology and clinical presentations: JACC state-of-the-art review[J]. J Am Coll Cardiol, 2020, 76(14):1690-1702. DOI: 10.1016/j.jacc.2020.08.014 . |
| 3 | CRAICI I M, WAGNER S J, WEISSGERBER T L, et al. Advances in the pathophysiology of pre-eclampsia and related podocyte injury[J]. Kidney Int, 2014, 86(2):275-285. DOI: 10.1038/ki.2014.17 . |
| 4 | ROBERTS J M, KING T L, BARTON J R, et al. Care plan for individuals at risk for preeclampsia: shared approach to education, strategies for prevention, surveillance, and follow-up[J]. Am J Obstet Gynecol, 2023: S0002-9378(23)00260. DOI: 10.1016/j.ajog.2023.04.023 . |
| 5 | GATFORD K L, ANDRAWEERA P H, ROBERTS C T, et al. Animal models of preeclampsia: causes, consequences, and interventions[J]. Hypertension, 2020, 75(6):1363-1381. DOI: 10.1161/HYPERTENSIONAHA.119.14598 . |
| 6 | LI J, LAMARCA B, RECKELHOFF J F. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model[J]. Am J Physiol Heart Circ Physiol, 2012, 303(1): H1-H8. DOI: 10.1152/ajpheart.00117.2012 . |
| 7 | 肖仲琳, 杨青, 张键, 等. 子痫前期动物模型的研究进展[J]. 生物化学与生物物理进展, 2016, 43(6): 563-569. DOI: 10.16476/j.pibb.2016.0011 |
| XIAO Z L, YANG Q, ZHANG J, et al. Research progress of animal models of preeclampsia[J]. Prog Biochem Biophys, 2016, 43(6): 563-569. DOI: 10.16476/j.pibb.2016.0011 . | |
| 8 | UZUN M, GENCER M, TURKON H, et al. Effects of melatonin on blood pressure, oxidative stress and placental expressions of TNFα, IL-6, VEGF and sFlt-1 in RUPP rat model of preeclampsia[J]. Arch Med Res, 2017, 48(7):592-598. DOI: 10.1016/j.arcmed.2017.08.007 . |
| 9 | SHI M T, YANG X F, SUN L, et al. Comparison of different modified operations in the reduced uteroplacental perfusion pressure rat model of preeclampsia[J]. J Reprod Immunol, 2023, 156:103815. DOI: 10.1016/j.jri.2023.103815 . |
| 10 | BAKRANIA B A, GEORGE E M, GRANGER J P. Animal models of preeclampsia: investigating pathophysiology and therapeutic targets[J]. Am J Obstet Gynecol, 2022, 226(2S): S973-S987. DOI: 10.1016/j.ajog.2020.10.025 . |
| 11 | PEAKER M. Gestation period and litter size in the goat[J]. Br Vet J, 1978, 134(4):379-383. DOI: 10.1016/s0007-1935(17)33441-3 . |
| 12 | SMITH M C, SHERMAN D M. Goat Medicine[M]. 2nd ed. Hoboken: Blackwell Pub Professional, 2009. |
| 13 | 吴正一. 实验外科技术: 羊的全身麻醉方法[J]. 上海口腔医学, 2000, 9(1): 42-44. DOI: 10.3969/j.issn.1006-7248.2000.01.015 . |
| WU Z Y. Techniques in experimental surgery—general anesthesia of the goat[J]. Shanghai J Stomatol, 2000, 9(1): 42-44. DOI: 10.3969/j.issn.1006-7248.2000.01.015 . | |
| 14 | PRACTICE A C O O. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists[J]. Int J Gynaecol Obstet, 2002, 77(1):67-75. |
| 15 | JACKSON P, COCKCROFT P. Clinical examination of farm animals[M]. Hoboken: Blackwell Science Ltd, 2002. |
| 16 | 杨怡珂, 漆洪波. 美国妇产科医师学会(ACOG)"妊娠期高血压和子痫前期指南2019版"要点解读(第一部分)[J]. 中国实用妇科与产科杂志, 2019, 35(8):895-899. DOI: 10.19538/j.fk2019080112 . |
| YANG Y K, QI H B. Interpretation of the American college of obstetricians and gynecologists (ACOG) guidelines for pregnancy-induced hypertension and preeclampsia (2019 edition) (part I)[J]. Chin J Pract Gynecol Obstet, 2019, 35(8):895-899. DOI: 10.19538/j.fk2019080112 . | |
| 17 | PHIPPS E A, THADHANI R, BENZING T, et al. Pre-eclampsia: pathogenesis, novel diagnostics and therapies[J]. Nat Rev Nephrol, 2019, 15(5):275-289. DOI: 10.1038/s41581-019-0119-6 . |
| 18 | MILLER D, MOTOMURA K, GALAZ J, et al. Cellular immune responses in the pathophysiology of preeclampsia[J]. J Leukoc Biol, 2021, 111(1):237-260. DOI: 10.1002/JLB.5RU1120-787RR . |
| 19 | JUNG E, ROMERO R, YEO L, et al. The etiology of preeclampsia[J]. Am J Obstet Gynecol, 2022, 226(2S): S844-S866. DOI: 10.1016/j.ajog.2021.11.1356 . |
| 20 | 周晶, 严国锋, 罗章源, 等. 子痫前期大鼠模型建立及相关指标监测[J]. 实验动物与比较医学, 2015, 35(6): 448-452. DOI: 10.3969/j.issn.1674-5817.2015.06.004 . |
| ZHOU J, YAN G F, LUO Z Y, et al. Establishment of preeclampsia model in rat and detection on related indicators[J]. Lab Anim Comp Med, 2015, 35(6): 448-452. DOI: 10.3969/j.issn.1674-5817.2015.06.004 . | |
| 21 | PAAUW N D, JOLES J A, SPRADLEY F T, et al. Exposure to placental ischemia impairs postpartum maternal renal and cardiac function in rats[J]. Am J Physiol Regul Integr Comp Physiol, 2017, 312(5): R664-R670. DOI: 10.1152/ajpregu.00510.2016 . |
| 22 | CORDINA M, BHATTI S, FERNANDEZ M, et al. Maternal hemoglobin at 27-29 weeks' gestation and severity of pre-eclampsia[J]. J Matern Fetal Neonatal Med, 2015, 28(13):1575-1580. DOI: 10.3109/14767058.2014.961006 . |
| 23 | Anon. Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222[J]. Obstet Gynecol, 2020, 135(6): e237-e260. DOI: 10.1097/AOG.0000000000003891 . |
| 24 | HEIDA K Y, BOTS M L, DE GROOT C J, et al. Cardiovascular risk management after reproductive and pregnancy-related disorders: a Dutch multidisciplinary evidence-based guideline[J]. Eur J Prev Cardiol, 2016, 23(17):1863-1879. DOI: 10.1177/2047487316659573 . |
| 25 | RICHARDS C, SESPEREZ K, CHHOR M, et al. Characterisation of cardiac health in the reduced uterine perfusion pressure model and a 3D cardiac spheroid model, of preeclampsia[J]. Biol Sex Differ, 2021, 12(1):31. DOI: 10.1186/s13293-021-00376-1 . |
| [1] | 刘亚益, 贾云凤, 左一鸣, 张军平, 吕仕超. 心气阴两虚证动物模型的构建方法与评价进展[J]. 实验动物与比较医学, 2025, 45(4): 411-421. |
| [2] | 赵鑫, 王晨曦, 石文清, 娄月芬. 斑马鱼在炎症性肠病机制及药物研究中的应用进展[J]. 实验动物与比较医学, 2025, 45(4): 422-431. |
| [3] | 李会萍, 高洪彬, 温金银, 杨锦淳. 疾病动物模型数字化图谱数据库平台的构建与初步应用[J]. 实验动物与比较医学, 2025, 45(3): 300-308. |
| [4] | 潘颐聪, 蒋汶洪, 胡明, 覃晓. 慢性肾脏病大鼠主动脉钙化模型的术式优化及效果评价[J]. 实验动物与比较医学, 2025, 45(3): 279-289. |
| [5] | 陈钰涵, 陈瑾玲, 李欣, 区燕华, 王斯, 陈镜伊, 王兴易, 袁嘉丽, 段媛媛, 羊忠山, 牛海涛. 基于中西医临床病证特点的重症肌无力动物模型分析[J]. 实验动物与比较医学, 2025, 45(2): 176-186. |
| [6] | 连辉, 姜艳玲, 刘佳, 张玉立, 谢伟, 薛晓鸥, 李健. 异常子宫出血大鼠模型的构建与评价[J]. 实验动物与比较医学, 2025, 45(2): 130-146. |
| [7] | 罗世雄, 张赛, 陈慧. 常见哮喘动物模型的建立方法与评价研究进展[J]. 实验动物与比较医学, 2025, 45(2): 167-175. |
| [8] | 王碧莹, 鲁家铄, 昝桂影, 陈若松, 柴景蕊, 刘景根, 王瑜珺. 啮齿类动物药物成瘾模型的构建方法和应用进展[J]. 实验动物与比较医学, 2025, 45(2): 158-166. |
| [9] | 费彬, 郭文科, 郭建平. 疝疾病动物模型研究及新型疝修补材料应用进展[J]. 实验动物与比较医学, 2025, 45(1): 55-66. |
| [10] | 杨家豪, 丁纯蕾, 钱风华, 孙旗, 姜旭升, 陈雯, 沈梦雯. 脓毒症相关脏器损伤动物模型研究进展[J]. 实验动物与比较医学, 2024, 44(6): 636-644. |
| [11] | 孙效容, 苏丹, 贵文娟, 陈玥. 手术诱导大鼠中重度膝骨关节炎模型的建立与评价[J]. 实验动物与比较医学, 2024, 44(6): 597-604. |
| [12] | 田芳, 潘滨, 史佳怡, 徐燕意, 李卫华. 大气细颗粒物PM2.5暴露动物模型建立方法及在生殖毒性研究中的应用进展[J]. 实验动物与比较医学, 2024, 44(6): 626-635. |
| [13] | 赵小娜, 王鹏, 叶茂青, 曲新凯. 应用Triacsin C构建新型高血糖肥胖小鼠心功能减退模型[J]. 实验动物与比较医学, 2024, 44(6): 605-612. |
| [14] | 涂颖欣, 纪依澜, 王菲, 杨东明, 王冬冬, 孙芷馨, 戴悦欣, 王言吉, 阚广捍, 吴斌, 赵德明, 杨利峰. 小型猪后肢去负荷模拟失重模型的建立与组织损伤研究[J]. 实验动物与比较医学, 2024, 44(5): 475-486. |
| [15] | 黄冬妍, 吴建辉. 生殖毒理学研究动物模型的建立方法及应用评价[J]. 实验动物与比较医学, 2024, 44(5): 550-559. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||