













实验动物与比较医学 ›› 2024, Vol. 44 ›› Issue (5): 567-576.DOI: 10.12300/j.issn.1674-5817.2024.079
郑卿勇1,2( )(
)( ), 李腾飞3, 许建国1,2, 周泳佳3, 马智超4, 王娜5, 李莫兰5, 杨雯景5, 吴佩润4, 王海东6, 田金徽1,2(
), 李腾飞3, 许建国1,2, 周泳佳3, 马智超4, 王娜5, 李莫兰5, 杨雯景5, 吴佩润4, 王海东6, 田金徽1,2( )(
)( )
)
收稿日期:2024-06-03
修回日期:2024-08-08
出版日期:2024-10-25
发布日期:2024-10-25
通讯作者:
田金徽(1978—),男,博士,教授,研究方向:网状Meta分析与卫生技术评估。E-mail:tjh996@163.com。ORCID:0000-0002-3859-9587作者简介:郑卿勇(1998—),男,博士研究生,研究方向:循证医学与医学信息学。E-mail:easonzz@foxmail.com。ORCID:0000-0002-9480-0169;
李腾飞(1998—),男,硕士研究生,研究方向:循证护理。E-mail:ltf980102@163.com。ORCID:0009-0005-1168-3075
基金资助:
ZHENG Qingyong1,2( )(
)( ), LI Tengfei3, XU Jianguo1,2, ZHOU Yongjia3, MA Zhichao4, WANG Na5, LI Molan5, YANG Wenjing5, WU Peirun4, WANG Haidong6, TIAN Jinhui1,2(
), LI Tengfei3, XU Jianguo1,2, ZHOU Yongjia3, MA Zhichao4, WANG Na5, LI Molan5, YANG Wenjing5, WU Peirun4, WANG Haidong6, TIAN Jinhui1,2( )(
)( )
)
Received:2024-06-03
Revised:2024-08-08
Published:2024-10-25
Online:2024-10-25
Contact:
TIAN Jinhui (ORCID: 0000-0002-3859-9587), E-mail: tjh996@163.com摘要:
动物实验证据整合是生物医学研究中的关键环节,为疾病机制的深入研究与新药开发等提供了重要的先验信息。目前,动物模型在模拟人类疾病方面发挥着不可替代的作用,但动物实验证据整合在实践中仍面临诸多挑战,包括受重视程度不足、研究设计的异质性明显、高发表偏倚、与临床研究实践存在差距等。本文首先指出动物实验原始研究证据的现存问题,包括动物模型的选择和适用性、动物实验研究设计的考量、动物实验证据转化的影响因素等方面;然后介绍了多种动物实验证据整合方法的应用进展,如系统评价与Meta分析、系统评价再评价/伞形综述、范围综述、证据图谱等;最后探讨了目前动物实验证据整合面临的主要挑战以及针对性的改进策略,旨在提高动物实验研究成果向临床实践转化的效率,并推动循证医学的发展。未来,通过不断优化原始实验研究方案与证据整合实践,有望逐步构建一个更为科学、高效的动物实验证据综合环境,为临床试验与人类健康事业作出更大的贡献。
中图分类号:
郑卿勇,李腾飞,许建国,等. 动物实验证据整合方法研究的进展与挑战[J]. 实验动物与比较医学, 2024, 44(5): 567-576. DOI: 10.12300/j.issn.1674-5817.2024.079.
ZHENG Qingyong,LI Tengfei,XU Jianguo,et al. Advances and Challenges in the Research of Integration Methods of Animal Experimental Evidence[J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 567-576. DOI: 10.12300/j.issn.1674-5817.2024.079.
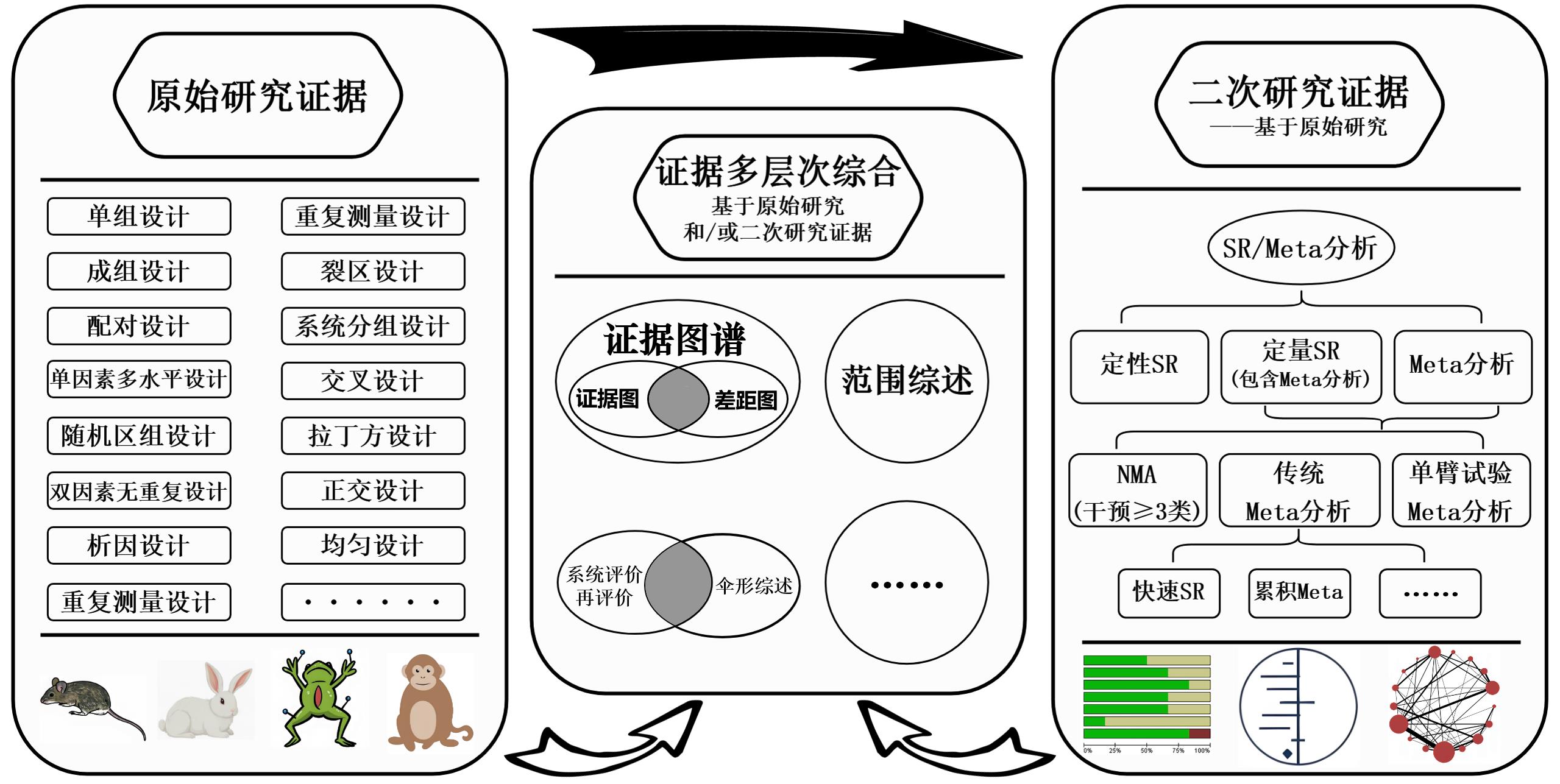
图1 动物实验证据整合方法现状注:SR指系统评价;NMA指网状Meta分析。
Figure 1 Current methods for integrating evidence from animal experimentsNote:SR, systematic review; NMA, network meta-analysis.

图2 动物实验各类型研究开展数量的分布趋势(2000—2023年)注:SR指系统评价;本研究数量是基于中国知网(CNKI)与PubMed数据库的综合结果。
Figure 2 Distribution trends of various types of animal experimental studies (2000-2023)Note: SR, systematic review. The number of studies is based on a combined result from China National Knowledge Infrastructure (CNKI) and PubMed databases.
整合方法 Integration methods | 应用特点 Application characteristics | 实例参考 Examples |
|---|---|---|
系统评价与Meta分析 Systematic review and meta-analysis | 通过严谨的研究设计和方法学,对现有的动物实验数据进行系统性综述与定量分析(部分),以提供最高级别的证据。Meta分析可综合多个研究的结果,对其进行定量整合,揭示干预措施的总体效果 | Perna A, et al/1996[ |
单臂试验Meta分析 Meta-analysis of single-arm trials | 通常用于关注单个群体中事件的发生率,特别适用于处理稀有事件或样本量较小的研究 | Alonso S, et al/2016[ |
累积Meta分析 Cumulative meta-analysis | 将研究资料作为连续性的统一体,评估随时间累积研究结果的动态变化趋势 | Inchuai R, et al/2021[ |
定性系统评价/Meta整合 Qualitative systematic review/meta-synthesis | 关注研究结果的叙述性合成,适用于研究设计差异过大或数据类型不适合定量分析的情境 | Pommergaard H C, et al/2011[ |
快速系统评价 Rapid review | 高效、加速版的系统评价方法,适用于紧急决策或时间敏感情况下的证据综合 | Ciapponi A, et al/2011[ |
网状Meta分析 Network meta-analysis | 结合直接和间接证据,综合多个比较组的结果,评估不同干预措施之间的相对效果 | Yao M, et al /2015[ |
系统评价再评价/伞形综述 Overview of systematic reviews/ umbrella review | 对已有的多个系统评价进行再评价,部分会再次进行统计分析,提供某一领域的全面证据概览 | Hirst J A, et al /2014[ |
范围综述 Scoping review | 映射某一研究领域的文献分布,识别研究的数量和特征,揭示知识空白,通常不进行质量评估和定量分析 | Gardner E G, et al /2019[ |
证据图谱 Evidence mapping | 主要包括证据图与证据差距图,其系统性地检索和分析现有证据,通过表格或图形形式展示研究现状和证据差距,支持决策制定和研究规划 | Koch S E, et al /2022[ moola S, et al /2021[ |
表1 动物实验研究证据的主要整合方法概览
Table 1 Overview of main methods for synthesizing evidence from animal experiment studies
整合方法 Integration methods | 应用特点 Application characteristics | 实例参考 Examples |
|---|---|---|
系统评价与Meta分析 Systematic review and meta-analysis | 通过严谨的研究设计和方法学,对现有的动物实验数据进行系统性综述与定量分析(部分),以提供最高级别的证据。Meta分析可综合多个研究的结果,对其进行定量整合,揭示干预措施的总体效果 | Perna A, et al/1996[ |
单臂试验Meta分析 Meta-analysis of single-arm trials | 通常用于关注单个群体中事件的发生率,特别适用于处理稀有事件或样本量较小的研究 | Alonso S, et al/2016[ |
累积Meta分析 Cumulative meta-analysis | 将研究资料作为连续性的统一体,评估随时间累积研究结果的动态变化趋势 | Inchuai R, et al/2021[ |
定性系统评价/Meta整合 Qualitative systematic review/meta-synthesis | 关注研究结果的叙述性合成,适用于研究设计差异过大或数据类型不适合定量分析的情境 | Pommergaard H C, et al/2011[ |
快速系统评价 Rapid review | 高效、加速版的系统评价方法,适用于紧急决策或时间敏感情况下的证据综合 | Ciapponi A, et al/2011[ |
网状Meta分析 Network meta-analysis | 结合直接和间接证据,综合多个比较组的结果,评估不同干预措施之间的相对效果 | Yao M, et al /2015[ |
系统评价再评价/伞形综述 Overview of systematic reviews/ umbrella review | 对已有的多个系统评价进行再评价,部分会再次进行统计分析,提供某一领域的全面证据概览 | Hirst J A, et al /2014[ |
范围综述 Scoping review | 映射某一研究领域的文献分布,识别研究的数量和特征,揭示知识空白,通常不进行质量评估和定量分析 | Gardner E G, et al /2019[ |
证据图谱 Evidence mapping | 主要包括证据图与证据差距图,其系统性地检索和分析现有证据,通过表格或图形形式展示研究现状和证据差距,支持决策制定和研究规划 | Koch S E, et al /2022[ moola S, et al /2021[ |
| 1 | 张连峰, 崔韶. 国内外实验动物模型概览[J]. 科技导报, 2017, 35(24):27-31. DOI: 10.3981/j.issn.1000-7857.2017.24.002 . |
| ZHANG L F, CUI S. An overview of laboratory animal models at home and abroad[J]. Sci Technol Rev, 2017, 35(24):27-31. DOI: 10.3981/j.issn.1000-7857.2017.24.002 . | |
| 2 | SANDERCOCK P, ROBERTS I. Systematic reviews of animal experiments[J]. Lancet, 2002, 360(9333):586. DOI: 10.1016/S0140-6736(02)09812-4 . |
| 3 | 贺争鸣, 邢瑞昌, 方喜业, 等. 论实验动物福利、动物实验与动物实验替代方法[J]. 实验动物科学与管理, 2005, 22(1):61-64.DOI:10.3969/j.issn.1006-6179.2005.01.021 . |
| HE Z M, XING R C, FANG X Y, et al. Laboratory animal welfare, animal experiment and replacement method[J]. Lab Anim Sci Adm, 2005, 22(1):61-64.DOI:10.3969/j.issn.1006-6179.2005.01.021 . | |
| 4 | HOOIJMANS C R, ROVERS M M, DE VRIES R B M, et al. SYRCLE's risk of bias tool for animal studies[J]. BMC Med Res Methodol, 2014, 14:43. DOI: 10.1186/1471-2288-14-43 . |
| 5 | 陈匡阳, 王亚楠, 赵雅琴, 等. 国内动物实验系统评价/Meta分析研究的现状分析[J]. 中国循证医学杂志, 2015, 15(4):414-418. DOI: 10.7507/1672-2531.20150070 . |
| CHEN K Y, WANG Y N, ZHAO Y Q, et al. Analyzing the systematic review/meta-analysis of animal studies published in Chinese journals[J]. Chin J Evid Based Med, 2015, 15(4):414-418. DOI: 10.7507/1672-2531.20150070 . | |
| 6 | 尚志忠, 姜彦彪, 赵冰, 等. 动物实验Meta分析的数据处理[J]. 中国循证心血管医学杂志, 2019, 11(12):1437-1440, 1445. DOI: 10.3969/j.issn.1674-4055.2019.12.06 . |
| SHANG Z Z, JIANG Y B, ZHAO B, et al. Data processing of Meta-analysis in animal studies[J]. Chin J Evid Based Cardiovasc Med, 2019, 11(12):1437-1440, 1445. DOI: 10.3969/j.issn.1674-4055.2019.12.06 . | |
| 7 | HOOIJMANS C R, INTHOUT J, RITSKES-HOITINGA M, et al. Meta-analyses of animal studies: an introduction of a valuable instrument to further improve healthcare[J]. ILAR J, 2014, 55(3):418-426. DOI: 10.1093/ilar/ilu042 . |
| 8 | VESTERINEN H M, SENA E S, EGAN K J, et al. Meta-analysis of data from animal studies: a practical guide[J]. J Neurosci Methods, 2014, 221:92-102. DOI: 10.1016/j.jneumeth.2013. 09.010 . |
| 9 | 曾茂贵, 郑沁鈊. 中药药理研究中证候动物模型的选择和应用[J]. 福建中医药, 2007, 38(3):60-62. DOI: 10.3969/j.issn.1000-338X.2007.03.043 . |
| ZENG M G, ZHENG Q X. Selection and application of syndrome animal model in pharmacological research of traditional Chinese medicine[J]. Fujian J Tradit Chin Med, 2007, 38(3):60-62. DOI: 10.3969/j.issn.1000-338X.2007.03.043 . | |
| 10 | 胡凯燕, 邢丽娜, 姜彦彪, 等. 促进临床前动物实验系统评价发展, 提高其成果的转化和利用[J]. 中国循证心血管医学杂志, 2019, 11(12):1423-1425. DOI: 10.3969/j.issn.1674-4055.2019.12.03 . |
| HU K Y, XING L N, JIANG Y B, et al. Promoting the development of preclinical animal experimental systematic reviews, and improving the transformation and utilization of its results[J]. Chin J Evid Based Cardiovasc Med, 2019, 11(12):1423-1425. DOI: 10.3969/j.issn.1674-4055.2019.12.03 . | |
| 11 | VAMATHEVAN J J, HALL M D, HASAN S, et al. Minipig and beagle animal model genomes aid species selection in pharmaceutical discovery and development[J]. Toxicol Appl Pharmacol, 2013, 270(2):149-157. DOI: 10.1016/j.taap. 2013. 04.007 . |
| 12 | 刘晓辰, 付维力. 骨关节炎动物模型的选择[J]. 中国组织工程研究, 2020, 24(11):1769-1776. DOI: 10.3969/j.issn.2095-4344.1995 . |
| LIU X C, FU W L. Selection of animal models of osteoarthritis[J]. Chin J Tissue Eng Res, 2020, 24(11):1769-1776. DOI: 10.3969/j.issn.2095-4344.1995 . | |
| 13 | 中国研究型医院学会医学动物实验专家委员会. 自发性脑出血动物模型选择及临床前药物试验指南(2024年版)[J]. 实验动物与比较医学, 2024, 44(1):3-30. DOI: 10.12300/j.issn.1674-5817.2024.001 . |
| Committee of Experts on Medical Animal Experiments, Chinese Research Hospital Association. Guidelines for the selection of animal models and preclinical drug trials for spontaneous intracerebral hemorrhage (2024 edition)[J]. Lab Anim Comp Med, 2024, 44(1):3-30. DOI: 10.12300/j.issn.1674-5817.2024.001 . | |
| 14 | 吴玥, 王珏, 冯婷婷, 等. 基于动物模型的药物筛选数据库的建立[J]. 中国实验动物学报, 2023, 31(6):778-786. DOI: 10.3969/j.issn.1005-4847.2023.06.010 . |
| WU Y, WANG J, FENG T T, et al. Construction of a drug screening database based on animal models[J]. Acta Lab Animalis Sci Sin, 2023, 31(6):778-786. DOI: 10.3969/j.issn.1005-4847.2023.06.010 . | |
| 15 | 丁雪梅, 白春艳, 张晓君, 等. 动物科学和动物医学专业常用的试验设计方法的选择[J]. 黑龙江畜牧兽医, 2020(6):37-44. DOI: 10.13881/j.cnki.hljxmsy.2019.09.0261 . |
| DING X M, BAI C Y, ZHANG X J, et al. Selection of commonly used experimental design methods in animal science and veterinary medicine[J]. Heilongjiang Anim Sci Vet Med, 2020(6):37-44. DOI: 10.13881/j.cnki.hljxmsy.2019.09.0261 . | |
| 16 | 邹华, 田东波, 刘春磊, 等. 不同剂量红霉素在博来霉素诱导的小鼠肺纤维化中的疗效观察[J]. 深圳中西医结合杂志, 2017, 27(18):16-18, 199. DOI: 10.16458/j.cnki.1007-0893.2017.18.007 . |
| ZOU H, TIAN D B, LIU C L, et al. Therapeutic effect of erythromycin in different doses on bleomycin-induced pulmonary fibrosis in mice[J]. Shenzhen J Integr Tradit Chin West Med, 2017, 27(18):16-18, 199. DOI: 10.16458/j.cnki.1007-0893.2017.18.007 . | |
| 17 | PERCIE DU SERT N, HURST V, AHLUWALIA A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research[J]. PLoS Biol, 2020, 18(7): e3000410. DOI: 10.1371/journal.pbio.3000410 . |
| 18 | PERCIE DU SERT N, AHLUWALIA A, ALAM S, et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0[J]. PLoS Biol, 2020, 18(7): e3000411. DOI: 10.1371/journal.pbio.3000411 . |
| 19 | LANDIS S C, AMARA S G, ASADULLAH K, et al. A call for transparent reporting to optimize the predictive value of preclinical research[J]. Nature, 2012, 490(7419):187-191. DOI: 10.1038/nature11556 . |
| 20 | 胡锴克, 徐冶. 人源化动物模型的研究进展[J]. 吉林医药学院学报, 2021, 42(1):42-44. |
| HU K K, XU Y. Research progress of humanized animal model[J]. J Jilin Med Univ, 2021, 42(1):42-44. | |
| 21 | 路群, 顾觉奋. 药物高通量筛选技术应用研究进展[J]. 今日药学, 2010, 20(2):2-5, 15. DOI:CNKI:SUN:YAXU.0.2010-02-005 . |
| LU Q, GU J F. Research progress on the application of drug Qualcomm screening technology[J]. Pharm Today, 2010, 20(2):2-5, 15. DOI:CNKI:SUN:YAXU.0.2010-02-005 . | |
| 22 | 袁顺, 王宁, 孟丹丹, 等. 系统生物学在中医证候模型评价方法中的应用与研究进展[J]. 中国中西医结合杂志, 2020, 40(10):1252-1257. DOI: 10.7661/j.cjim.20200904.335 . |
| YUAN S, WANG N, MENG D D, et al. Application and progress of systems biology in evaluation of TCM syndrome models[J]. Chin J Integr Tradit West Med, 2020, 40(10):1252-1257. DOI: 10.7661/j.cjim.20200904.335 . | |
| 23 | POUND P, EBRAHIM S, SANDERCOCK P, et al. Where is the evidence that animal research benefits humans?[J]. BMJ, 2004, 328(7438):514-517. DOI: 10.1136/bmj.328.7438.514 . |
| 24 | PERNA A, REMUZZI G. Abnormal permeability to proteins and glomerular lesions: a meta-analysis of experimental and human studies[J]. Am J Kidney Dis, 1996, 27(1):34-41. DOI: 10.1016/s0272-6386(96)90028-1 . |
| 25 | ALONSO S, DOHOO I, LINDAHL J, et al. Prevalence of tuberculosis, brucellosis and trypanosomiasis in cattle in Tanzania: a systematic review and meta-analysis[J]. Anim Health Res Rev, 2016, 17(1):16-27. DOI: 10.1017/S146625231600013X . |
| 26 | INCHUAI R, WEERAKUN S, NGUYEN H N, et al. Global prevalence of chlamydial infections in reptiles: a systematic review and meta-analysis[J]. Vector Borne Zoonotic Dis, 2021, 21(1):32-39. DOI: 10.1089/vbz.2020.2654 . |
| 27 | POMMERGAARD H C, ROSENBERG J, SCHUMACHER-PETERSEN C, et al. Choosing the best animal species to mimic clinical colon anastomotic leakage in humans: a qualitative systematic review[J]. Eur Surg Res, 2011, 47(3):173-181. DOI: 10.1159/000330748 . |
| 28 | CIAPPONI A, BARDACH A, MAZZONI A, et al. Safety of components and platforms of COVID-19 vaccines considered for use in pregnancy: a rapid review[J]. Vaccine, 2021, 39(40):5891-5908. DOI: 10.1016/j.vaccine.2021.08.034 . |
| 29 | YAO M, YANG L, WANG J, et al. Neurological recovery and antioxidant effects of curcumin for spinal cord injury in the rat: a network meta-analysis and systematic review[J]. J Neurotrauma, 2015, 32(6):381-391. DOI: 10.1089/neu.2014.3520 . |
| 30 | HIRST J A, HOWICK J, ARONSON J K, et al. The need for randomization in animal trials: an overview of systematic reviews[J]. PLoS One, 2014, 9(6): e98856. DOI: 10.1371/journal.pone.0098856 . |
| 31 | GARDNER E G, KELTON D, POLJAK Z, et al. A rapid scoping review of Middle East respiratory syndrome coronavirus in animal hosts[J]. Zoonoses Public Health, 2019, 66(1):35-46. DOI: 10.1111/zph.12537 . |
| 32 | KOCH S E, DE KORT B J, HOLSHUIJSEN N, et al. Animal studies for the evaluation of in situ tissue-engineered vascular grafts - a systematic review, evidence map, and meta-analysis[J]. NPJ Regen Med, 2022, 7(1):17. DOI: 10.1038/s41536-022-00211-0 . |
| 33 | MOOLA S, BERI D, SALAM A, et al. Leptospirosis prevalence and risk factors in India: evidence gap maps[J]. Trop Doct, 2021, 51(3):415-421. DOI: 10.1177/00494755211005203 . |
| 34 | 熊玮, 魏强, 刘雪梅. 动物实验研究的系统评价简介[J]. 中国循证医学杂志, 2005, 5(2):161-163, 173. DOI: 10.3969/j.issn.1672-2531.2005.02.016 . |
| XIONG W, WEI Q, LIU X M. Introduction to systematic reviews of animal studies[J]. Chin J Evid Based Med, 2005, 5(2):161-163, 173. DOI: 10.3969/j.issn.1672-2531.2005.02.016 . | |
| 35 | 赵霏, 唐晓宇, 寇城坤, 等. 动物实验系统评价/Meta分析的质量和报告特征[J]. 中国循证医学杂志, 2018, 18(8):871-877. DOI: 10.7507/1672-2531.201803091 . |
| ZHAO F, TANG X Y, KOU C K, et al. Methodological and reporting quality of systematic review/meta-analysis of animal studies[J]. Chin J Evid Based Med, 2018, 18(8):871-877. DOI: 10.7507/1672-2531.201803091 . | |
| 36 | 陈匡阳, 屈丽娜, 胡芳, 等. 动物实验系统评价/Meta分析检索策略报告情况调查[J]. 中国循证医学杂志, 2016, 16(3):348-353. DOI: 10.7507/1672-2531.20160054 . |
| CHEN K Y, QU L N, HU F, et al. The search strategy of systematic review/meta-analysis of animal research: a survey[J]. Chin J Evid Based Med, 2016, 16(3):348-353. DOI: 10.7507/1672-2531.20160054 . | |
| 37 | 许家科, 赵璐璐, 廖绪亮, 等. 循证构建动物实验系统评价制作流程[J]. 中国循证医学杂志, 2017, 17(11):1357-1364. DOI: 10.7507/1672-2531.201707133 . |
| XU J K, ZHAO L L, LIAO X L, et al. Systematic development of a standard process for systematic reviews of animal experimental studies[J]. Chin J Evid Based Med, 2017, 17(11):1357-1364. DOI: 10.7507/1672-2531.201707133 . | |
| 38 | 赵冰, 胡凯燕, 曾宪涛, 等. 中医药动物实验系统评价的报告指南[J]. 中国循证医学杂志, 2022, 8(5):508-516. DOI:10.7507/1672-2531.202201116 . |
| ZHAO B, HU K Y, ZENG X T, et al. Reporting guideline for systematic reviews of animal experiments in the field of traditional Chinese medicine[J]. Chin J Evid Based Med, 2022, 8(5):508-516. DOI:10.7507/1672-2531.202201116 . | |
| 39 | 刘曼, 陈文松, 刘玉秀, 等. 单组率研究稀疏数据的Meta分析方法[J]. 中国循证儿科杂志, 2020, 15(6):471-475. DOI: 10.3969/j.issn.1673-5501.2020.06.016 . |
| LIU M, CHEN W S, LIU Y X, et al. Meta-analysis method of sparse data in single group rate research[J]. Chin J Evid Based Pediatr, 2020, 15(6):471-475. DOI: 10.3969/j.issn.1673-5501.2020.06.016 . | |
| 40 | 赵景波. 累积Meta分析方法及其在临床医学研究中的应用[J]. 循证医学, 2002, 2(3):167-171. DOI: 10.3969/j.issn.1671-5144.2002.03.020 . |
| ZHAO J B. Cumulative meta-analysis method and its application in research of clinical medicine[J]. J Evid Based Med, 2002, 2(3):167-171. DOI: 10.3969/j.issn.1671-5144.2002.03.020 . | |
| 41 | SONG F J, LOKE Y K, WALSH T, et al. Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews[J]. BMJ, 2009, 338: b1147. DOI: 10.1136/bmj.b1147 . |
| 42 | GATES M, GATES A, PIEPER D, et al. Reporting guideline for overviews of reviews of healthcare interventions: development of the PRIOR statement[J]. BMJ, 2022, 378: e070849. DOI: 10.1136/bmj-2022-070849 . |
| 43 | COLQUHOUN H L, LEVAC D, O'BRIEN K K, et al. Scoping reviews: time for clarity in definition, methods, and reporting[J]. J Clin Epidemiol, 2014, 67(12):1291-1294. DOI: 10.1016/j.jclinepi.2014.03.013 . |
| 44 | SNILSTVEIT B, VOJTKOVA M, BHAVSAR A, et al. Evidence & Gap Maps: a tool for promoting evidence informed policy and strategic research agendas[J]. J Clin Epidemiol, 2016, 79:120-129. DOI: 10.1016/j.jclinepi.2016.05.015 . |
| 45 | 李艳飞, 李秀霞, 李睿, 等. 证据图谱的制作与报告[J]. 中国循证医学杂志, 2020, 20(9):1098-1103. DOI: 10.7507/1672-2531.202001079 . |
| LI Y F, LI X X, LI R, et al. Generation and reporting of evidence mapping[J]. Chin J Evid Based Med, 2020, 20(9):1098-1103. DOI: 10.7507/1672-2531.202001079 . | |
| 46 | REICHLIN T S, VOGT L, WÜRBEL H. The researchers' view of scientific rigor-survey on the conduct and reporting of in vivo research[J]. PLoS One, 2016, 11(12): e0165999. DOI: 10.1371/journal.pone.0165999 . |
| 47 | SMITH A J, CLUTTON R E, LILLEY E, et al. PREPARE: guidelines for planning animal research and testing[J]. Lab Anim, 2018, 52(2):135-141. DOI: 10.1177/0023677217724823 . |
| 48 | ZHU X, LIU Q, CAO M Q, et al. Reporting quality and risk of bias assessment of animal research on Chaihu-Shugan-San for depression: a systematic review[J]. Heliyon, 2023, 9(8): e19232. DOI: 10.1016/j.heliyon.2023.e19232 . |
| 49 | MARTIĆ-KEHL M I, WERNERY J, FOLKERS G, et al. Quality of animal experiments in anti-angiogenic cancer drug development: a systematic review[J]. PLoS One, 2015, 10(9): e0137235. DOI: 10.1371/journal.pone.0137235 . |
| 50 | LIU J Y, DAI Y, HE Y X, et al. Effect of berberine on cognitive function and β-amyloid precursor protein in Alzheimer's disease models: a systematic review and meta-analysis[J]. Front Pharmacol, 2023, 14:1301102. DOI: 10.3389/fphar.2023.1301102 . |
| 51 | FU Z M, SU X J, ZHOU Q, et al. Protective effects and possible mechanisms of catalpol against diabetic nephropathy in animal models: a systematic review and meta-analysis[J]. Front Pharmacol, 2023, 14:1192694. DOI: 10.3389/fphar.2023.1192694 . |
| 52 | 翁鸿, 王颖, 李柄辉, 等. 系统评价与Meta分析的类型及制作步骤[J]. 同济大学学报(医学版), 2019, 40(2):248-253. DOI: 10.16118/j.1008-0392.2019.02.022 . |
| WENG H, WANG Y, LI B H, et al. Types and steps of systematic review and meta-analysis: a brief introduction[J]. J Tongji Univ Med Sci, 2019, 40(2):248-253. DOI: 10.16118/j.1008-0392.2019.02.022 . | |
| 53 | MANDALIA K, MOUSAD A, WELBORN B, et al. Scaffold- and graft-based biological augmentation of rotator cuff repair: an updated systematic review and meta-analysis of preclinical and clinical studies for 2010-2022[J]. J Shoulder Elbow Surg, 2023, 32(9):1784-1800. DOI: 10.1016/j.jse.2023.03.031 . |
| 54 | WU Y, LI T L, LI P C, et al. Effects of Shenmai injection against chronic heart failure: a meta-analysis and systematic review of preclinical and clinical studies[J]. Front Pharmacol, 2023, 14:1338975. DOI: 10.3389/fphar.2023.1338975 . |
| [1] | 郑卿勇, 杨冬华, 马智超, 周姿余, 陆洋, 王晶宇, 邢丽娜, 康迎英, 杜莉, 赵春香, 狄宝山, 田金徽. 动物实验系统评价与Meta分析报告的规范撰写建议[J]. 实验动物与比较医学, 2025, 45(4): 496-507. |
| [2] | 闵凡贵, 富宏坤, 刘永刚, 刘香梅, 刘忠华, 李垚, 陶雨风. 感染性动物实验的福利与伦理特殊要求[J]. 实验动物与比较医学, 2025, 45(2): 239-246. |
| [3] | 费彬, 郭文科, 郭建平. 疝疾病动物模型研究及新型疝修补材料应用进展[J]. 实验动物与比较医学, 2025, 45(1): 55-66. |
| [4] | 李腾飞, 郑卿勇, 许建国, 李艺羿, 周泳佳, 徐彩花, 张明悦, 田杰祥, 王钢, 田金徽. 提高动物实验系统评价/Meta分析的证据确定性:GRADE方法的实证研究[J]. 实验动物与比较医学, 2025, 45(1): 101-111. |
| [5] | 杨家豪, 丁纯蕾, 钱风华, 孙旗, 姜旭升, 陈雯, 沈梦雯. 脓毒症相关脏器损伤动物模型研究进展[J]. 实验动物与比较医学, 2024, 44(6): 636-644. |
| [6] | 《实验动物与比较医学》编辑委员会. 动物实验与比较医学研究论文出版规范清单(2024年版)[J]. 实验动物与比较医学, 2024, 44(5): 577-582. |
| [7] | 马政文, 李夏莹, 刘晓宇, 李垚, 王剑, 卢今, 陈国元, 卢晓, 白玉, 卢选成, 刘永刚, 庞万勇, 陶雨风. 《动物研究:体内实验报告》即ARRIVE 2.0指南的解释和阐述(五)[J]. 实验动物与比较医学, 2024, 44(1): 105-114. |
| [8] | 苗金环, 徐霞, 周璐, 成海燕, 何燕. 基于VOSviewer的中医护理技术动物实验可视化分析[J]. 实验动物与比较医学, 2023, 43(6): 626-635. |
| [9] | 李夏莹, 田永路, 刘晓宇, 卢选成, 陈国元, 卢晓, 白玉, 高静, 李垚, 韦玉生, 庞万勇, 陶雨风. 《动物研究:体内实验报告》即ARRIVE 2.0指南的解释和阐述(四)[J]. 实验动物与比较医学, 2023, 43(6): 659-668. |
| [10] | 王硕, 吕昀徽, 王小康, 张朕豪, 崔永春. 体外膜肺氧合动物实验平台质量评价指标体系的构建与验证[J]. 实验动物与比较医学, 2023, 43(6): 604-611. |
| [11] | 张睿, 吕美豫, 张建军, 刘金莲, 陈彦, 黄志强, 刘尧, 周澜华. 痤疮动物模型建立及评价研究进展[J]. 实验动物与比较医学, 2023, 43(4): 398-405. |
| [12] | 刘晓宇, 卢选成, 师晓萌, 张雨舟, 吕超, 陈国元, 卢晓, 白玉, 高静, 李垚, 刘永刚, 陶雨风, 庞万勇. 《动物研究:体内实验报告》即ARRIVE 2.0指南的解释与阐述(三)[J]. 实验动物与比较医学, 2023, 43(4): 446-456. |
| [13] | 陈国元, 卢晓, 白玉, 于灵芝, 乔颖, 王剑, 卢今, 刘晓宇, 卢选成, 高静, 李垚, 庞万勇. 《动物研究:体内实验报告》即ARRIVE 2.0指南的解释和阐述(二)[J]. 实验动物与比较医学, 2023, 43(3): 323-331. |
| [14] | 王剑, 卢今, 马政文, 陈国元, 卢晓, 白玉, 刘晓宇, 卢选成, 高静, 李垚, 庞万勇. 《动物研究:体内实验报告》即ARRIVE 2.0指南的解释和阐述(一)[J]. 实验动物与比较医学, 2023, 43(2): 213-224. |
| [15] | 丁相荣, 霍姝汭, 代解杰. 甲型流感病毒对人与实验动物神经系统影响的研究进展[J]. 实验动物与比较医学, 2023, 43(2): 180-185. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||