













实验动物与比较医学 ›› 2023, Vol. 43 ›› Issue (2): 213-224.DOI: 10.12300/j.issn.1674-5817.2023.043
所属专题: 动物实验设计及统计学方法
王剑1( ), 卢今1, 马政文1, 陈国元2, 卢晓3, 白玉4, 刘晓宇5, 卢选成6, 高静7, 李垚1, 庞万勇8(
), 卢今1, 马政文1, 陈国元2, 卢晓3, 白玉4, 刘晓宇5, 卢选成6, 高静7, 李垚1, 庞万勇8( )(
)( )
)
收稿日期:2023-03-30
修回日期:2023-04-20
出版日期:2023-04-25
发布日期:2023-04-25
通讯作者:
庞万勇(1974—),男,兽医学博士,研究方向:实验动物医学。E-mail: pang1yong@outlook.com。ORCID:0000-0002-0724-2016作者简介:王 剑(1986—),男,兽医学博士,实验师,研究方向:实验动物医学。E-mail: wangjian3976@shsmu.edu.cn
Jian WANG1( ), Jin LU1, Zhengwen MA1, Guoyuan CHEN2, Xiao LU3, Yu BAI4, Xiaoyu LIU5, Xuancheng LU6, Jing GAO7, Yao LI1, Wanyong Pang8(
), Jin LU1, Zhengwen MA1, Guoyuan CHEN2, Xiao LU3, Yu BAI4, Xiaoyu LIU5, Xuancheng LU6, Jing GAO7, Yao LI1, Wanyong Pang8( )(
)( )
)
Received:2023-03-30
Revised:2023-04-20
Published:2023-04-25
Online:2023-04-25
Contact:
PANG Wanyong (ORCID: 0000-0002-0724-2016), E-mail: pang1yong@outlook.com摘要:
提高生物医学研究结果的可重复性是一项重大挑战,研究人员透明且准确地报告其研究过程有利于读者对该研究结果的可靠性进行评估,进而重复该实验或在该成果的基础上进一步探索。ARRIVE 2.0指南是2019年英国国家3Rs中心(NC3Rs)组织发布的一份适用于任何与活体动物研究报告相关的指导性清单,用以提高动物体内实验设计、实验实施和实验报告的规范性,以及动物实验结果的可靠性、可重复性和临床转化率。ARRIVE 2.0指南的使用不仅可以丰富动物实验研究报告的细节,确保动物实验结果信息被充分评估和利用,还可以使读者准确且清晰地了解作者所表述的内容,促进基础研究评审过程的透明化和完整性。目前,ARRIVE 2.0指南已经被国际生物医学期刊广泛采纳。本文是在国际期刊遵循ARRIVE 2.0指南的最佳实践基础上,对2020年发表于PLoS Biology期刊上的ARRIVE 2.0指南完整解读版(原文请见
中图分类号:
王剑,卢今,马政文,等. 《动物研究:体内实验报告》即ARRIVE 2.0指南的解释和阐述(一)[J]. 实验动物与比较医学, 2023, 43(2): 213-224. DOI: 10.12300/j.issn.1674-5817.2023.043.
Jian WANG,Jin LU,Zhengwen MA,et al. Explanation and Elaboration for the ARRIVE Guidelines 2.0—Reporting Animal Research and In Vivo Experiments (Ⅰ)[J]. Laboratory Animal and Comparative Medicine, 2023, 43(2): 213-224. DOI: 10.12300/j.issn.1674-5817.2023.043.
术语名称 Terminology name | 含义 Content |
|---|---|
偏倚 Bias | 对干预的真实效果的过高或过低估计。偏倚是由实验设计、实施或分析的不足引起,从而导致误差的引入 |
统计描述和统计推断 Descriptive and inferential statistics | 统计描述用于总结数据,通常包括集中趋势(如平均值或中位数)的测量和离散程度(如标准差或范围)的测量。统计推断用于对从中抽取样本的总体进行概括。假设检验如方差分析(ANOVA)、Mann-Whitney检验或t检验等属于统计推断的范畴 |
效应量 Effect size | 组间差异或变量之间关系强度的定量测量 |
实验单元 Experimental unit | 独立于所有其他单元而接受干预的生物实体,这样就可以将任何两个实验单元分配给不同的处理组。有时也被称为随机化单元。 |
外部效度 External validity | 某一特定研究的结果能够应用或推广到其他研究、研究条件、动物品系/物种或人类的程度 |
假阴性 False negative | 当备择假设(H1)为真时,却得到无统计学意义的结果。在统计学中,它被称为第Ⅱ类错误 |
假阳性 False positive | 当零假设(H0)为真时,却得到具有统计学意义的结果。在统计学中,它被称为第Ⅰ类错误 |
自变量 Independent variable | 研究人员控制的变量(如处理、条件、时间)或样本的属性(如性别)或技术特征(如批次、笼、样本收集)等可能会影响结果测量的变量。自变量可以是科研中所关注的变量,也可以是干扰变量。自变量也被称为预测变量 |
内部效度 Internal validity | 某一特定研究的结果在多大程度上可以归因于实验干预的效果,而不是其他一些未知的因素(如该研究的设计、实施或分析的不足所引入的偏倚) |
干扰变量 Nuisance variable | 干扰变量是指在研究中出现的一类变量,不是研究的主要关注点,但可能对研究结果测量产生影响并增加变异性,因此需要在实验设计或分析中加以考虑。此外,如果它们与关注的自变量有关联,就成为混杂因素,因为这会引入偏倚。在实验设计(以防止它们成为混杂因素)和分析(解释变异性,有时是降低偏倚)中都应对干扰变量加以考虑。 例如,可将干扰变量作为区组因素或协变量 |
零假设和备择假设 Null and alternative hypotheses | 零假设(H0)是指没有影响/效应,如各组之间的差异或变量之间的关联。备择假设(H1)是假设存在某种效应 |
结果测量/结局变量 Outcome measure | 在研究过程中记录的、用以评估处理或实验干预效果的任何变量。它也被称为因变量、响应变量 |
统计效力/检验效能 Power | 对于预先定义的有生物学意义的效应量,如果效应真实存在(即零假设被正确拒绝),统计检验将检测出效应的概率 |
样本量 Sample size | 每组的实验单元数量,也被称为n |
表1 ARRIVE 2.0指南所附统计学术语
Table 1 Statistical terminology attached to the ARRIVE 2.0 guidelines
术语名称 Terminology name | 含义 Content |
|---|---|
偏倚 Bias | 对干预的真实效果的过高或过低估计。偏倚是由实验设计、实施或分析的不足引起,从而导致误差的引入 |
统计描述和统计推断 Descriptive and inferential statistics | 统计描述用于总结数据,通常包括集中趋势(如平均值或中位数)的测量和离散程度(如标准差或范围)的测量。统计推断用于对从中抽取样本的总体进行概括。假设检验如方差分析(ANOVA)、Mann-Whitney检验或t检验等属于统计推断的范畴 |
效应量 Effect size | 组间差异或变量之间关系强度的定量测量 |
实验单元 Experimental unit | 独立于所有其他单元而接受干预的生物实体,这样就可以将任何两个实验单元分配给不同的处理组。有时也被称为随机化单元。 |
外部效度 External validity | 某一特定研究的结果能够应用或推广到其他研究、研究条件、动物品系/物种或人类的程度 |
假阴性 False negative | 当备择假设(H1)为真时,却得到无统计学意义的结果。在统计学中,它被称为第Ⅱ类错误 |
假阳性 False positive | 当零假设(H0)为真时,却得到具有统计学意义的结果。在统计学中,它被称为第Ⅰ类错误 |
自变量 Independent variable | 研究人员控制的变量(如处理、条件、时间)或样本的属性(如性别)或技术特征(如批次、笼、样本收集)等可能会影响结果测量的变量。自变量可以是科研中所关注的变量,也可以是干扰变量。自变量也被称为预测变量 |
内部效度 Internal validity | 某一特定研究的结果在多大程度上可以归因于实验干预的效果,而不是其他一些未知的因素(如该研究的设计、实施或分析的不足所引入的偏倚) |
干扰变量 Nuisance variable | 干扰变量是指在研究中出现的一类变量,不是研究的主要关注点,但可能对研究结果测量产生影响并增加变异性,因此需要在实验设计或分析中加以考虑。此外,如果它们与关注的自变量有关联,就成为混杂因素,因为这会引入偏倚。在实验设计(以防止它们成为混杂因素)和分析(解释变异性,有时是降低偏倚)中都应对干扰变量加以考虑。 例如,可将干扰变量作为区组因素或协变量 |
零假设和备择假设 Null and alternative hypotheses | 零假设(H0)是指没有影响/效应,如各组之间的差异或变量之间的关联。备择假设(H1)是假设存在某种效应 |
结果测量/结局变量 Outcome measure | 在研究过程中记录的、用以评估处理或实验干预效果的任何变量。它也被称为因变量、响应变量 |
统计效力/检验效能 Power | 对于预先定义的有生物学意义的效应量,如果效应真实存在(即零假设被正确拒绝),统计检验将检测出效应的概率 |
样本量 Sample size | 每组的实验单元数量,也被称为n |
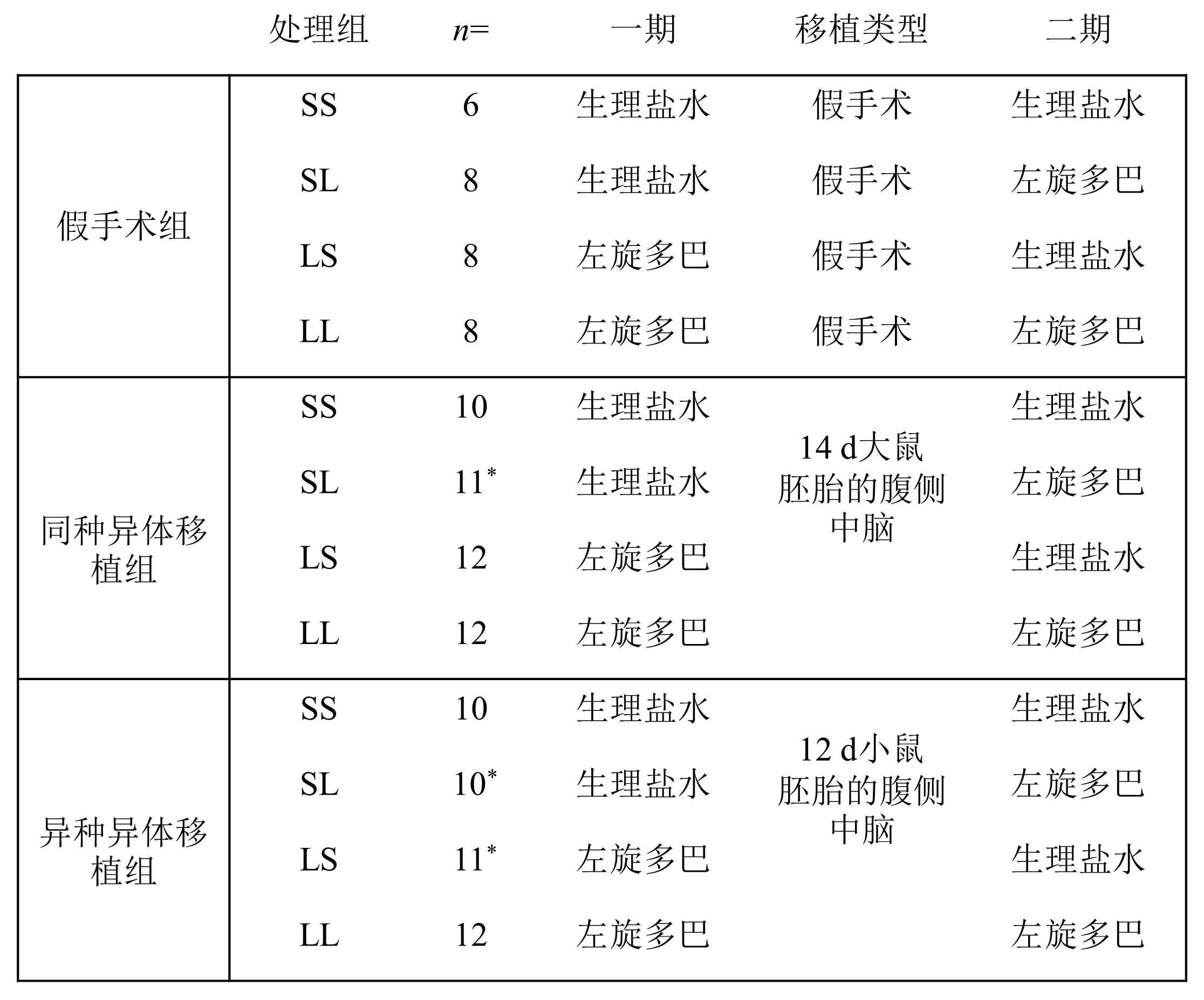
图2 文献[32]中每组动物的处理和移植方案注:*表示这些组最初有12只动物,但一些动物患上了与实验无关的肿瘤,因此被从研究中移除。
Figure 2 The scheme of treatment and transplantation for each group of animals in study [32]Note:*These groups initially consisted of 12 animals, but some animals developed tumors unrelated to the experiment and were therefore removed from the study.
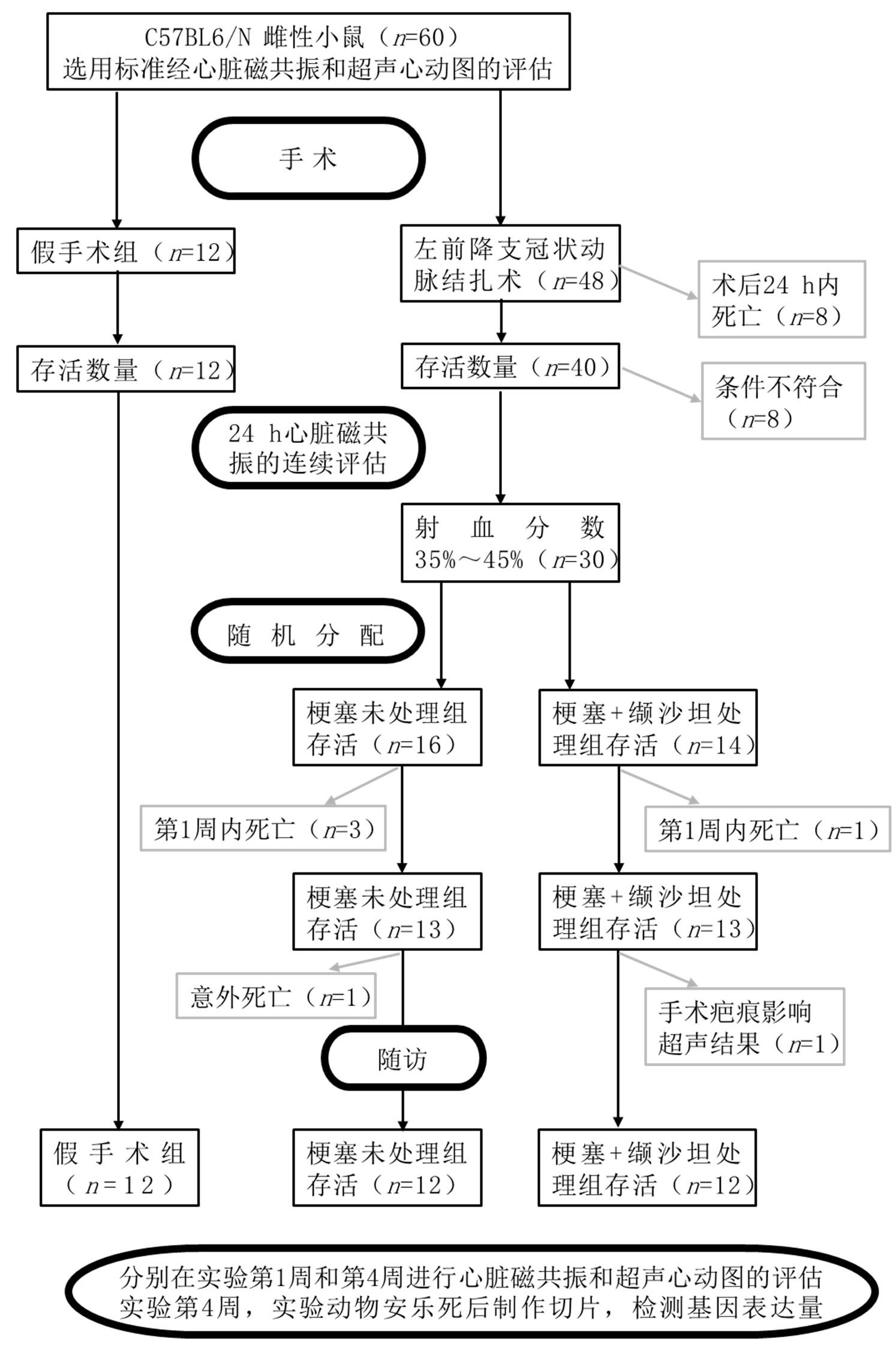
图3 文献[59]中实验方案及其所使用、死亡和纳入的动物数量
Figure 3 Flow chart showing the experimental protocol with the number of animals used, died and included in the study [59]
| 1 | KILKENNY C, PARSONS N, KADYSZEWSKI E, et al. Survey of the quality of experimental design, statistical analysis and reporting of research using animals[J]. PLoS One, 2009, 4(11): e7824. DOI: 10.1371/journal.pone.0007824 . |
| 2 | FLÓREZ-VARGAS O, BRASS A, KARYSTIANIS G, et al. Bias in the reporting of sex and age in biomedical research on mouse models[J]. eLife, 2016, 5: e13615. DOI: 10.7554/eLife. 13615 . |
| 3 | WEISSGERBER T L, GARCIA-VALENCIA O, GAROVIC V D, et al. Why we need to report more than 'Data were Analyzed by t-tests or ANOVA'[J]. eLife, 2018, 7: e36163. DOI: 10.7554/eLife. 36163 . |
| 4 | MACLEOD M R, LAWSON MCLEAN A, KYRIAKOPOULOU A, et al. Correction: risk of bias in reports of in vivo research: a focus for improvement[J]. PLoS Biol, 2015, 13(11): e1002301. DOI: 10.1371/journal.pbio.1002301 . |
| 5 | KILKENNY C, BROWNE W J, CUTHILL I C, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research[J]. Osteoarthritis Cartilage, 2012, 20(4):256-260. DOI: 10.1016/j.joca.2021.02.010 . |
| 6 | BAKER D, LIDSTER K, SOTTOMAYOR A, et al. Two years later: journals are not yet enforcing the ARRIVE guidelines on reporting standards for pre-clinical animal studies[J]. PLoS Biol, 2014, 12(1): e1001756. DOI: 10.1371/journal.pbio.1001756 . |
| 7 | GULIN J E N, ROCCO D M, GARCÍA-BOURNISSEN F. Quality of reporting and adherence to ARRIVE guidelines in animal studies for chagas disease preclinical drug research: a systematic review[J]. PLoS Negl Trop Dis, 2015, 9(11): e0004194. DOI: 10.1371/journal.pntd.0004194 . |
| 8 | AVEY M T, MOHER D, SULLIVAN K J, et al. The devil is in the details: incomplete reporting in preclinical animal research[J]. PLoS One, 2016, 11(11): e0166733. DOI: 10.1371/journal.pone.0166733 . |
| 9 | REICHLIN T S, VOGT L, WÜRBEL H. The researchers' view of scientific rigor-survey on the conduct and reporting of in vivo research[J]. PLoS One, 2016, 11(12): e0165999. DOI: 10.1371/journal.pone.0165999 . |
| 10 | LEUNG V, ROUSSEAU-BLASS F, BEAUCHAMP G, et al. ARRIVE has not ARRIVEd: support for the ARRIVE (Animal Research: reporting of in vivo Experiments) guidelines does not improve the reporting quality of papers in animal welfare, analgesia or anesthesia[J]. PLoS One, 2018, 13(5): e0197882. DOI: 10.1371/journal.pone.0197882 . |
| 11 | HAIR K, MACLEOD M R, SENA E S, et al. A randomised controlled trial of an Intervention to Improve Compliance with the ARRIVE guidelines (IICARus)[J]. Res Integr Peer Rev, 2019, 4:12. DOI: 10.1186/s41073-019-0069-3 . |
| 12 | MCGRATH J C, LILLEY E. Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP[J]. Br J Pharmacol, 2015, 172(13):3189-3193. DOI: 10.1111/bph.12955 . |
| 13 | PERCIE DU SERT N, HURST V, AHLUWALIA A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research[J]. BMC Vet Res, 2020,16(1):242. DOI: 10.1186/s12917-020-02451-y . |
| 14 | DU SERT N P, AHLUWALIA A, ALAM S, et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0[J]. PLoS Biol, 2020, 18(7): e3000411. DOI: 10.1371/journal.pbio.3000411 . |
| 15 | 张俊彦, 刘晓宇, 李垚, 等. 动物实验研究报告的国际指南ARRIVE 2.0介绍及期刊实施计划[J]. 实验动物与比较医学, 2023, 43(1): 86-94.DOI:10.12300/j.issn.1674-5817.2023.014 . |
| ZHANG J Y, LIU X Y, LI Y, et al. Introduction to the international guide for animal research reporting ARRIVE 2.0, and its implementation plan in the journal[J]. Lab Anim Comp Med, 2023, 43(1): 86-94.DOI:10.12300/j.issn.1674-5817.2023.014 . | |
| 16 | FESTING M F W, ALTMAN D G. Guidelines for the design and statistical analysis of experiments using laboratory animals[J]. ILAR J, 2002, 43(4):244-258. DOI: 10.1093/ilar.43.4.244 . |
| 17 | BATE S T, CLARK R A. The design and statistical analysis of animal experiments[M]. Cambridge: Cambridge University Press, 2014. |
| 18 | RUXTON G D, COLEGRAVE N. Experimental design for the life sciences[M]. Oxford: Oxford University Press, 2017. |
| 19 | PERCIE DU SERT N, BAMSEY I, BATE S T, et al. The experimental design assistant[J]. PLoS Biol, 2017, 15(9): e2003779. DOI: 10.1371/journal.pbio.2003779 . |
| 20 | The BMJ Scientific misconduct[EB/OL]. [2020-1-10]. https://www.bmj.com/about-bmj/resourcesauthors/forms-policies-and-checklists/scientific-misconduct. |
| 21 | NGUYEN T T, BAZZOLI C, MENTRÉ F. Design evaluation and optimisation in crossover pharmacokinetic studies analysed by nonlinear mixed effects models[J]. Stat Med, 2012, 31(11-12):1043-1058. DOI: 10.1002/sim.4390 . |
| 22 | HILL D, CHEN L P, SNAAR-JAGALSKA E, et al. Embryonic zebrafish xenograft assay of human cancer metastasis[J]. F1000Research, 2018, 7:1682. DOI: 10.12688/f1000research.16659.2 . |
| 23 | BURDGE G C, LILLYCROP K A, JACKSON A A, et al. The nature of the growth pattern and of the metabolic response to fasting in the rat are dependent upon the dietary protein and folic acid intakes of their pregnant dams and post-weaning fat consumption[J]. Br J Nutr, 2008, 99(3):540-549. DOI: 10.1017/S0007114507815819 . |
| 24 | LAZIC S E, CLARKE-WILLIAMS C J, MUNAFÒ M R. What exactly is 'N' in cell culture and animal experiments?[J]. PLoS Biol, 2018, 16(4): e2005282. DOI: 10.1371/journal.pbio.2005282 . |
| 25 | NC3Rs. Experimental unit 2015 [EB/OL]. [2019-3-21]. https://eda.nc3rs.org.uk/experimental-designunit. |
| 26 | LAZIC S E. The problem of pseudoreplication in neuroscientific studies: is it affecting your analysis?[J]. BMC Neurosci, 2010, 11:5. DOI: 10.1186/1471-2202-11-5 . |
| 27 | HURLBERT S H. Pseudoreplication and the design of ecological field experiments[J]. Ecol Monogr, 1984, 54(2):187-211. DOI: 10.2307/1942661 . |
| 28 | KWAN S T C, KING J H, GRENIER J K, et al. Maternal choline supplementation during normal murine pregnancy alters the placental epigenome: results of an exploratory study[J]. Nutrients, 2018, 10(4):417. DOI: 10.3390/nu10040417 . |
| 29 | KARP N A, MASON J, BEAUDET A L, et al. Prevalence of sexual dimorphism in mammalian phenotypic traits[J]. Nat Commun, 2017, 8:15475. DOI: 10.1038/ncomms15475 . |
| 30 | RIBEIRO F, VASQUEZ L A, FERNANDES J B K, et al. Feeding level and frequency for freshwater angelfish[J]. R Bras Zootec, 2012, 41(6):1550-1554. DOI: 10.1590/S1516-35982012-000600033 . |
| 31 | GRASSELLI G, ROSSI S, MUSELLA A, et al. Abnormal NMDA receptor function exacerbates experimental autoimmune encephalomyelitis[J]. Br J Pharmacol, 2013, 168(2):502-517. DOI: 10.1111/j.1476-5381.2012.02178.x . |
| 32 | LUDIVINE S, BREGER, . Influence of chronic L-DOPA treatment on immune response following allogeneic and xenogeneic graft in a rat model of Parkinson's disease[J]. Brain Behav Immun, 2017, 61:155-164. DOI: 10.1016/j.bbi. 2016. 11.014 . |
| 33 | VAHIDY F, SCHÄBITZ W R, FISHER M, et al. Reporting standards for preclinical studies of stroke therapy[J]. Stroke, 2016, 47(10):2435-2438. DOI: 10.1161/STROKEAHA.116.013643 . |
| 34 | MUHLHAUSLER B S, BLOOMFIELD F H, GILLMAN M W. Whole animal experiments should be more like human randomized controlled trials[J]. PLoS Biol, 2013, 11(2): e1001481. DOI: 10.1371/journal.pbio.1001481 . |
| 35 | JENNIONS M D, MØLLER A P. A survey of the statistical power of research in behavioral ecology and animal behavior[J]. Behav Ecol, 2003, 14(3):438-445. DOI: 10.1093/beheco/14.3.438 . |
| 36 | BUTTON K S, IOANNIDIS J P A, MOKRYSZ C, et al. Power failure: why small sample size undermines the reliability of neuroscience[J]. Nat Rev Neurosci, 2013, 14(5):365-376. DOI: 10.1038/nrn3475 . |
| 37 | WÜRBEL H. More than 3Rs: the importance of scientific validity for harm-benefit analysis of animal research[J]. Lab Anim (NY), 2017, 46(4):164-166. DOI: 10.1038/laban.1220 . |
| 38 | TEAM R. R: a language and environment for statistical computing[M]. Vienna, Austria: R Foundation for Statistical Computing, 2017. |
| 39 | PENG C Y, LONG H Y, ABACI S. Power analysis software for educational researchers[J]. J Exp Educ, 2012, 80:113-136. DOI: 10.1080/00220973.2011.647115 . |
| 40 | CHARAN J, KANTHARIA N D. How to calculate sample size in animal studies?[J]. J Pharmacol Pharmacother, 2013, 4(4):303-306. DOI: 10.4103/0976-500X.119726 . |
| 41 | FESTING M F. On determining sample size in experiments involving laboratory animals[J]. Lab Anim, 2018, 52(4):341-350. DOI: 10.1177/0023677217738268 . |
| 42 | FREEDMAN L S. An analysis of the controversy over classical one-sided tests[J]. Clin Trials, 2008, 5(6):635-640. DOI: 10.1177/1740774508098590 . |
| 43 | RUXTON G D, NEUHÄUSER M. When should we use one-tailed hypothesis testing?[J]. Methods Ecol Evol, 2010, 1(2):114-117. DOI: 10.1111/j.2041-210X.2010.00014x . |
| 44 | REYNOLDS P S. When power calculations won't do: Fermi approximation of animal numbers[J]. Lab Anim (NY), 2019, 48(9):249-253. DOI: 10.1038/s41684-019-0370-2 . |
| 45 | Bate ST. How to decide your sample size when the power calculation is not straightforward [EB/OL]. [2018-8-2]. https://www.nc3rs.org.uk/news/howdecide-your-sample-size-when-power-calculation-not-straightforward. |
| 46 | BUSTAMANTE R, DAZA M A, CANFRÁN S, et al. Comparison of the postoperative analgesic effects of cimicoxib, buprenorphine and their combination in healthy dogs undergoing ovariohysterectomy[J]. Vet Anaesth Analg, 2018, 45(4):545-556. DOI: 10.1016/j.vaa.2018.01.003 . |
| 47 | SPIN J, OLIVEIRA G, SPIN-NETO R, et al. Histomorphometric evaluation of the association between bioglass and lyophilized bovine bone in the treatment of critical bone defects created on rat calvaria: a pilot study[J]. Rev Odontol UNESP, 2015, 44(1):37-43. DOI: 10.1590/1807-2577.1020 . |
| 48 | RICE A S C, MORLAND R, HUANG W, et al. Transparency in the reporting of in vivo pre-clinical pain research: The relevance and implications of the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines[J].Scand J Pain, 2013, 4(2):58-62. DOI: 10.1016/j.sjpain.2013.02.002 . |
| 49 | SALKIND N J. Encyclopedia of research design[M]. Thousand Oaks, Calif: SAGE Publications, 2010. |
| 50 | WORKMAN P, ABOAGYE E O, BALKWILL F, et al. Guidelines for the welfare and use of animals in cancer research[J]. Br J Cancer, 2010, 102(11):1555-1577. DOI: 10.1038/sj.bjc.6605642 . |
| 51 | SENA E S, JEFFREYS A L, COX S F, et al. The benefit of hypothermia in experimental ischemic stroke is not affected by pethidine[J]. Int J Stroke, 2013, 8(3):180-185. DOI: 10.1111/j.1747-4949.2012.00834.x . |
| 52 | KAFKAFI N, AGASSI J, CHESLER E J, et al. Reproducibility and replicability of rodent phenotyping in preclinical studies[J]. Neurosci Biobehav Rev, 2018, 87:218-232. DOI: 10.1016/j.neubiorev.2018.01.003 . |
| 53 | SCOTT S, KRANZ J E, COLE J, et al. Design, power, and interpretation of studies in the standard murine model of ALS[J]. Amyotroph Lateral Scler, 2008, 9(1):4-15. DOI: 10.1080/17482960701856300 . |
| 54 | KANG H. The prevention and handling of the missing data[J]. Korean J Anesthesiol, 2013, 64(5):402-406. DOI: 10.4097/kjae.2013.64.5.402 . |
| 55 | DAVID ALLISON P. Missing data[M]. Thousand Oaks, Calif:Sage Publications, 2002. |
| 56 | JAKOBSEN J C, GLUUD C, WETTERSLEV J, et al. When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts[J]. BMC Med Res Methodol, 2017, 17(1):162. DOI: 10.1186/s12874-017-0442-1 . |
| 57 | HOLMAN C, PIPER S K, GRITTNER U, et al. Where have all the rodents gone? the effects of attrition in experimental research on cancer and stroke[J]. PLoS Biol, 2016, 14(1): e1002331. DOI: 10.1371/journal.pbio.1002331 . |
| 58 | GENTHER-SCHROEDER O N, BRANINE M E, HANSEN S L. Effects of increasing supplemental dietary Zn concentration on growth performance and carcass characteristics in finishing steers fed ractopamine hydrochloride[J]. J Anim Sci, 2018, 96(5):1903-1913. DOI: 10.1093/jas/sky094 . |
| 59 | CASTIGLIONI L, COLAZZO F, FONTANA L, et al. Evaluation of left ventricle function by regional fractional area change (RFAC) in a mouse model of myocardial infarction secondary to valsartan treatment[J]. PLoS One, 2015, 10(8):e0135778. DOI:10.1371/journal.pone.0135778 . |
| 60 | BRENT L J N, HEILBRONNER S R, HORVATH J E, et al. Genetic origins of social networks in rhesus macaques[J]. Sci Rep, 2013, 3:1042. DOI: 10.1038/srep01042 . |
| 61 | GOUVEIA K, HURST J L. Optimising reliability of mouse performance in behavioural testing: the major role of non-aversive handling[J]. Sci Rep, 2017, 7:44999. DOI: 10.1038/srep44999 . |
| [1] | 郑卿勇, 杨冬华, 马智超, 周姿余, 陆洋, 王晶宇, 邢丽娜, 康迎英, 杜莉, 赵春香, 狄宝山, 田金徽. 动物实验系统评价与Meta分析报告的规范撰写建议[J]. 实验动物与比较医学, 2025, 45(4): 496-507. |
| [2] | 闵凡贵, 富宏坤, 刘永刚, 刘香梅, 刘忠华, 李垚, 陶雨风. 感染性动物实验的福利与伦理特殊要求[J]. 实验动物与比较医学, 2025, 45(2): 239-246. |
| [3] | 李腾飞, 郑卿勇, 许建国, 李艺羿, 周泳佳, 徐彩花, 张明悦, 田杰祥, 王钢, 田金徽. 提高动物实验系统评价/Meta分析的证据确定性:GRADE方法的实证研究[J]. 实验动物与比较医学, 2025, 45(1): 101-111. |
| [4] | 郑卿勇, 李腾飞, 许建国, 周泳佳, 马智超, 王娜, 李莫兰, 杨雯景, 吴佩润, 王海东, 田金徽. 动物实验证据整合方法研究的进展与挑战[J]. 实验动物与比较医学, 2024, 44(5): 567-576. |
| [5] | 《实验动物与比较医学》编辑委员会. 动物实验与比较医学研究论文出版规范清单(2024年版)[J]. 实验动物与比较医学, 2024, 44(5): 577-582. |
| [6] | 马政文, 李夏莹, 刘晓宇, 李垚, 王剑, 卢今, 陈国元, 卢晓, 白玉, 卢选成, 刘永刚, 庞万勇, 陶雨风. 《动物研究:体内实验报告》即ARRIVE 2.0指南的解释和阐述(五)[J]. 实验动物与比较医学, 2024, 44(1): 105-114. |
| [7] | 苗金环, 徐霞, 周璐, 成海燕, 何燕. 基于VOSviewer的中医护理技术动物实验可视化分析[J]. 实验动物与比较医学, 2023, 43(6): 626-635. |
| [8] | 李夏莹, 田永路, 刘晓宇, 卢选成, 陈国元, 卢晓, 白玉, 高静, 李垚, 韦玉生, 庞万勇, 陶雨风. 《动物研究:体内实验报告》即ARRIVE 2.0指南的解释和阐述(四)[J]. 实验动物与比较医学, 2023, 43(6): 659-668. |
| [9] | 王硕, 吕昀徽, 王小康, 张朕豪, 崔永春. 体外膜肺氧合动物实验平台质量评价指标体系的构建与验证[J]. 实验动物与比较医学, 2023, 43(6): 604-611. |
| [10] | 刘晓宇, 卢选成, 师晓萌, 张雨舟, 吕超, 陈国元, 卢晓, 白玉, 高静, 李垚, 刘永刚, 陶雨风, 庞万勇. 《动物研究:体内实验报告》即ARRIVE 2.0指南的解释与阐述(三)[J]. 实验动物与比较医学, 2023, 43(4): 446-456. |
| [11] | 陈国元, 卢晓, 白玉, 于灵芝, 乔颖, 王剑, 卢今, 刘晓宇, 卢选成, 高静, 李垚, 庞万勇. 《动物研究:体内实验报告》即ARRIVE 2.0指南的解释和阐述(二)[J]. 实验动物与比较医学, 2023, 43(3): 323-331. |
| [12] | 丁相荣, 霍姝汭, 代解杰. 甲型流感病毒对人与实验动物神经系统影响的研究进展[J]. 实验动物与比较医学, 2023, 43(2): 180-185. |
| [13] | 张俊彦, 刘晓宇, 李垚, 陈国元, 卢晓, 白玉, 卢选成, 庞万勇, 吴宝金. 动物实验研究报告的国际指南ARRIVE 2.0介绍及期刊实施计划[J]. 实验动物与比较医学, 2023, 43(1): 86-94. |
| [14] | 刘香梅, 马中春, 富宏坤, 高峰, 陶雨风. 毒理检测领域实验室认可能力范围表述浅谈[J]. 实验动物与比较医学, 2022, 42(6): 526-530. |
| [15] | 王小康, 赵若瑾, 吕昀徽, 岳广新, 刘尚雨, 贺婷, 彭鹏, 孟亮, 李巨波, 张宝杰, 申晨, 崔永春, 王欣. 动物在体心脏传导系统标测技术的研究生教学新模式探索[J]. 实验动物与比较医学, 2022, 42(5): 466-471. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||