













实验动物与比较医学 ›› 2024, Vol. 44 ›› Issue (4): 357-373.DOI: 10.12300/j.issn.1674-5817.2024.008
吴玥1( ), 李璐2, 张阳2, 王珏1, 冯婷婷1, 李依桐1, 王凯2, 孔琪1(
), 李璐2, 张阳2, 王珏1, 冯婷婷1, 李依桐1, 王凯2, 孔琪1( )(
)( )
)
收稿日期:2024-01-16
修回日期:2024-04-10
出版日期:2024-09-06
发布日期:2024-08-25
通讯作者:
孔琪(1978—),男,博士,研究员,研究方向:比较医学及生物信息学。E-mail: kongqi@cnilas.org。ORCID: 0000-0003-2867-7382作者简介:吴玥(1993—),女,硕士,助理研究员,研究方向:比较医学及相关数据库建设。E-mail: wuyue@cnilas.org。ORCID: 0000-0001-5874-8263基金资助:
WU Yue1( ), LI Lu2, ZHANG Yang2, WANG Jue1, FENG Tingting1, LI Yitong1, WANG Kai2, KONG Qi1(
), LI Lu2, ZHANG Yang2, WANG Jue1, FENG Tingting1, LI Yitong1, WANG Kai2, KONG Qi1( )(
)( )
)
Received:2024-01-16
Revised:2024-04-10
Published:2024-08-25
Online:2024-09-06
Contact:
KONG Qi (ORCID: 0000-0003-2867-7382), E-mail: kongqi@cnilas.org摘要:
目的 分析各个公共数据库中可感染人的冠状病毒感染动物模型的组学数据资源情况,包括数据分布、数据集数量、数据类型、物种、品系、研究内容等,从而深入理解冠状病毒的生物学特征和致病机制,为研究有效的治疗方案和预防措施奠定基础。 方法 定义特定病毒名称、时间范围和物种等检索策略与纳入排除标准,检索GEO、ArrayExpress等大型公共组学数据库。根据不同字段类型进行二次过滤,获取更精确的数据列表。建立组学数据文本库,进行文献计量学分析,构建共现网络图,分析不同研究主题、技术方法和涉及物种之间的关联强度。同时,分析研究涉及的细胞类型、器官和参与的生物途径,以进一步阐明病原体与宿主之间的致病相互作用。 结果 含有冠状病毒组学数据的公共数据库有20余个,以新型冠状病毒感染组学数据为主。常用物种为人、小鼠、仓鼠和猴,常用病毒株为Wuhan-Hu-1和USA-WA1/2020。此外,人类相关研究主要集中于气道上皮细胞和Calu-3细胞,动物模型(如小鼠、猕猴与雪貂)则多采用肺组织。表达谱数据显示感染后参与炎症、细胞因子反应、补体途径、细胞损伤、增殖和分化等通路基因显著上调。蛋白组学研究揭示,在不同感染阶段的患者样本中磷酸化蛋白质组、泛素组和全蛋白质组具有显著变化。特定蛋白质类别,包括病毒受体和蛋白酶、转录因子、细胞因子、凝血系统相关蛋白、血管生成相关蛋白及纤维化标志物等六类蛋白均在冠状病毒感染后发生改变。此外,代谢组数据提示磷酸胆碱、磷酸乙醇胺、花生四烯酸和油酸可作为潜在的代谢标志物。表观组学研究结果显示,m6A甲基化在新冠病毒复制、感染和传播过程中发挥作用,并且对宿主细胞-病毒互作产生影响。N、S、非结构蛋白2和3泛素化最为显著。微生物组学研究趋势表明,肠道和废水中的微生物群落正在成为新的研究重点。 结论 冠状病毒组学数据类型丰富,模型与细胞类型多样。根据不同病毒的特征,造模物种和技术方法的选择具有差异性。研究冠状病毒感染动物模型的多组学数据可以揭示宿主-病原体之间的关键相互作用,发现生物标志物和潜在的治疗靶点,为深入理解冠状病毒的生物学特性和感染机制提供丰富信息。
中图分类号:
吴玥,李璐,张阳,等. 冠状病毒感染动物模型组学数据集成分析[J]. 实验动物与比较医学, 2024, 44(4): 357-373. DOI: 10.12300/j.issn.1674-5817.2024.008.
WU Yue,LI Lu,ZHANG Yang,et al. Integrative Analysis of Omics Data in Animal Models of Coronavirus Infection[J]. Laboratory Animal and Comparative Medicine, 2024, 44(4): 357-373. DOI: 10.12300/j.issn.1674-5817.2024.008.
数据库名称 Database name | 网址 URL | 简介 Brief description |
|---|---|---|
| Biostudies | https://www.ebi.ac.uk/biostudies/ | 包含生物学研究数据集,以及与EMBL-EBI数据库或其他公共数据库的数据链接 |
| ENA (European Nucleotide Archive) | https://www.ebi.ac.uk/ena | 核酸测序原始数据、序列拼装和功能注释信息 |
| PRIDE (Proteomics Identifications Database) | https://www.ebi.ac.uk/pride | 蛋白质组学质谱数据存储平台包含蛋白质鉴定、翻译后修饰和光谱数据 |
| GEO (Gene Expression Omnibus) | https://www.ncbi.nlm.nih.gov/geo/ | 存储各种高通量实验数据的公共数据库,以表达谱、芯片数据为主 |
| EGA (European Genome-Phenome Archive) | https://ega-archive.org | 存储生物医学研究中产生的遗传和表型数据 |
| ArrayExpress | https://www.ebi.ac.uk/arrayexpress/ | 主要包括微阵列芯片和高通量测序数据,与GEO数据库类似 |
| iProX (Integrated Proteome Resources) | https://www.iprox.org | 国家蛋白质科学中心网站,收录蛋白质组学相关数据集 |
MassIVE (Mass Spectrometry Interactive Virtual Environment) | https://massive.ucsd.edu/ProteoSAFe/static/massive.jsp | 储存蛋白、多肽、质谱数据 |
| FAIRDOMHub | https://fairdomhub.org/ | 共享多种类型的科学研究数据集、模型或研究过程和成果数据 |
| BioModels | https://www.ebi.ac.uk/biomodels/ | 储存生物和医学相关的数学模型、生理学和药学相关机制模型 |
| MetaboLights | https://www.ebi.ac.uk/metabolights/ | 储存代谢组学和相关衍生信息的数据库,包含代谢物的结构、浓度、功能数据 |
| dbGap (Database of Genotypes and Phenotypes) | https://dbgap.ncbi.nlm.nih.gov/ | 储存基因型和表型互作数据 |
| PanoramaPublic | https://panoramaweb.org/ | 储存蛋白质组学和小分子数据 |
| GNPS (Global Natural Products Social Molecular Networking) | https://gnps.ucsd.edu/ | 储存原始、处理或注释过的质谱数据 |
Metabolomics Workbench | https://www.metabolomicsworkbench.org/ | 代谢组学元数据和实验数据的公共数据库,包含不同物种的质谱(MS)和核磁共振(NMR)光谱数据 |
| ExpressionAtlas | https://www.ebi.ac.uk/gxa/home | 提供不同条件下基因表达信息,包含芯片与转录组数据 |
jPOST (Japan Proteome Standard Repository Database) | https://jpostdb.org/ | 包含蛋白质组数据及分析结果数据 |
| EVA (European Variation Archive) | https://www.ebi.ac.uk/eva/ | 包含不同物种遗传变异数据 |
| NODE (National Omics Data Encyclopedia) | https://www.biosino.org/node/ | 储存多组学数据资源,包括测序数据、蛋白质组学数据、代谢组学数据及荧光成像数据 |
| PeptideAtlas | https://peptideatlas.org/ | 收录质谱、蛋白质组学数据。主要为人、小鼠、酵母数据 |
表1 含有冠状病毒组学数据的公共数据库
Table 1 Public databases containing coronavirus omics data
数据库名称 Database name | 网址 URL | 简介 Brief description |
|---|---|---|
| Biostudies | https://www.ebi.ac.uk/biostudies/ | 包含生物学研究数据集,以及与EMBL-EBI数据库或其他公共数据库的数据链接 |
| ENA (European Nucleotide Archive) | https://www.ebi.ac.uk/ena | 核酸测序原始数据、序列拼装和功能注释信息 |
| PRIDE (Proteomics Identifications Database) | https://www.ebi.ac.uk/pride | 蛋白质组学质谱数据存储平台包含蛋白质鉴定、翻译后修饰和光谱数据 |
| GEO (Gene Expression Omnibus) | https://www.ncbi.nlm.nih.gov/geo/ | 存储各种高通量实验数据的公共数据库,以表达谱、芯片数据为主 |
| EGA (European Genome-Phenome Archive) | https://ega-archive.org | 存储生物医学研究中产生的遗传和表型数据 |
| ArrayExpress | https://www.ebi.ac.uk/arrayexpress/ | 主要包括微阵列芯片和高通量测序数据,与GEO数据库类似 |
| iProX (Integrated Proteome Resources) | https://www.iprox.org | 国家蛋白质科学中心网站,收录蛋白质组学相关数据集 |
MassIVE (Mass Spectrometry Interactive Virtual Environment) | https://massive.ucsd.edu/ProteoSAFe/static/massive.jsp | 储存蛋白、多肽、质谱数据 |
| FAIRDOMHub | https://fairdomhub.org/ | 共享多种类型的科学研究数据集、模型或研究过程和成果数据 |
| BioModels | https://www.ebi.ac.uk/biomodels/ | 储存生物和医学相关的数学模型、生理学和药学相关机制模型 |
| MetaboLights | https://www.ebi.ac.uk/metabolights/ | 储存代谢组学和相关衍生信息的数据库,包含代谢物的结构、浓度、功能数据 |
| dbGap (Database of Genotypes and Phenotypes) | https://dbgap.ncbi.nlm.nih.gov/ | 储存基因型和表型互作数据 |
| PanoramaPublic | https://panoramaweb.org/ | 储存蛋白质组学和小分子数据 |
| GNPS (Global Natural Products Social Molecular Networking) | https://gnps.ucsd.edu/ | 储存原始、处理或注释过的质谱数据 |
Metabolomics Workbench | https://www.metabolomicsworkbench.org/ | 代谢组学元数据和实验数据的公共数据库,包含不同物种的质谱(MS)和核磁共振(NMR)光谱数据 |
| ExpressionAtlas | https://www.ebi.ac.uk/gxa/home | 提供不同条件下基因表达信息,包含芯片与转录组数据 |
jPOST (Japan Proteome Standard Repository Database) | https://jpostdb.org/ | 包含蛋白质组数据及分析结果数据 |
| EVA (European Variation Archive) | https://www.ebi.ac.uk/eva/ | 包含不同物种遗传变异数据 |
| NODE (National Omics Data Encyclopedia) | https://www.biosino.org/node/ | 储存多组学数据资源,包括测序数据、蛋白质组学数据、代谢组学数据及荧光成像数据 |
| PeptideAtlas | https://peptideatlas.org/ | 收录质谱、蛋白质组学数据。主要为人、小鼠、酵母数据 |
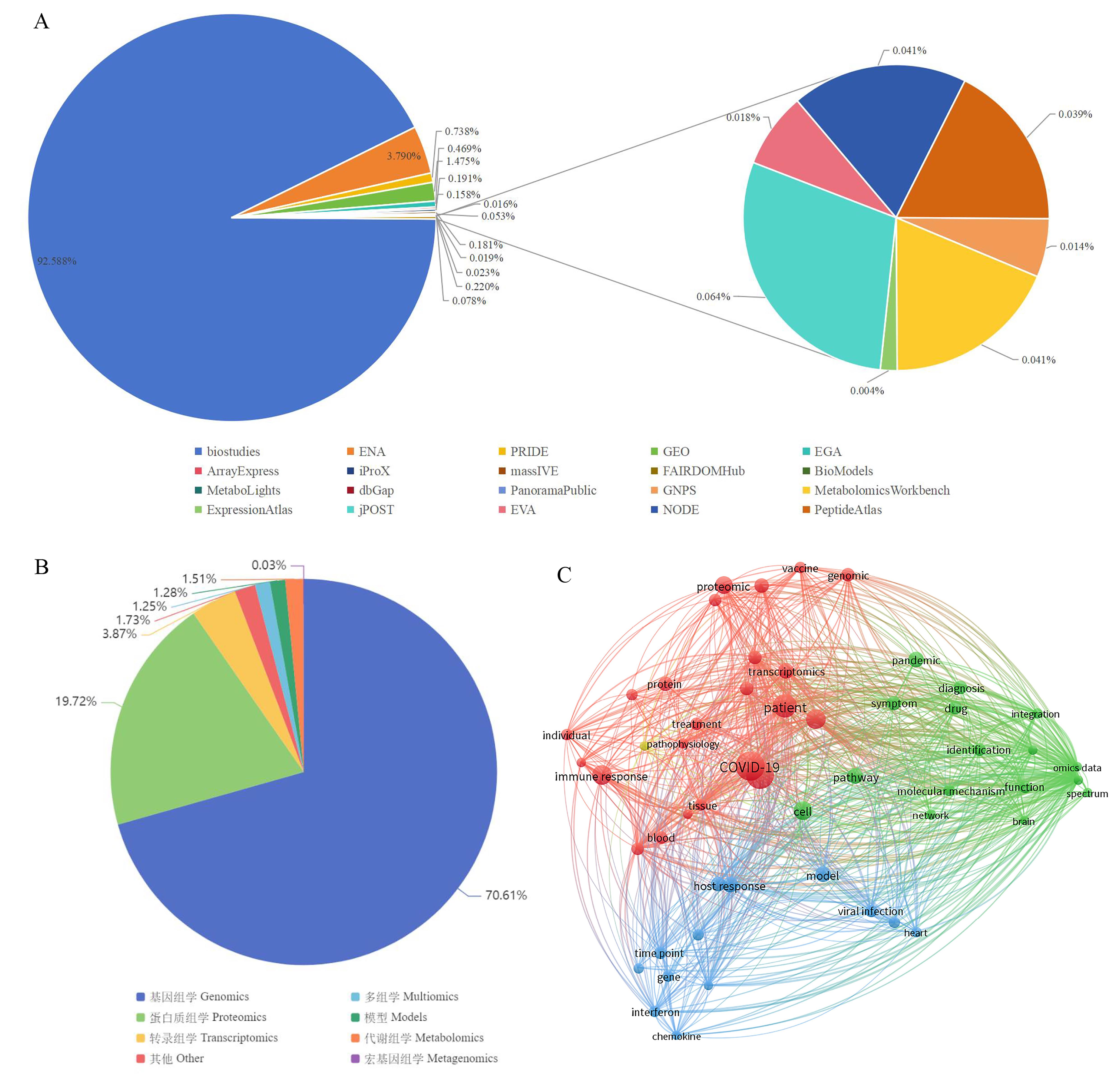
图1 公共数据库中冠状病毒多组学数据统计及文本共现网络分析注:A,公共数据库中冠状病毒组学数据分布;B,OmicsDI数据库中新冠病毒数据类型;C,新冠病毒多组学文本共现网络分析。
Figure 1 Statistics and text co-occurrence network analysis of coronavirus multi-omics data in public databasesNote:A, Distribution of coronavirus omics data in public databases; B, Data types of SARS-CoV-2 in OmicsDI; C, Text co-occurrence network analysis of multi-omics related to SARS-CoV-2.
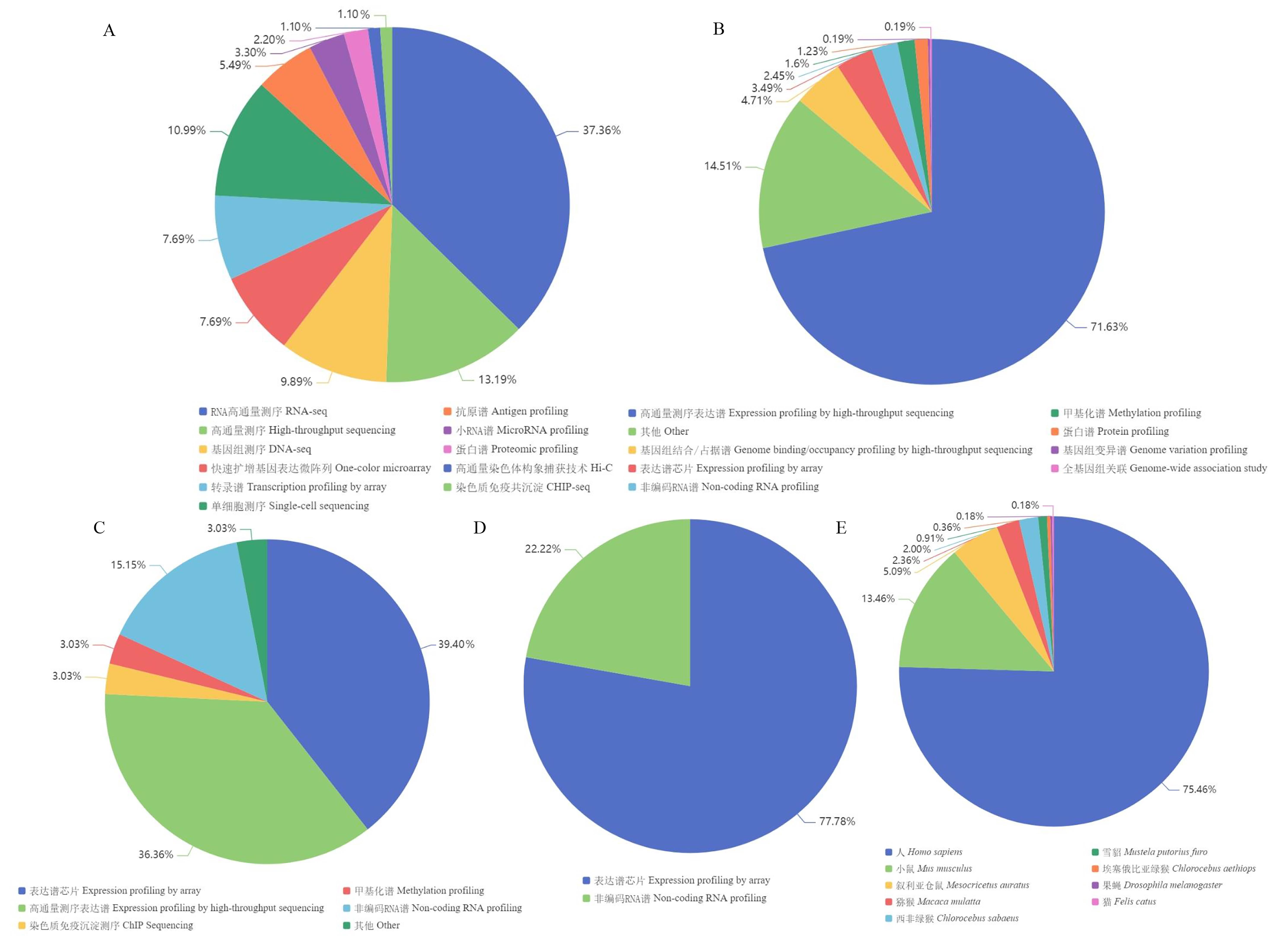
图2 ArrayExpress、GEO及DDBJ数据库中冠状病毒组学数据统计及分析注:A, ArrayExpress数据库中新冠病毒数据类型;B, GEO数据库中新冠病毒数据类型;C, GEO数据库中MERS数据类型;D, GEO数据库中SARS数据类型;E, DDBJ数据库中新冠病毒数据的物种分布。
Figure 2 Statistics and analysis of coronavirus omics data in ArrayExpress, GEO, and DDBJNote:A, Data types of SARS-CoV-2 in ArrayExpress; B, Data types of SARS-CoV-2 in GEO; C, Data types of MERS in GEO; D, Data types of SARS in GEO; E, Species distribution of SARS-CoV-2 data in DDBJ.
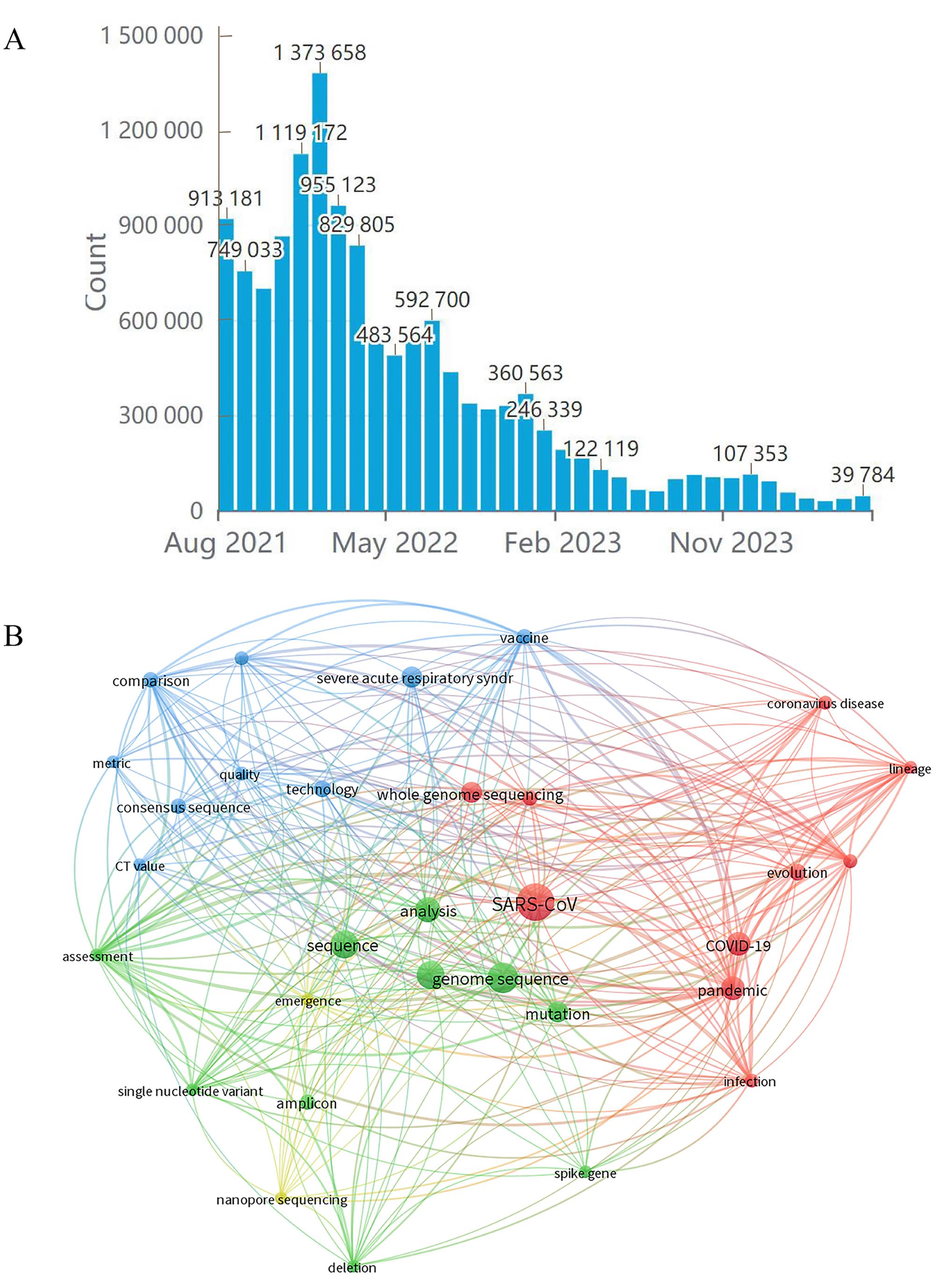
图3 新冠病毒基因组学的数据统计及分析注:A,GISAID数据库中新冠病毒序列数据变化情况;B,新冠病毒基因组学文本共现网络分析。
Figure 3 Statistics and analysis of SARS-CoV-2 genomics dataNote: A, Changes of SARS-CoV-2 sequence data in GISAID; B, Text co-occurrence network analysis of SARS-CoV-2 genomics.
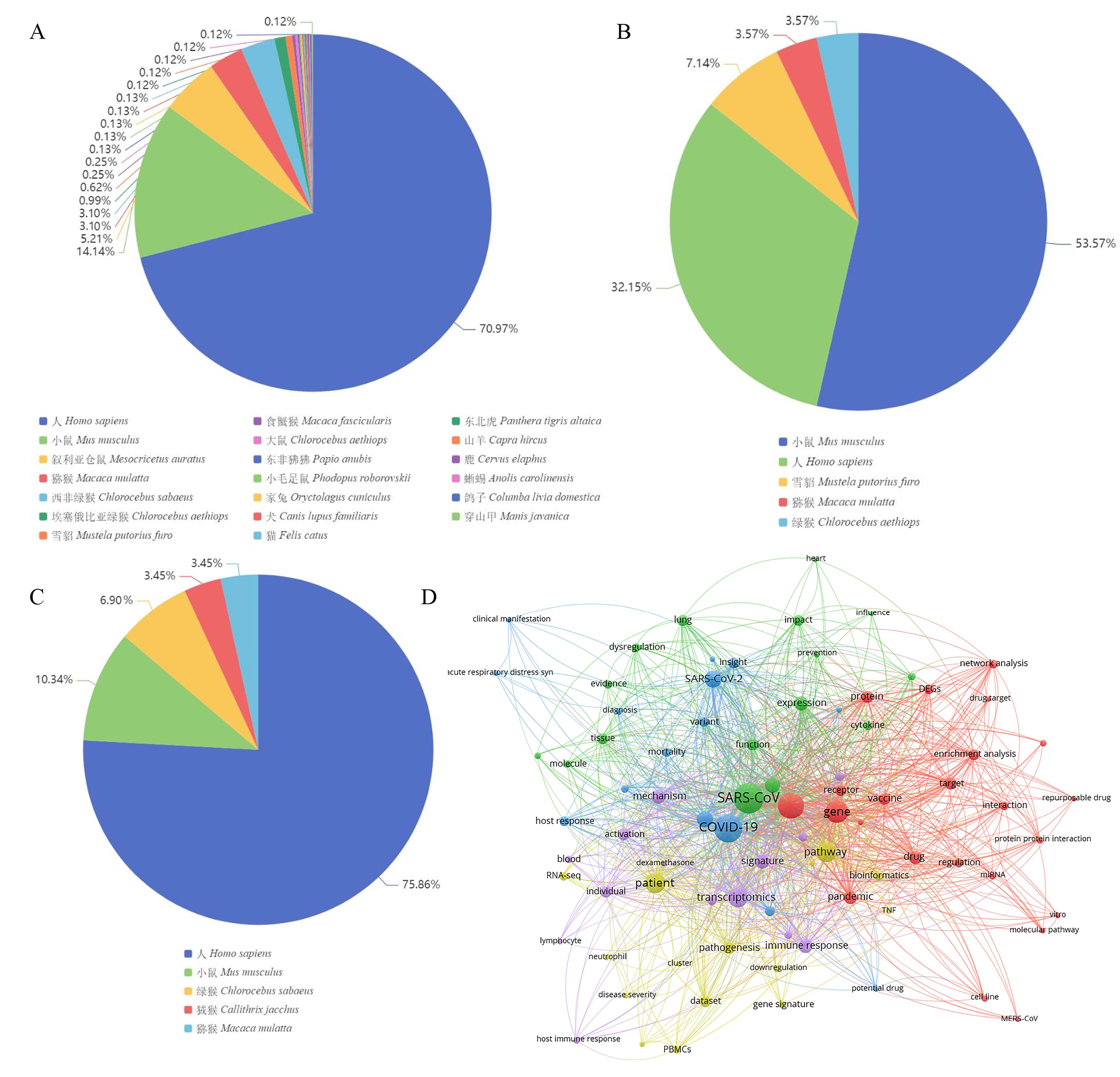
图4 GEO数据库中冠状病毒表达谱统计及分析注:A,GEO数据库中新冠病毒表达谱物种分布;B,GEO数据库中SARS表达谱物种分布;C,GEO数据库中MERS表达谱物种分布;D,新冠病毒转录组学文本共现网络分析。
Figure 4 Statistics and analysis of coronavirus expression profiles in GEONote: A, Species distribution of SARS-CoV-2 expression profiles in GEO; B, Species distribution of SARS expression profiles in GEO; C, Species distribution of MERS expression profiles in GEO; D, Text co-occurrence network analysis of SARS-CoV-2 in transcriptomics.
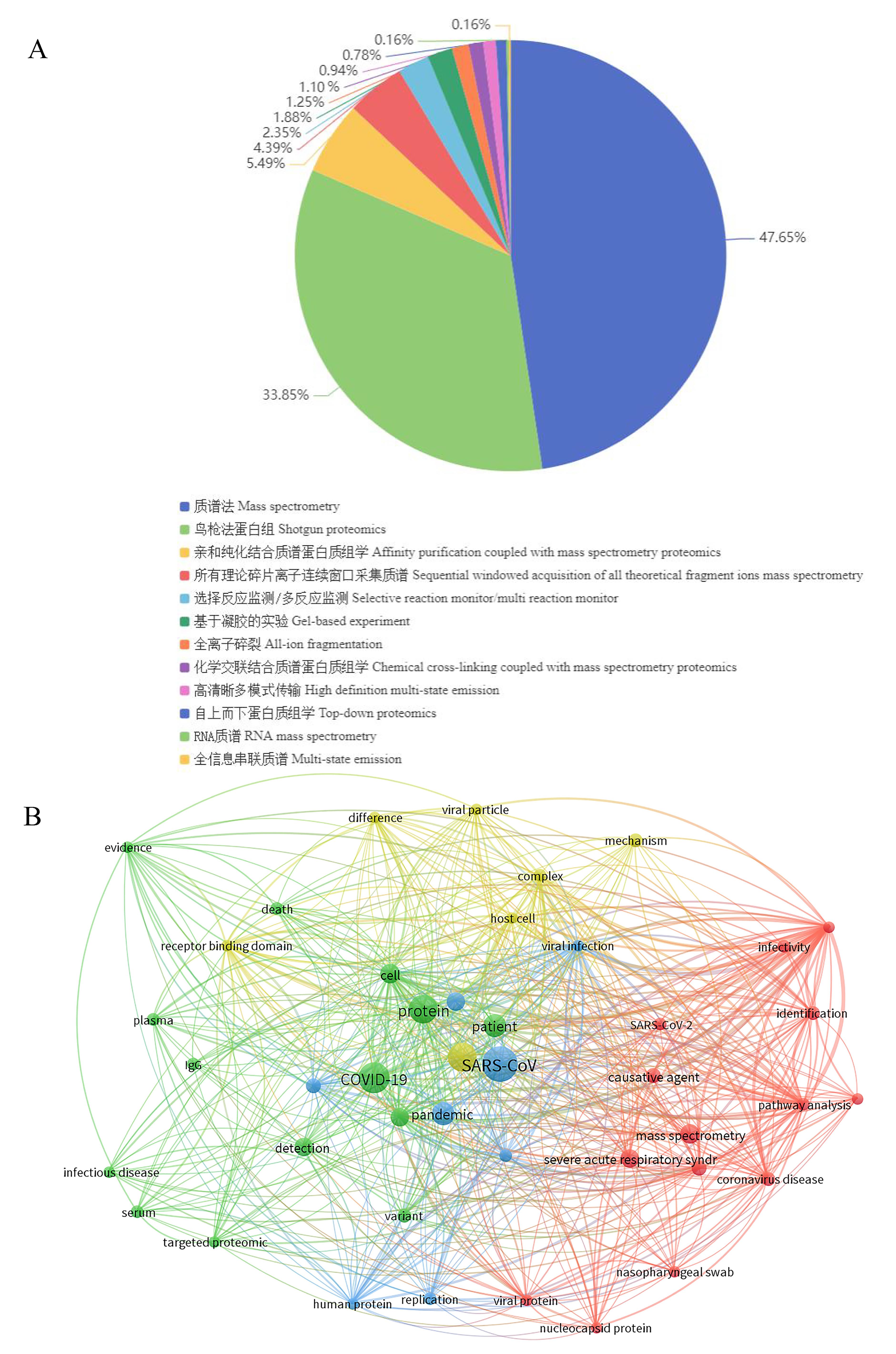
图5 冠状病毒蛋白质组学数据统计及文本共现网络分析注:A,冠状病毒蛋白质组学数据类型;B,新冠病毒蛋白质组学文本共现网络分析。
Figure 5 Statistics and text co-occurrence networkNote: A,Types of coronavirus proteomics data; B, Text co-occurrence network analysis of SARS-CoV-2 proteomics.analysis of coronavirus proteomics data
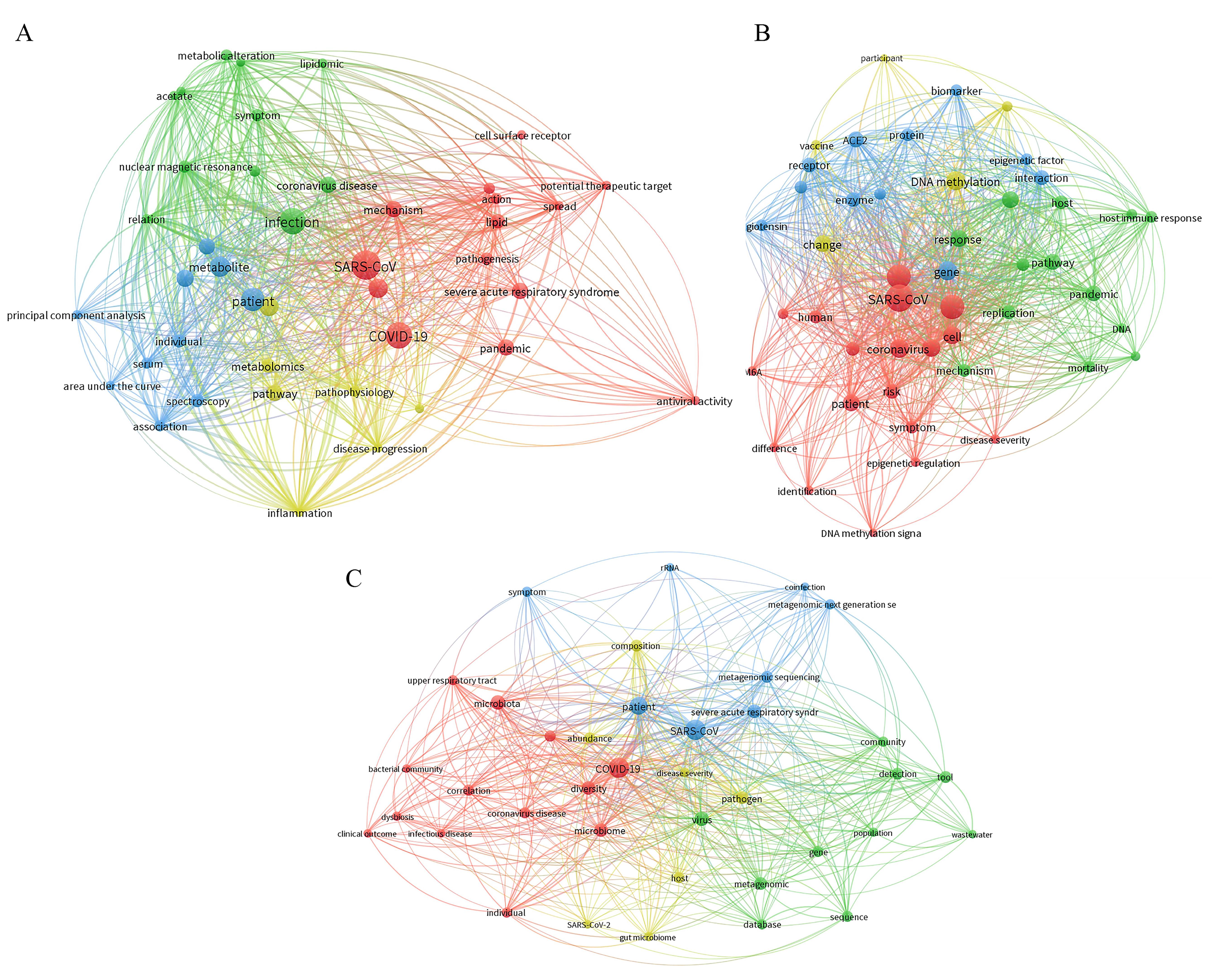
图6 新冠病毒代谢组学(A)、表观组学(B)、微生物组学(C)的文本共现网络分析
Figure 6 Text co-occurrence network analysis of SARS-CoV-2 metabolomics (A), epigenomics (B), and microbiomics (C)
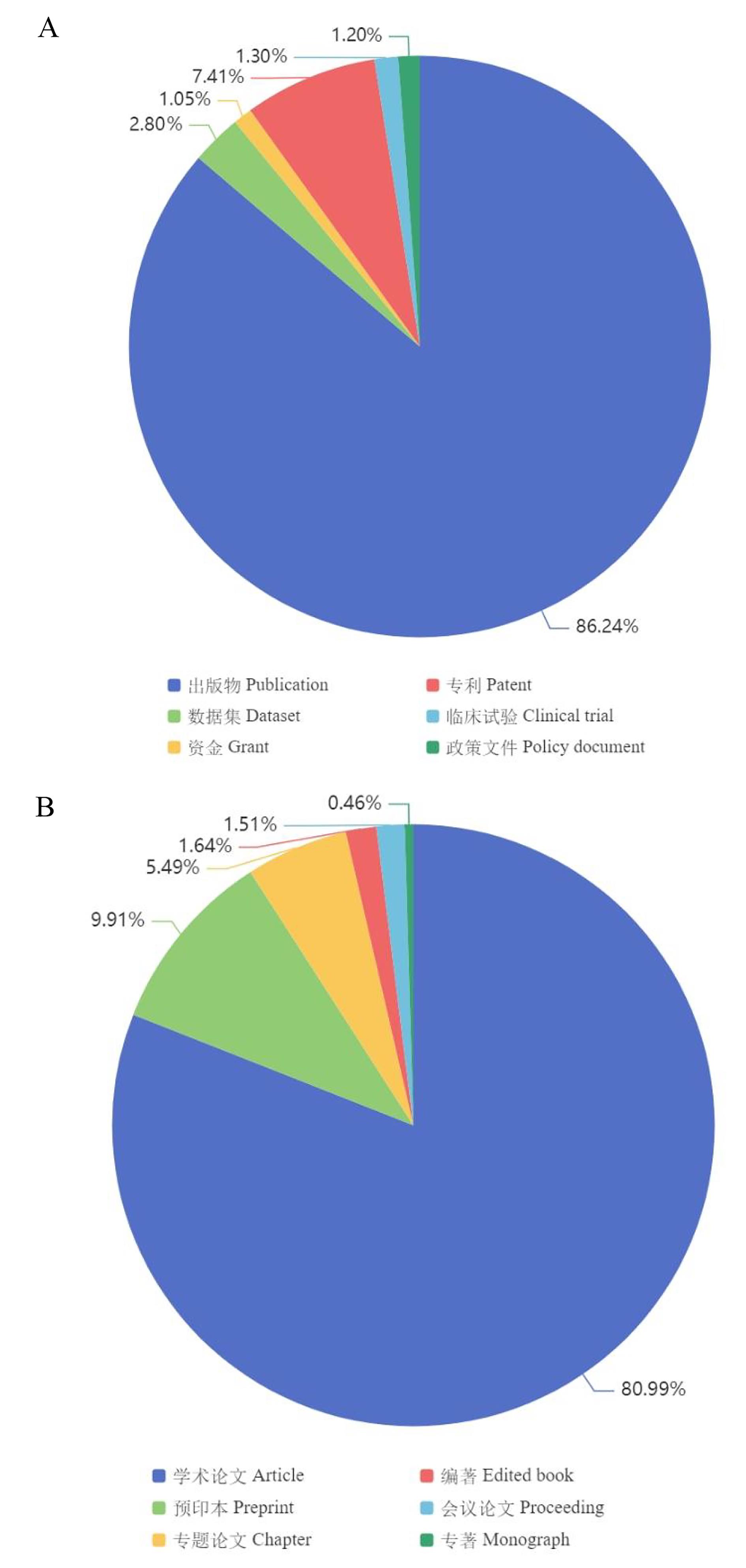
图7 冠状病毒相关数据及文献分布注:A,Dimension数据库中冠状病毒相关数据类型;B,Dimension数据库中冠状病毒相关出版物类型。
Figure 7 Distribution of coronavirus-related data and literatureNote:A, Types of coronavirus-related data in Dimension; B, Types of coronavirus-related publications in Dimension.
| 1 | KHALIFA S A M, MOHAMED B S, ELASHAL M H, et al. Comprehensive overview on multiple strategies fighting COVID-19[J]. Int J Environ Res Public Health, 2020, 17(16):5813. DOI: 10.3390/ijerph17165813 . |
| 2 | SHEHATA M M, GOMAA M R, ALI M A, et al. Middle East respiratory syndrome coronavirus: a comprehensive review[J]. Front Med, 2016, 10(2):120-136. DOI: 10.1007/s11684-016-0430-6 . |
| 3 | RANA R, TRIPATHI A, KUMAR N, et al. A comprehensive overview on COVID-19: future perspectives[J]. Front Cell Infect Microbiol, 2021, 11:744903. DOI: 10.3389/fcimb. 2021.744903 . |
| 4 | CHAN J F, KOK K H, ZHU Z, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan[J]. Emerg Microbes Infect, 2020, 9(1):221-236. DOI: 10.1080/22221751.2020.1719902 . |
| 5 | SHI W F, LI J, ZHOU H, et al. Pathogen genomic surveillance elucidates the origins, transmission and evolution of emerging viral agents in China[J]. Sci China Life Sci, 2017, 60(12):1317-1330. DOI: 10.1007/s11427-017-9211-0 . |
| 6 | DAS J K, TRADIGO G, VELTRI P, et al. Data science in unveiling COVID-19 pathogenesis and diagnosis: evolutionary origin to drug repurposing[J]. Brief Bioinform, 2021, 22(2):855-872. DOI: 10.1093/bib/bbaa420 . |
| 7 | SAYERS E W, BECK J, BOLTON E E, et al. Database resources of the National Center for Biotechnology Information[J]. Nucleic Acids Res, 2024, 52(D1): D33-D43. DOI: 10.1093/nar/gkad1044 . |
| 8 | O'SULLIVAN C, BUSBY B, MIZRACHI I K. Managing sequence data[J]. Methods Mol Biol, 2017, 1525:79-106. DOI: 10.1007/978-1-4939-6622-6_4 . |
| 9 | SHUMWAY M, COCHRANE G, SUGAWARA H. Archiving next generation sequencing data[J]. Nucleic Acids Res, 2010, 38(Database issue): D870-D871. DOI: 10.1093/nar/gkp1078 . |
| 10 | WILKINSON M D, DUMONTIER M, AALBERSBERG I JAN, et al. Addendum: the FAIR Guiding Principles for scientific data management and stewardship[J]. Sci Data, 2019, 6(1):6. DOI: 10.1038/s41597-019-0009-6 . |
| 11 | MONTALDO C, MESSINA F, ABBATE I, et al. Multi-omics approach to COVID-19: a domain-based literature review[J]. J Transl Med, 2021, 19(1):501. DOI: 10.1186/s12967-021-03168-8 . |
| 12 | VENKATAKRISHNAN A J, PURANIK A, ANAND A, et al. Knowledge synthesis of 100 million biomedical documents augments the deep expression profiling of coronavirus receptors[J]. Elife, 2020, 9: e58040. DOI: 10.7554/eLife.58040 . |
| 13 | KOLENC Ž, PIRIH N, GRETIC P, et al. Top trends in multiomics research: evaluation of 52 published studies and new ways of thinking terminology and visual displays[J]. OMICS, 2021, 25(11):681-692. DOI: 10.1089/omi.2021.0160 . |
| 14 | 周大琼, 曹继华, 任力锋, 等. 基因芯片数据库GEO与ArrayExpress的使用及比较分析[J]. 中国现代医学杂志, 2014, 24(12):38-42. DOI:10.3969/j.issn.1005-8982.2014.12.009 . |
| ZHOU D Q, CAO J H, REN L F, et al. Usage and comparative analysis of GEO and ArrayExpress microarray database[J]. China J Mod Med, 2014, 24(12):38-42. DOI:10.3969/j.issn.1005- 8982.2014.12.009 . | |
| 15 | MURILLO J, VILLEGAS L M, ULLOA-MURILLO L M, et al. Recent trends on omics and bioinformatics approaches to study SARS-CoV-2: a bibliometric analysis and mini-review[J]. Comput Biol Med, 2021, 128:104162. DOI: 10.1016/j.compbiomed.2020.104162 . |
| 16 | VAN ECK N J, WALTMAN L. Software survey: VOSviewer, a computer program for bibliometric mapping[J]. Scientometrics, 2010, 84(2):523-538. DOI: 10.1007/s11192-009-0146-3 . |
| 17 | DASS G, VU M T, XU P, et al. The omics discovery REST interface[J]. Nucleic Acids Res, 2020, 48(W1): W380-W384. DOI: 10.1093/nar/gkaa326 . |
| 18 | BARH D, TIWARI S, WEENER M E, et al. Multi-omics-based identification of SARS-CoV-2 infection biology and candidate drugs against COVID-19[J]. Comput Biol Med, 2020, 126:104051. DOI: 10.1016/j.compbiomed.2020.104051 . |
| 19 | GUO M Y, XIONG M Y, PENG J Y, et al. Multi-omics for COVID-19: driving development of therapeutics and vaccines[J]. Natl Sci Rev, 2023, 10(9): nwad161. DOI: 10.1093/nsr/nwad161 . |
| 20 | DRAKE K A, TALANTOV D, TONG G J, et al. Multi-omic profiling reveals early immunological indicators for identifying COVID-19 Progressors[J]. Clin Immunol, 2023, 256:109808. DOI: 10.1016/j.clim.2023.109808 . |
| 21 | LI C X, GAO J, ZHANG Z C, et al. Multiomics integration-based molecular characterizations of COVID-19[J]. Brief Bioinform, 2022, 23(1): bbab485. DOI: 10.1093/bib/bbab485 . |
| 22 | SARAVANAN K A, PANIGRAHI M, KUMAR H, et al. Role of genomics in combating COVID-19 pandemic[J]. Gene, 2022, 823:146387. DOI: 10.1016/j.gene.2022.146387 . |
| 23 | ROUCHKA E C, CHARIKER J H, CHUNG D. Variant analysis of 1,040 SARS-CoV-2 genomes[J]. PLoS One, 2020, 15(11): e0241535. DOI: 10.1371/journal.pone.0241535 . |
| 24 | GAO C C, LI M, DENG W, et al. Differential transcriptomic landscapes of multiple organs from SARS-CoV-2 early infected rhesus macaques[J]. Protein Cell, 2022, 13(12):920-939. DOI: 10.1007/s13238-022-00915-5 . |
| 25 | RUAN Q R, YANG K, WANG W X, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China[J]. Intensive Care Med, 2020, 46(5):846-848. DOI: 10.1007/s00134-020-05991-x . |
| 26 | KIM J A, KIM S H, SEO J S, et al. Temporal transcriptome analysis of SARS-CoV-2-infected lung and spleen in human ACE2-transgenic mice[J]. Mol Cells, 2022, 45(12):896-910. DOI: 10.14348/molcells.2022.0089 . |
| 27 | DALY J L, SIMONETTI B, KLEIN K, et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection[J]. Science, 2020, 370(6518):861-865. DOI: 10.1126/science.abd3072 . |
| 28 | O'DONNELL K L, PINSKI A N, CLANCY C S, et al. Pathogenic and transcriptomic differences of emerging SARS-CoV-2 variants in the Syrian golden hamster model[J]. EBioMedicine, 2021, 73:103675. DOI: 10.1016/j.ebiom. 2021. 103675 . |
| 29 | DESAI N, NEYAZ A, SZABOLCS A, et al. Temporal and spatial heterogeneity of host response to SARS-CoV-2 pulmonary infection[J]. Nat Commun, 2020, 11(1):6319. DOI: 10.1038/s41467-020-20139-7 . |
| 30 | OH T, KIM G, BAEK S H, et al. Comparative spatial transcriptomic profiling of severe acute respiratory syndrome coronavirus 2 Delta and Omicron variants infections in the lungs of cynomolgus macaques[J]. J Med Virol, 2023, 95(6): e28847. DOI: 10.1002/jmv.28847 . |
| 31 | ESPOSITO S, D'ABROSCA G, ANTOLAK A, et al. Host and viral zinc-finger proteins in COVID-19[J]. Int J Mol Sci, 2022, 23(7):3711. DOI: 10.3390/ijms23073711 . |
| 32 | GUPTA R K, ROSENHEIM J, BELL L C, et al. Blood transcriptional biomarkers of acute viral infection for detection of pre-symptomatic SARS-CoV-2 infection: a nested, case-control diagnostic accuracy study[J]. Lancet Microbe, 2021, 2(10): e508-e517. DOI: 10.1016/S2666-5247(21)00146-4 . |
| 33 | ZIEGLER C G K, MIAO V N, OWINGS A H, et al. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19[J]. Cell, 2021, 184(18):4713-4733.e22. DOI: 10.1016/j.cell.2021.07.023 . |
| 34 | NIE X, QIAN L J, SUN R, et al. Multi-organ proteomic landscape of COVID-19 autopsies[J]. Cell, 2021, 184(3):775-791.e14. DOI: 10.1016/j.cell.2021.01.004 . |
| 35 | MESSNER C B, DEMICHEV V, WENDISCH D, et al. Ultra-high-throughput clinical proteomics reveals classifiers of COVID-19 infection[J]. Cell Syst, 2020, 11(1):11-24.e4. DOI: 10.1016/j.cels.2020.05.012 . |
| 36 | BOJKOVA D, KLANN K, KOCH B, et al. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets[J]. Nature, 2020, 583(7816):469-472. DOI: 10.1038/s41586-020-2332-7 . |
| 37 | XU J J, ZHOU M, LUO P, et al. Plasma metabolomic profiling of patients recovered from coronavirus disease 2019 (COVID-19) with pulmonary sequelae 3 months after discharge[J]. Clin Infect Dis, 2021, 73(12):2228-2239. DOI: 10.1093/cid/ciab147 . |
| 38 | SHEN B, YI X, SUN Y T, et al. Proteomic and metabolomic characterization of COVID-19 patient sera[J]. Cell, 2020, 182(1):59-72.e15. DOI: 10.1016/j.cell.2020.05.032 . |
| 39 | KARPE A V, NGUYEN T V, SHAH R M, et al. A time-series metabolomic analysis of SARS-CoV-2 infection in a ferret model[J]. Metabolites, 2022, 12(11):1151. DOI: 10.3390/metabo12111151 . |
| 40 | JIA H L, LIU C W, LI D T, et al. Metabolomic analyses reveal new stage-specific features of COVID-19[J]. Eur Respir J, 2022, 59(2):2100284. DOI: 10.1183/13993003.00284-2021 . |
| 41 | VALDÉS A, MORENO L O, RELLO S R, et al. Metabolomics study of COVID-19 patients in four different clinical stages[J]. Sci Rep, 2022, 12(1):1650. DOI: 10.1038/s41598-022-05667-0 . |
| 42 | THOMAS T, STEFANONI D, REISZ J A, et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status[J]. JCI Insight, 2020, 5(14): e140327. DOI: 10.1172/jci.insight.140327 . |
| 43 | MA J L, DENG Y W, ZHANG M H, et al. The role of multi-omics in the diagnosis of COVID-19 and the prediction of new therapeutic targets[J]. Virulence, 2022, 13(1):1101-1110. DOI: 10.1080/21505594.2022.2092941 . |
| 44 | KGATLE M M, LAWAL I O, MASHABELA G, et al. COVID-19 is a multi-organ aggressor: epigenetic and clinical marks[J]. Front Immunol, 2021, 12:752380. DOI: 10.3389/fimmu. 2021.752380 . |
| 45 | BALNIS J, MADRID A, HOGAN K J, et al. Persistent blood DNA methylation changes one year after SARS-CoV-2 infection[J]. Clin Epigenetics, 2022, 14(1):94. DOI: 10.1186/s13148-022-01313-8 . |
| 46 | STUKALOV A, GIRAULT V, GRASS V, et al. Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV[J]. Nature, 2021, 594(7862):246-252. DOI: 10.1038/s41586-021-03493-4 . |
| 47 | PHILLIPS S, MISHRA T, KHADKA S, et al. Epitranscriptomic N 6-methyladenosine profile of SARS-CoV-2-infected human lung epithelial cells[J]. Microbiol Spectr, 2023, 11(1): e0394322. DOI: 10.1128/spectrum.03943-22 . |
| 48 | MA S L, ZHANG F, ZHOU F X, et al. Metagenomic analysis reveals oropharyngeal microbiota alterations in patients with COVID-19[J]. Signal Transduct Target Ther, 2021, 6(1):191. DOI: 10.1038/s41392-021-00614-3 . |
| 49 | MAEDA Y, MOTOOKA D, KAWASAKI T, et al. Longitudinal alterations of the gut mycobiota and microbiota on COVID-19 severity[J]. BMC Infect Dis, 2022, 22(1):572. DOI: 10.1186/s12879-022-07358-7 . |
| 50 | ZUO T, ZHANG F, LUI G C Y, et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization[J]. Gastroenterology, 2020, 159(3):944-955.e8. DOI: 10.1053/j.gastro.2020.05.048 . |
| 51 | LIU Q, MAK J W Y, SU Q, et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome[J]. Gut, 2022, 71(3):544-552. DOI: 10.1136/gutjnl-2021-325989 . |
| 52 | NIES L D, GALATA V, MARTIN-GALLAUSIAUX C, et al. Altered infective competence of the human gut microbiome in COVID-19[J]. Microbiome, 2023, 11(1):46. DOI: 10.1186/s40168-023-01472-7 . |
| 53 | XU X G, ZHANG W, GUO M Q, et al. Integrated analysis of gut microbiome and host immune responses in COVID-19[J]. Front Med, 2022, 16(2):263-275. DOI: 10.1007/s11684-022-0921-6 . |
| 54 | PANG Z Q, ZHOU G Y, CHONG J, et al. Comprehensive meta-analysis of COVID-19 global metabolomics datasets[J]. Metabolites, 2021, 11(1):44. DOI: 10.3390/metabo11010044 . |
| 55 | LU T, WANG Y R, GUO T N. Multi-omics in COVID-19: seeing the unseen but overlooked in the clinic[J]. Cell Rep Med, 2022, 3(3):100580. DOI: 10.1016/j.xcrm.2022.100580 . |
| 56 | CEN X P, WANG F G, HUANG X H, et al. Towards precision medicine: Omics approach for COVID-19[J]. Biosaf Health, 2023, 5(2):78-88. DOI: 10.1016/j.bsheal.2023.01.002 . |
| 57 | MIRALLES A, BRUY T, WOLCOTT K, et al. Repositories for taxonomic data: where we are and what is missing[J]. Syst Biol, 2020, 69(6):1231-1253. DOI: 10.1093/sysbio/syaa026 . |
| 58 | HAAS P, MURALIDHARAN M, KROGAN N J, et al. Proteomic approaches to study SARS-CoV-2 biology and COVID-19 pathology[J]. J Proteome Res, 2021, 20(2):1133-1152. DOI: 10.1021/acs.jproteome.0c00764 . |
| 59 | YANG J J, YAN Y Z, ZHONG W. Application of omics technology to combat the COVID-19 pandemic[J]. MedComm, 2021, 2(3):381-401. DOI: 10.1002/mco2.90 . |
| 60 | RAY S, SRIVASTAVA S. COVID-19 pandemic: hopes from proteomics and multiomics research[J]. OMICS, 2020, 24(8):457-459. DOI: 10.1089/omi.2020.0073 . |
| 61 | ZHU Z J, ZHANG S N, WANG P, et al. A comprehensive review of the analysis and integration of omics data for SARS-CoV-2 and COVID-19[J]. Brief Bioinform, 2022, 23(1): bbab446. DOI: 10.1093/bib/bbab446 . |
| 62 | DONG X J, LIU C Y, DOZMOROV M. Review of multi-omics data resources and integrative analysis for human brain disorders[J]. Brief Funct Genomics, 2021, 20(4):223-234. DOI: 10.1093/bfgp/elab024 . |
| 63 | 吴玥, 向志光, 高苒, 等. 冠状病毒感染动物模型比较转录组学数据库的建立[J]. 中国实验动物学报, 2022, 30(1):92-99. DOI: 10.3969/j.issn.1005-4847.2022.01.012 . |
| WU Y, XIANG Z G, GAO R, et al. Establishment of a comparative transcriptomics database of coronavirus infected animal models[J]. Acta Lab Anim Sci Sin, 2022, 30(1):92-99. DOI: 10.3969/j.issn.1005-4847.2022.01.012 . | |
| 64 | 吴玥, 王珏, 薛婧, 等. 心脏病动物模型比较转录组学数据库的构建[J]. 中国比较医学杂志, 2023, 33(3):75-81. DOI: 10.3969/j.issn.1671-7856.2023.03.010 . |
| WU Y, WANG J, XUE J, et al. Establishment of a comparative transcriptomics database of heart disease animal models[J]. Chin J Comp Med, 2023, 33(3):75-81. DOI: 10.3969/j.issn.1671-7856.2023.03.010 . | |
| 65 | 孔琪, 夏霞宇, 秦川. 实验动物品系数据库的建立[J]. 中国比较医学杂志, 2015, 25(4):78-83. DOI: 10.3969/j.issn.1671.7856.2015.004.016 . |
| KONG Q, XIA X Y, QIN C. Establishment of the laboratory animal strain database[J]. Chin J Comp Med, 2015, 25(4):78-83. DOI: 10.3969/j.issn.1671.7856.2015.004.016 . | |
| 66 | 吴玥, 魏强, 张连峰, 等. 比较医学大数据平台的建立[J]. 中国比较医学杂志, 2022, 32(12):57-65. DOI: 10.3969/j.issn.1671-7856.2022.12.008 . |
| WU Y, WEI Q, ZHANG L F, et al. Establishment the comparative medicine big-data platform[J]. Chin J Comp Med, 2022, 32(12):57-65. DOI: 10.3969/j.issn.1671-7856.2022.12.008 . | |
| 67 | 吴玥, 薛婧, 魏强, 等. 国家动物模型资源共享信息平台的建立[J]. 中国实验动物学报, 2022, 30(8):1080-1086. DOI: 10.3969/j.issn.1005-4847.2022.08.009 . |
| WU Y, XUE J, WEI Q, et al. Establishment of national infrastructure for an animal model resource platform[J]. Acta Lab Anim Sci Sin, 2022, 30(8):1080-1086. DOI: 10.3969/j.issn.1005-4847.2022.08.009 . | |
| 68 | 吴玥, 王珏, 冯婷婷, 等. 基于动物模型的药物筛选数据库的建立[J]. 中国实验动物学报, 2023, 31(6):778-786. DOI: 10.3969/j.issn.1005-4847.2023.06.010 . |
| WU Y, WANG J, FENG T T, et al. Construction of a drug screening database based on animal models[J]. Acta Lab Anim Sci Sin, 2023, 31(6):778-786. DOI: 10.3969/j.issn.1005-4847.2023.06.010 . | |
| 69 | WU Y, WANG J, XUE J, et al. Flu-CED: A comparative transcriptomics database of influenza virus-infected human and animal models[J]. Animal Model Exp Med,2024,2:2-12. DOI: 10.1002/ame2.12384 . |
| [1] | 郑艺清, 邓亚胜, 范燕萍, 梁天薇, 黄慧, 刘永辉, 倪召兵, 林江. 基于数据挖掘的盆腔炎性疾病动物模型应用分析[J]. 实验动物与比较医学, 2024, 44(4): 405-418. |
| [2] | 丁天送, 谢京红, 杨斌, 李河桥, 乔一倬, 陈心如, 田纹凡, 李佳佩, 张婉怡, 李帆旋. 复发性流产动物模型特点评价与应用分析[J]. 实验动物与比较医学, 2024, 44(4): 393-404. |
| [3] | 姚广源, 董平, 吴昊, 柏梅, 党嬴, 王悦, 胡凯. 长骨骨折动物模型的研究进展[J]. 实验动物与比较医学, 2024, 44(3): 289-296. |
| [4] | 包方奇, 屠海烨, 方明笋, 张倩, 陈民利. 基于动物模型的高尿酸肾病病理及分子机制研究进展[J]. 实验动物与比较医学, 2024, 44(2): 180-191. |
| [5] | 张莉, 匡宇, 韩凌霞. 人与其他动物椎间盘解剖和组织学结构的比较医学研究进展[J]. 实验动物与比较医学, 2024, 44(2): 192-201. |
| [6] | 梁天薇, 邓亚胜, 黄慧, 荣娜, 刘鑫, 王玉洁, 林江. 围绝经期综合征动物模型的制备方法及评价指标分析[J]. 实验动物与比较医学, 2024, 44(1): 74-84. |
| [7] | . 自发性脑出血动物模型选择及临床前药物试验指南(2024年版)[J]. 实验动物与比较医学, 2024, 44(1): 3-30. |
| [8] | 刘欣, 石少波, 张翠, 杨波, 曲川. 小鼠自体动静脉内瘘端侧吻合模型的建立与评价[J]. 实验动物与比较医学, 2023, 43(6): 595-603. |
| [9] | 万颖寒, 顾也欣, 袁雨浓, 汤忞, 鲁立. FDA现代化法案2.0给疾病动物模型发展带来的启示和思考[J]. 实验动物与比较医学, 2023, 43(5): 472-481. |
| [10] | 谢淑武, 沈如凌, 林金杏, 范春. 雄性不育药物研发相关实验动物模型建立和应用进展[J]. 实验动物与比较医学, 2023, 43(5): 504-511. |
| [11] | 陈艳娟, 沈如凌. 模式动物疾病模型在结直肠癌医学研究中的应用进展[J]. 实验动物与比较医学, 2023, 43(5): 512-523. |
| [12] | 张睿, 吕美豫, 张建军, 刘金莲, 陈彦, 黄志强, 刘尧, 周澜华. 痤疮动物模型建立及评价研究进展[J]. 实验动物与比较医学, 2023, 43(4): 398-405. |
| [13] | 卢今, 王剑, 朱莲, 严国锋, 马政文, 李垚, 戴建军, 朱寅秋, 周晶. 山羊先兆子痫疾病模型的构建及母体生物学特性评价[J]. 实验动物与比较医学, 2023, 43(4): 371-380. |
| [14] | 俞佳慧, 巩倩, 庄乐南. 肺动脉高压动物模型及其在药物研究中的应用进展[J]. 实验动物与比较医学, 2023, 43(4): 381-397. |
| [15] | 邓亚胜, 林江, 甘池伶, 曾官凤, 黄嘉茵, 邓慧芳, 麻颖贤, 韩丝银. 皮肤光老化动物模型制备要素和受试物数据的文献分析[J]. 实验动物与比较医学, 2023, 43(4): 406-414. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||