













实验动物与比较医学 ›› 2024, Vol. 44 ›› Issue (1): 31-41.DOI: 10.12300/j.issn.1674-5817.2023.118
郑建华, 法云智, 董巧燕, 邱业峰( )(
)( ), 陈菁青(
), 陈菁青( )(
)( )
)
收稿日期:2023-08-17
修回日期:2023-11-18
出版日期:2024-02-25
发布日期:2024-03-07
通讯作者:
邱业峰(1980—),男,博士,研究员,研究方向:实验动物模型创制研究。E-mail: qiuyefeng2001@163.com。ORCID: 0000-0002-5392-3293;作者简介:郑建华(1998—),男,硕士研究生,研究方向:实验动物模型创制研究。E-mail: zjhcbm0311@163.com
基金资助:
Jianhua ZHENG, Yunzhi FA, Qiaoyan DONG, Yefeng QIU( )(
)( ), Jingqing CHEN(
), Jingqing CHEN( )(
)( )
)
Received:2023-08-17
Revised:2023-11-18
Published:2024-02-25
Online:2024-03-07
Contact:
QIU Yefeng (ORCID:0000-0002-5392-3293), E-mail: qiuyefeng2001@163.com;摘要:
目的 通过模拟急性缺氧环境,建立实验性高原小鼠肠道应激损伤模型,为探讨高原急性胃肠病的致病机制以及防治措施奠定基础。 方法 根据体重按随机数字表法,将36只SPF级成年雄性BALB/c小鼠分为常氧24 h组、常氧72 h组、低氧24 h组和低氧72 h组,每组9只。常氧对照组小鼠饲养于常规屏障环境中;低氧应激组饲养于屏障环境中的低氧舱内,氧气浓度设定为10%以模拟高原环境,分别应激24 h和72 h,建立急性缺氧所致肠道损伤模型。造模结束后,称量小鼠体重,用1%戊巴比妥钠麻醉后断颈处死各组小鼠,采集十二指肠和结肠组织并进行HE染色后观察肠道组织病理形态,通过蛋白质印迹和免疫组织化学法检测肠道组织中紧密连接相关蛋白表达水平,应用实时荧光定量PCR检测炎性细胞因子和趋化因子的mRNA表达水平,采用TUNEL染色法检测肠上皮细胞凋亡活性等指标,从而对该模型的肠道损伤相关表型进行评价。 结果 与常氧组相比,低氧24 h组和低氧72 h组的小鼠表现为体重减轻,十二指肠绒毛长度变短、隐窝结构异常、绒毛/隐窝比下降,结肠黏膜炎性细胞浸润、隐窝结构不规则。低氧24 h组和低氧72 h组小鼠的十二指肠和结肠组织中闭锁蛋白(Occludin)及闭锁小带蛋白(zonula occludens-1,ZO-1)表达水平明显下降(P<0.05),十二指肠组织中促凋亡蛋白Bax表达明显上调,抑凋亡蛋白Bcl-2表达明显下调(P<0.05),而且肠上皮细胞的凋亡活性显著增强(P<0.05)。此外,缺氧应激24 h和72 h后,小鼠十二指肠组织中白细胞介素(interlenkin,IL)-1β、IL-6、单核细胞趋化蛋白-1(monocyte chemoattractant protein-1,MCP-1)及肿瘤坏死因子-α(tumour necrosis factor-α,TNF-α)mRNA水平显著增高(P<0.05);缺氧应激24 h后,小鼠结肠组织中炎性细胞因子的表达水平无显著变化(P>0.05);但缺氧应激72 h后,小鼠结肠组织中促炎因子IL-1β、TNF-α、IL-6、MCP-1以及抗炎因子IL-10 mRNA水平显著增高(P<0.05)。 结论 利用低氧舱模拟高原急性缺氧环境可导致应激小鼠肠道组织结构异常、肠屏障功能障碍,并诱导肠上皮细胞凋亡,引发肠道炎性反应。这些结果表明急性缺氧应激肠道损伤小鼠模型构建成功。
中图分类号:
郑建华, 法云智, 董巧燕, 邱业峰, 陈菁青. 高原急性缺氧肠道应激损伤小鼠模型的构建与评价[J]. 实验动物与比较医学, 2024, 44(1): 31-41.
Jianhua ZHENG, Yunzhi FA, Qiaoyan DONG, Yefeng QIU, Jingqing CHEN. Construction and Evaluation of a Mouse Model with Intestinal Injury by Acute Hypoxic Stress in Plateau[J]. Laboratory Animal and Comparative Medicine, 2024, 44(1): 31-41.
基因 Gene | 正向引物 Forward primers(5ʹ→3ʹ) | 反向引物 Reverse primers(5ʹ→3ʹ) |
|---|---|---|
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
| IL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| TNF-α | CCTGTAGCCCACGTCGTAG | GGGAGTAGACAAGGTACAACCC |
| IL-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| MCP-1 | TAAAAACCTGGATCGGAACCAAA | GCATTAGCTTCAGATTTACGGGT |
| IL-10 | AGCCTTATCGGAAATGATCCAGT | GGCCTTGTAGACACCTTGGT |
表1 实时荧光定量PCR引物序列
Table 1 Sequence list of primers used in real-time fluorescent quantitative PCR
基因 Gene | 正向引物 Forward primers(5ʹ→3ʹ) | 反向引物 Reverse primers(5ʹ→3ʹ) |
|---|---|---|
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
| IL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| TNF-α | CCTGTAGCCCACGTCGTAG | GGGAGTAGACAAGGTACAACCC |
| IL-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| MCP-1 | TAAAAACCTGGATCGGAACCAAA | GCATTAGCTTCAGATTTACGGGT |
| IL-10 | AGCCTTATCGGAAATGATCCAGT | GGCCTTGTAGACACCTTGGT |
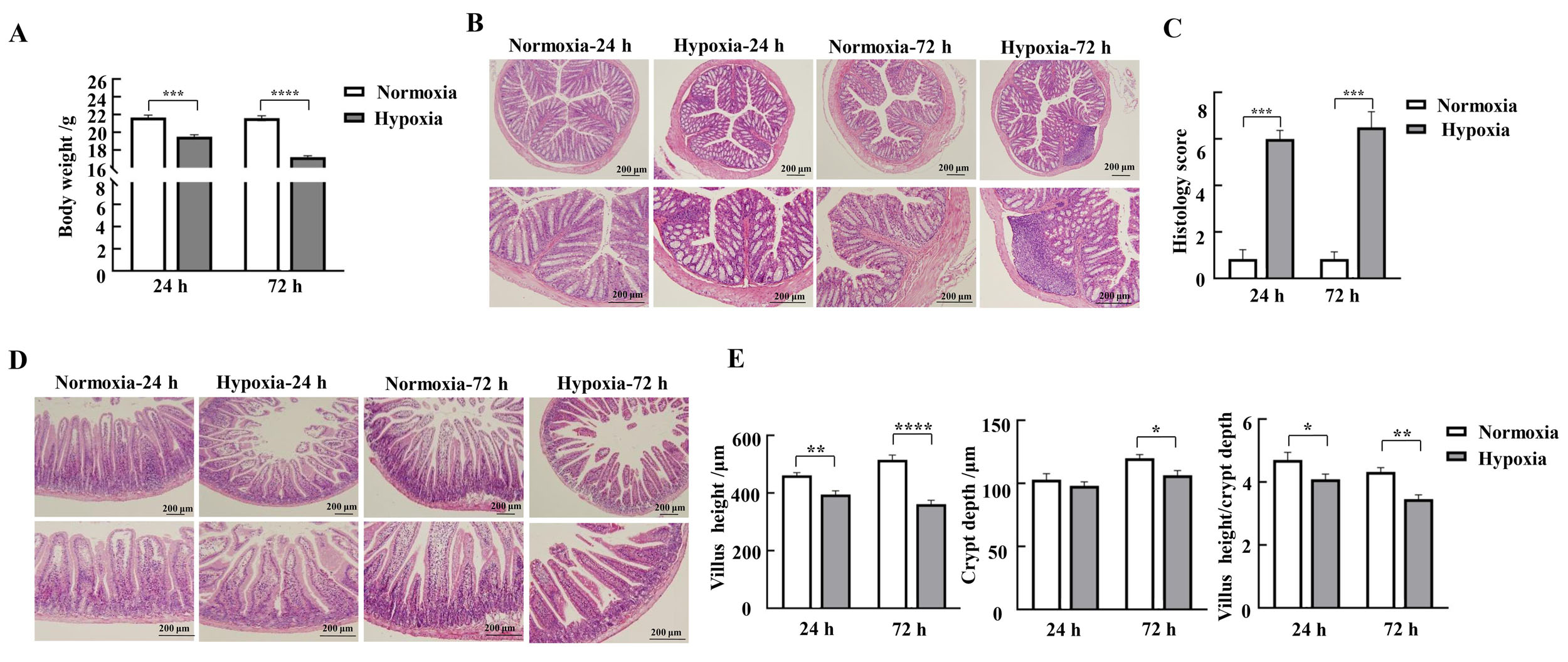
图1 缺氧应激对小鼠体重变化、结肠和十二指肠黏膜形态的影响注:A,各组小鼠体重变化(n=9);B~C,小鼠结肠HE染色结果及病理组织学评分(每组3只小鼠样本);D,小鼠十二指肠HE染色结果;E,小鼠十二指肠绒毛长度、隐窝深度和绒毛长度/隐窝深度比值(每组3只小鼠样本)。Normoxia为常氧对照组,Hypoxia为低氧应激组。数据表示为平均值±标准误;与对照组比较,*P<0.05,**P<0.01,***P<0.001,****P<0.000 1。
Figure 1 Effects of hypoxic stress on body weight changes and morphology of the mucosa of the colon and duodenum in miceNote:A, Changes in body weight of mice in each group (n=9); B-C, HE staining results and pathohistologic scores of mice colon (n=3); D, HE staining results of mice duodenum; E-G, Lengths of mice duodenal villi, depths of crypts, and values of villus length/crypt depth (n=3). Normoxia indicates normoxic control group, and Hypoxia indicates hypoxic stress group. Data are expressed as mean ± standard error; compared with normoxic control group, *P<0.05, **P<0.01, ***P<0.001, ****P<0.000 1.
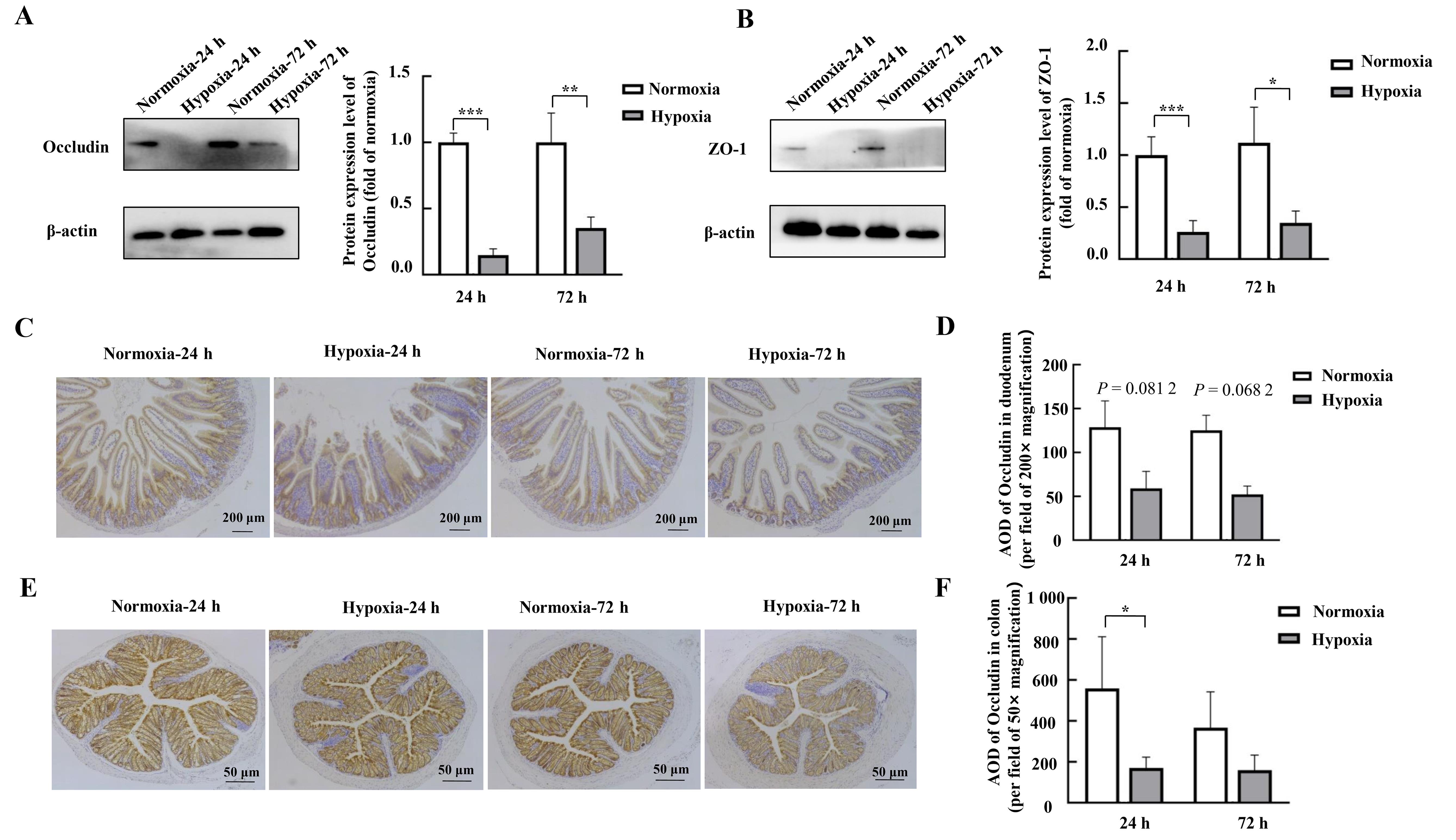
图2 蛋白质印迹和免疫组织化学法检测缺氧应激对小鼠十二指肠和结肠组织中紧密连接蛋白表达的影响注:A~B,蛋白质印迹法检测小鼠十二指肠组织中紧密连接蛋白Occludin、ZO-1的表达及其相对表达量分析(每组4只小鼠样本);C~D,免疫组织化学法检测小鼠十二指肠组织中紧密连接蛋白Occludin及其平均光密度值(AOD)(每组3只小鼠样本);E~F,免疫组织化学法检测小鼠结肠组织中紧密连接蛋白Occludin及其平均光密度值(AOD)(每组3只小鼠样本)。Normoxia为常氧对照组,Hypoxia为缺氧应激组。数据表示为平均值±标准误;与对照组比较,*P<0.05,**P<0.01,***P<0.001。
Figure 2 Effects of hypoxic stress on the expression of the tight junction protein in mouse duodenal and colonic tissues as detected by Western blotting and immunohistochemistryNote:A-B, Expression of tight junction proteins Occludin and ZO-1 in mouse duodenal tissues detected by Western blotting and their relative expression level analysis (n=4). C-D, Detection of the tight junction protein Occludin and its average optical densitye (AOD) in mouse duodenal tissues by immunohistochemistry (n=3). E-F, Detection of tight junction protein Occludin and its AOD in mouse colon tissues by immunohistochemistry (n=3). Normoxia indicates control normoxic group, and Hypoxia indicates hypoxic stress group. Data are expressed as mean ± standard error; compared with normoxic control group, *P<0.05, **P<0.01, ***P<0.001.
图3 实时荧光定量PCR检测缺氧应激对小鼠十二指肠和结肠组织中炎性细胞因子表达的影响注:A~B,小鼠十二指肠组织;C~D,小鼠结肠组织。Normoxia为常氧对照组,Hypoxia为缺氧应激组。数据表示为平均值±标准误,每组6只小鼠样本;与对照组比较,*P<0.05,**P<0.01,***P<0.001。
Figure 3 Effects of hypoxic stress on the expression of inflammatory cytokines in mouse duodenal and colonic tissues detected by real-time fluorescence quantitative PCRNote:A-B, Mouse duodenal tissues; C-D, Mouse colon tissues. Normoxia indicates normoxic control group, and Hypoxia indicates hypoxic stress group. Data are expressed as mean ± standard error; n = 6, compared with normoxic control group, *P<0.05, **P<0.01, ***P<0.001.
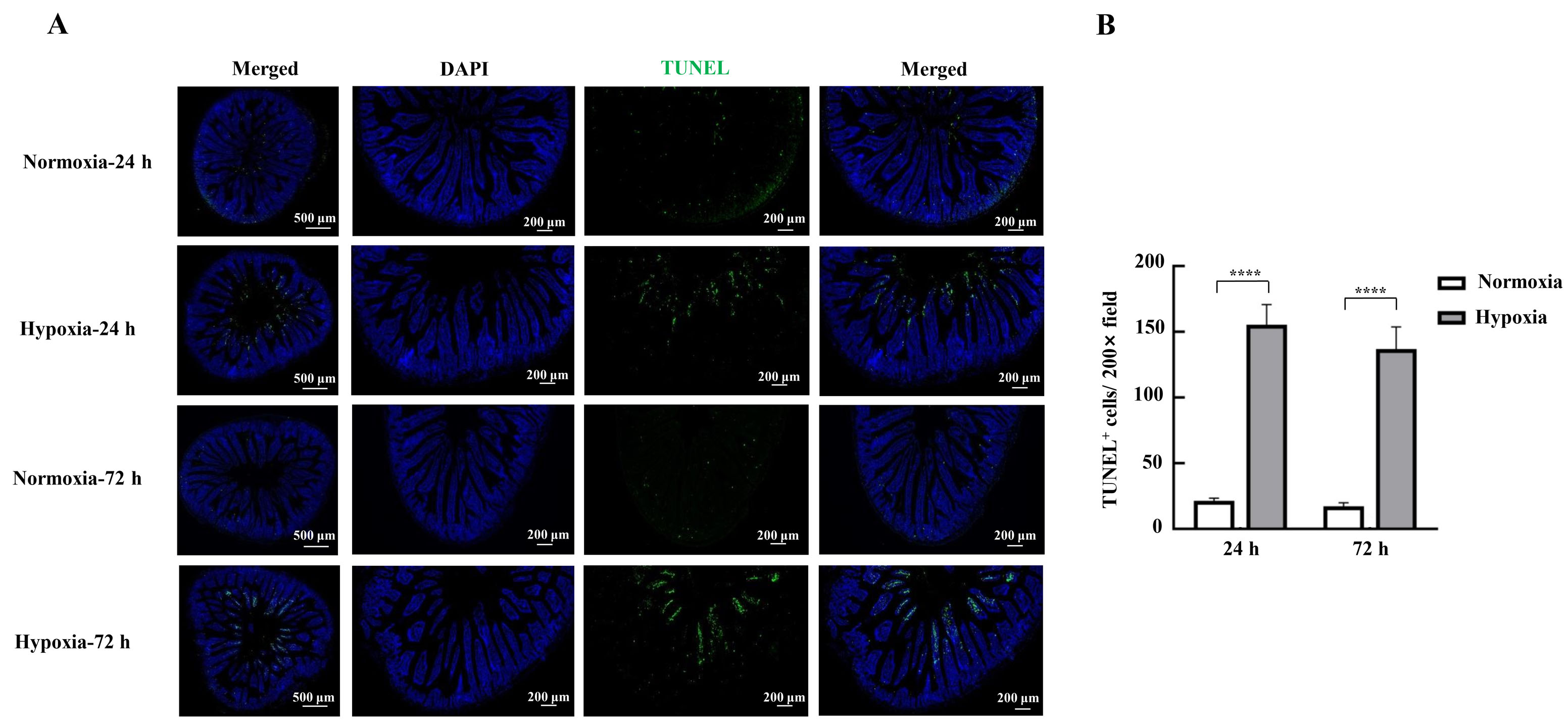
图4 TUNEL染色法检测缺氧应激对小鼠肠上皮细胞凋亡的影响注:A,小鼠十二指肠组织的TUNEL染色照片;B,TUNEL阳性细胞计数。Normoxia为常氧对照组,Hypoxia为缺氧应激组。数据表示为平均值±标准误;每组3只小鼠样本;与常氧对照组比较,****P<0.000 1。
Figure 4 Effects of hypoxic stress on apoptosis of mice intestinal epithelial cells detected by TUNEL staining methodNote:A, Photographs of TUNEL staining of mouse duodenal tissues; B, TUNEL-positive cell count. Normoxia indicates normoxic control group, and Hypoxia indicates hypoxic stress group. Data are expressed as mean ± standard error; n=3, compared with normoxic control group, ****P<0.000 1.
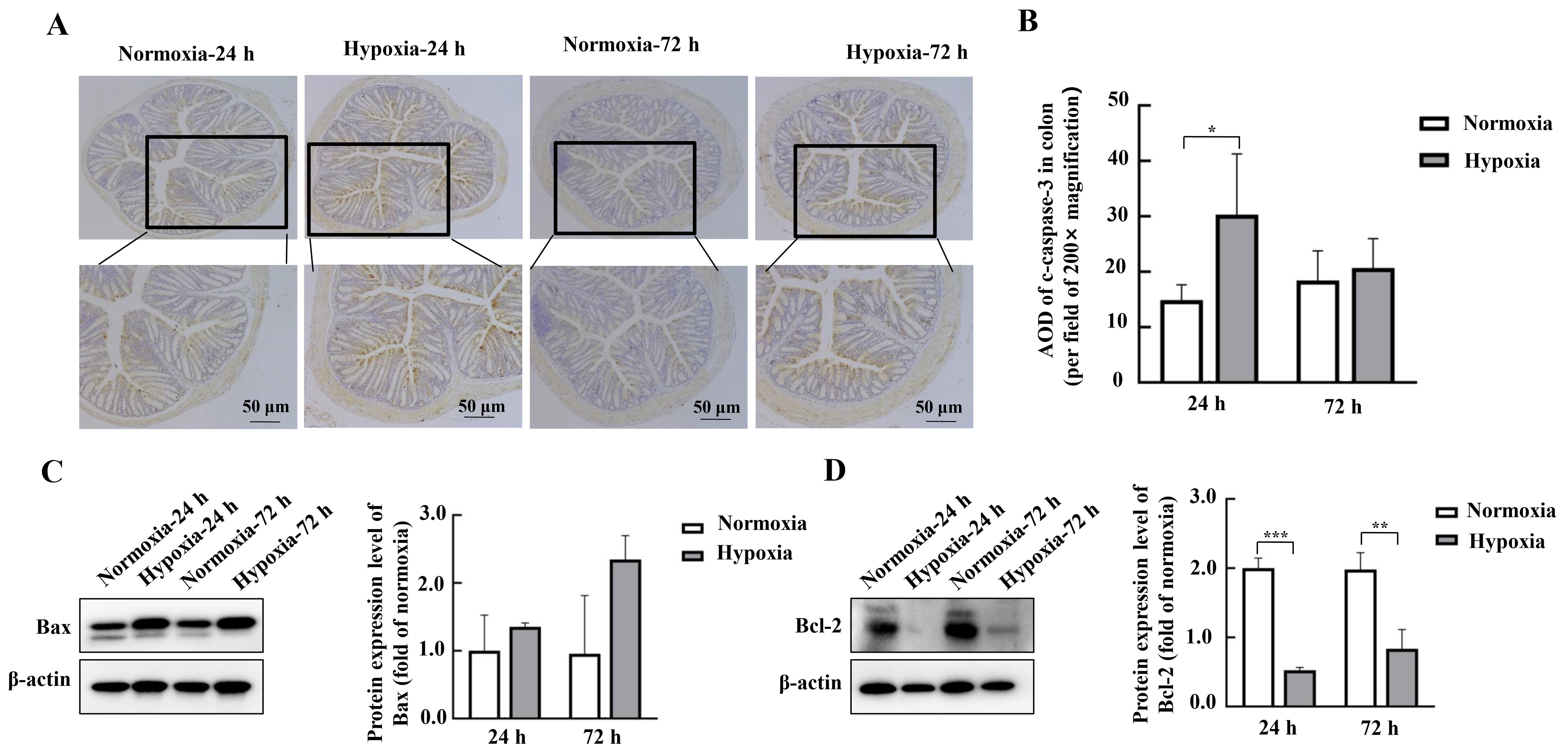
图5 蛋白质印迹和免疫组织化学法检测缺氧应激对小鼠肠道中凋亡相关蛋白表达的影响注:A~B,免疫组织化学法检测小鼠结肠组织中凋亡相关蛋白c-caspase-3及其平均光密度值(AOD)(每组3只小鼠样本);C~D,蛋白质印迹法检测小鼠十二指肠组织中凋亡相关蛋白Bax、Bcl-2的表达及其相对表达量分析(每组6只小鼠样本)。Normoxia为常氧对照组,Hypoxia为缺氧应激组。数据表示为平均值±标准误;与常氧对照组比较,*P<0.05,**P<0.01,***P<0.001。
Figure 5 Effects of hypoxic stress on the expression of intestinal apoptosis-related proteins in mouse colon detected by Western blotting and immunohistochemistryNote:A-B, Detection of apoptosis-related protein c-caspase-3 and its average optical density (AOD) in mouse colon tissues by immunohistochemistry (n=3). C-D, Expression of apoptosis-related proteins Bax and Bcl-2 and their relative expression analysis in mouse duodenal tissues detected by Western blotting method (n=6). Normoxia indicates normoxic control group, and Hypoxia indicates hypoxic stress group. Data are expressed as mean ± standard error; n=6, compared with normoxic control group, *P<0.05, **P<0.01, ***P<0.001.
| 1 | WANG F, ZHANG H, XU T, et al. Acute exposure to simulated high-altitude hypoxia alters gut microbiota in mice[J]. Arch Microbiol, 2022, 204(7):412. DOI: 10.1007/s00203-022-03031-4 . |
| 2 | NUSS R. Medical conditions and high-altitude travel[J]. N Engl J Med, 2022, 386(19):1866-1867. DOI: 10.1056/NEJMc 2203182 . |
| 3 | 王珍, 周亚洲, 王立坤, 等. 持续低氧条件下大鼠肠道微生物变化及其与心肌损伤的关联研究[J]. 微生物学报, 2023, 63(8):3054-3067. DOI: 10.13343/j.cnki.wsxb.20220862 . |
| WANG Z, ZHOU Y Z, WANG L K, et al. Alterations in gut microbiota and their associations with cardiac injury in rats after exposure to continuous normobaric hypoxia[J]. Acta Microbiol Sin, 2023, 63(8):3054-3067. DOI: 10.13343/j.cnki.wsxb.20220862 . | |
| 4 | KHANNA K, MISHRA K P, GANJU L, et al. High-Altitude-Induced alterations in Gut-Immune Axis: a review[J]. Int Rev Immunol, 2018, 37(2):119-126. DOI: 10.1080/08830185. 2017. 1407763 . |
| 5 | FRUEHAUF H, VAVRICKA S R, LUTZ T A, et al. Evaluation of acute mountain sickness by unsedated transnasal esophagogastroduodenoscopy at high altitude[J]. Clin Gastroenterol Hepatol, 2020, 18(10):2218-2225.e2. DOI: 10.1016/j.cgh.2019.11.036 . |
| 6 | HAMAD N, TRAVIS S P L. Weight loss at high altitude: pathophysiology and practical implications[J]. Eur J Gastroenterol Hepatol, 2006, 18(1):5-10. DOI: 10.1097/00042737-200601000-00002 . |
| 7 | LUO H, ZHOU D J, CHEN Z, et al. Establishment and evaluation of an experimental rat model for high-altitude intestinal barrier injury[J]. Exp Ther Med, 2017, 13(2):475-482. DOI: 10.3892/etm.2016.4012 . |
| 8 | MEIER D, COLLET T H, LOCATELLI I, et al. Does this patient have acute mountain sickness?[J]. JAMA, 2017, 318(18):1810. DOI: 10.1001/jama.2017.16192 . |
| 9 | MCKENNA Z J, GORINI PEREIRA F, GILLUM T L, et al. High-altitude exposures and intestinal barrier dysfunction[J]. Am J Physiol Regul Integr Comp Physiol, 2022, 322(3): R192-R203. DOI: 10.1152/ajpregu.00270.2021 . |
| 10 | WU T Y, DING S Q, LIU J L, et al. High-altitude gastrointestinal bleeding: an observation in Qinghai-Tibetan railroad construction workers on Mountain Tanggula[J]. World J Gastroenterol, 2007, 13(5):774-780. DOI: 10.3748/wjg.v13.i5.774 . |
| 11 | BISCHOFF S C, BARBARA G, BUURMAN W, et al. Intestinal permeability: a new target for disease prevention and therapy[J]. BMC Gastroenterol, 2014, 14:189. DOI: 10.1186/s12876-014-0189-7 . |
| 12 | KÖNIG J, WELLS J, CANI P D, et al. Human intestinal barrier function in health and disease[J]. Clin Transl Gastroenterol, 2016, 7(10): e196. DOI: 10.1038/ctg.2016.54 . |
| 13 | LOSHBAUGH J E, LOEPPKY J A, GREENE E R. Effects of acute hypobaric hypoxia on resting and postprandial superior mesenteric artery blood flow[J]. High Alt Med Biol, 2006, 7(1):47-53. DOI: 10.1089/ham.2006.7.47 . |
| 14 | MUSCH M W, CLARKE L L, MAMAH D, et al. T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+/K+-ATPase[J]. J Clin Invest, 2002, 110(11):1739-1747. DOI: 10.1172/JCI15695 . |
| 15 | DINMORE A J, EDWARDS J S, MENZIES I S, et al. Intestinal carbohydrate absorption and permeability at high altitude (5, 730 m)[J]. J Appl Physiol, 1994, 76(5):1903-1907. DOI: 10.1152/jappl.1994.76.5.1903 . |
| 16 | LI Y Y, WANG Y C, SHI F, et al. Phospholipid metabolites of the gut microbiota promote hypoxia-induced intestinal injury via CD1d-dependent γδ T cells[J]. Gut Microbes, 2022, 14(1):2096994. DOI: 10.1080/19490976.2022.2096994 . |
| 17 | BASNYAT B, MURDOCH D R. High-altitude illness[J]. Lancet, 2003, 361(9373):1967-1974. DOI: 10.1016/S0140-6736(03)13591-X . |
| 18 | KARL J P, BERRYMAN C E, YOUNG A J, et al. Associations between the gut microbiota and host responses to high altitude[J]. Am J Physiol Gastrointest Liver Physiol, 2018, 315(6): G1003-G1015. DOI: 10.1152/ajpgi.00253.2018 . |
| 19 | FAN X X, LI S, WU Z L, et al. Glycine supplementation to breast-fed piglets attenuates post-weaning jejunal epithelial apoptosis: a functional role of CHOP signaling[J]. Amino Acids, 2019, 51(3):463-473. DOI: 10.1007/s00726-018-2681-9 . |
| 20 | PATANKAR J V, BUBECK M, ACERA M G, et al. Breaking bad: necroptosis in the pathogenesis of gastrointestinal diseases[J]. Front Immunol, 2023, 14:1203903. DOI: 10.3389/fimmu.2023.1203903 . |
| 21 | ZHANG W, JIAO L F, LIU R X, et al. The effect of exposure to high altitude and low oxygen on intestinal microbial communities in mice[J]. PLoS One, 2018, 13(9): e0203701. DOI: 10.1371/journal.pone.0203701 . |
| 22 | HUANG X, ZHOU Y Z, ZHAO T, et al. A method for establishing the high-altitude cerebral edema (HACE) model by acute hypobaric hypoxia in adult mice[J]. J Neurosci Methods, 2015, 245:178-181. DOI: 10.1016/j.jneumeth. 2015.02.004 . |
| 23 | ZHOU Y Z, HUANG X, ZHAO T, et al. Hypoxia augments LPS-induced inflammation and triggers high altitude cerebral edema in mice[J]. Brain Behav Immun, 2017, 64:266-275. DOI: 10.1016/j.bbi.2017.04.013 . |
| 24 | HILL G W, GILLUM T L, LEE B J, et al. Prolonged treadmill running in normobaric hypoxia causes gastrointestinal barrier permeability and elevates circulating levels of pro- and anti-inflammatory cytokines[J]. Appl Physiol Nutr Metab, 2020, 45(4):376-386. DOI: 10.1139/apnm-2019-0378 . |
| 25 | LI M, HAN T Y, ZHANG W J, et al. Simulated altitude exercise training damages small intestinal mucosa barrier in the rats[J]. J Exerc Rehabil, 2018, 14(3):341-348. DOI: 10.12965/jer. 1835128.064 . |
| 26 | SHIH D Q, TARGAN S R. Insights into IBD pathogenesis[J]. Curr Gastroenterol Rep, 2009, 11(6):473-480. DOI: 10.1007/s11894-009-0072-9 . |
| 27 | LIU L, LIANG L P, YANG C H, et al. Extracellular vesicles of Fusobacterium nucleatum compromise intestinal barrier through targeting RIPK1-mediated cell death pathway[J]. Gut Microbes, 2021, 13(1):1-20. DOI: 10.1080/19490976. 2021. 1902718 . |
| 28 | PATANKAR J V, BECKER C. Cell death in the gut epithelium and implications for chronic inflammation[J]. Nat Rev Gastroenterol Hepatol, 2020, 17(9):543-556. DOI: 10.1038/s41575-020-0326-4 . |
| 29 | LIU Y L, XU Q, WANG Y, et al. Necroptosis is active and contributes to intestinal injury in a piglet model with lipopolysaccharide challenge[J]. Cell Death Dis, 2021, 12(1):62. DOI: 10.1038/s41419-020-03365-1 . |
| 30 | STRÄTER J, WELLISCH I, RIEDL S, et al. CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a possible role in ulcerative colitis[J]. Gastroenterology, 1997, 113(1):160-167. DOI: 10.1016/s0016-5085(97)70091-x . |
| 31 | OKUMURA R, TAKEDA K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis[J]. Exp Mol Med, 2017, 49(5): e338. DOI: 10.1038/emm.2017.20 . |
| 32 | CHENG E H, KIRSCH D G, CLEM R J, et al. Conversion of bcl-2 to a bax-like death effector by caspases[J]. Science, 1997, 278(5345):1966-1968. DOI: 10.1126/science.278.5345.1966 . |
| 33 | MCKENNA Z J, BELLOVARY B N, DUCHARME J B, et al. Circulating markers of intestinal barrier injury and inflammation following exertion in hypobaric hypoxia[J]. Eur J Sport Sci, 2023, 23(10):2002-2010. DOI: 10.1080/17461391. 2023. 2203107 . |
| 34 | MCKENNA Z J, FENNEL Z J, BERKEMEIER Q N, et al. Exercise in hypobaric hypoxia increases markers of intestinal injury and symptoms of gastrointestinal distress[J]. Exp Physiol, 2022, 107(4):326-336. DOI: 10.1113/EP090266 . |
| [1] | 梁敏, 郭洋, 王津津, 朱梦妍, 池骏, 陈艳娟, 王成稷, 喻智澜, 沈如凌. Dmd基因突变小鼠构建及在肌肉及免疫系统的表型验证[J]. 实验动物与比较医学, 2024, 44(1): 42-51. |
| [2] | 唐倩倩, 张秀莉, 常在. 清华大学实验动物中心小鼠自动饮水系统漏水情况统计分析[J]. 实验动物与比较医学, 2024, 44(1): 85-91. |
| [3] | 刘欣, 石少波, 张翠, 杨波, 曲川. 小鼠自体动静脉内瘘端侧吻合模型的建立与评价[J]. 实验动物与比较医学, 2023, 43(6): 595-603. |
| [4] | 王丹, 张晓璐, 王妍, 傅博, 王文栋, 刘京, 张甦寅, 武怡荷, 吴德国, 杜小燕, 战大伟, 章秀林, 李长龙. Big-BALB/c小鼠亚系选育前后的抗体制备效率比较研究[J]. 实验动物与比较医学, 2023, 43(6): 612-618. |
| [5] | 聂永强, 王朝霞. 濒危基因编辑小鼠品系拯救技术及其应用探讨[J]. 实验动物与比较医学, 2023, 43(6): 636-640. |
| [6] | 于灵芝, 谢建芸, 冯丽萍, 魏晓锋. 金黄色葡萄球菌荧光定量PCR检测方法的建立及其在大鼠、小鼠粪便检测中的应用[J]. 实验动物与比较医学, 2023, 43(5): 566-573. |
| [7] | 王成稷, 王珏, 王海杰, 陆炜晟, 史岩, 顾正页, 万鸣秋, 沈如凌. 乳胶血管灌注技术制作小鼠头面部静脉血管模型方法初探[J]. 实验动物与比较医学, 2023, 43(5): 574-578. |
| [8] | 黄缨, 韦思羽, 蔡莉, 强苏静, 李冬婷, 丁玉强. 供应商来源的实验大鼠和小鼠微生物监测结果分析:以复旦大学实验动物科学部为例[J]. 实验动物与比较医学, 2023, 43(4): 347-354. |
| [9] | 谈小倩, 杨颢, 唐慧青, 瞿伟, 李亮, 钱珍, 顾坚忠, 徐平, 肖君华. BALB/cA.Cg.SHJH hr 小鼠的培育及其相关遗传学特性分析[J]. 实验动物与比较医学, 2023, 43(4): 363-370. |
| [10] | 邓亚胜, 林江, 甘池伶, 曾官凤, 黄嘉茵, 邓慧芳, 麻颖贤, 韩丝银. 皮肤光老化动物模型制备要素和受试物数据的文献分析[J]. 实验动物与比较医学, 2023, 43(4): 406-414. |
| [11] | 王雪, 呼永河. 糖尿病小鼠模型的常见种类及其构建要素分析[J]. 实验动物与比较医学, 2023, 43(4): 415-421. |
| [12] | 黄慧, 邓亚胜, 梁天薇, 郑艺清, 范燕萍, 荣娜, 林江. 卵巢储备功能减退动物模型的造模方法评价与分析[J]. 实验动物与比较医学, 2023, 43(4): 422-428. |
| [13] | 翟珊珊, 梁亮, 曹颖颖, 李竹欣, 王青, 陶俊宇, 运晨霞, 冷静, 唐海波. 一例树鼩毛发上皮瘤的诊断及细胞生物学特性观察[J]. 实验动物与比较医学, 2023, 43(4): 440-445. |
| [14] | 潘志强, 农淄心, 谢海纳, 彭佩克. 顺铂对小鼠下丘脑-垂体-肾上腺/性腺轴功能的损伤作用及脱氢表雄酮的干预效应[J]. 实验动物与比较医学, 2023, 43(3): 229-242. |
| [15] | 郭文文, 赵亚, 王颖花, 刘可, 葛煦, 张延英, 汪永锋, 师长宏. 人参皂苷Rg1在小鼠创伤性脑损伤修复中的作用[J]. 实验动物与比较医学, 2023, 43(3): 243-252. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||