













实验动物与比较医学 ›› 2023, Vol. 43 ›› Issue (3): 229-242.DOI: 10.12300/j.issn.1674-5817.2022.182
• 实验动物与比较药理 • 下一篇
收稿日期:2022-11-23
修回日期:2023-02-14
出版日期:2023-06-25
发布日期:2023-06-25
作者简介:潘志强(1977—),男,博士,教授,博士生导师,研究方向:中医证候动物模型及其物质基础研究。E-mail:pzq527@163.com。ORCID:0000-0003-4143-2312
基金资助:
Zhiqiang PAN( )(
)( ), Zixin NONG, Haina XIE, Peike PENG
), Zixin NONG, Haina XIE, Peike PENG
Received:2022-11-23
Revised:2023-02-14
Published:2023-06-25
Online:2023-06-25
Contact:
PAN Zhiqiang (ORCID: 0000-0003-4143-2312), E-mail: pzq527@163.com摘要:
目的 研究顺铂(又名顺式-二氯二氨合铂,cis-dichlorodiamineplatinum,DDP)抑制小鼠类固醇激素合成的作用途径,并观察脱氢表雄酮(dehydroepiandrosterone,DHEA)的干预效应。 方法 将60只成年ICR小鼠随机分为3组:对照组、DDP建模组、DHEA治疗组,每组10只雄性和10只雌性小鼠。DDP建模组小鼠按2.5 mg·kg-1·d-1剂量腹腔注射DDP溶液,每3 d注射1次,共7次;造模同日,对照组小鼠腹腔注射等量的生理盐水;而DEHA治疗组小鼠在予以DDP处理的同时,按8.3 mg·kg-1·d-1剂量给予DHEA灌胃,连续给药21 d。通过旷场、抓力和转棒试验观察小鼠疲劳指标的变化,采用常规组织切片的HE染色法观察肾上腺、睾丸与卵巢组织形态的变化,运用高效液相色谱串联质谱方法检测小鼠血清类固醇激素的含量,运用实时荧光定量PCR和蛋白质印迹技术检测下丘脑、垂体、肾上腺、睾丸和卵巢组织中相关基因的mRNA和蛋白表达水平。 结果 与对照组比较,DDP建模组雄性与雌性小鼠的体重以及旷场运动能力均显著下降(P<0.05),同时雌性小鼠的抓力和转棒时间也均显著下降(P<0.05);而DHEA治疗组小鼠的上述疲劳指标均显著改善(P<0.05)。DDP建模组雄性小鼠睾丸组织中各级生精细胞排列紊乱且睾丸间质水肿,雌性小鼠卵巢组织中大量原始卵泡被激活,闭锁卵泡增多,卵泡颗粒细胞减少;而DHEA治疗组小鼠睾丸和卵巢受损伤表型均得到显著改善。与对照组比较,DDP建模组雄性与雌性小鼠血清睾酮、二氢睾酮含量均显著降低(P<0.01),雄性小鼠血清孕烯醇酮含量下降而皮质酮含量显著升高(P<0.05),雌性小鼠血清皮质酮含量显著降低(P<0.05);与DDP建模组比较,DHEA治疗组雄性小鼠的孕烯醇酮含量和雌性小鼠的孕酮含量均显著增高(P<0.05),而雌性小鼠的孕烯醇酮含量和雄性小鼠的孕酮含量均显著降低(P<0.05)。与对照组比较,DDP建模组雄性与雌性小鼠的肾上腺Cyp21a1、Cyp11a1基因和下丘脑Gnrh基因表达均显著下调(P<0.05);雌性小鼠肾上腺Hsd3b2基因,卵巢Star、Cyp11a1、Lhr基因,下丘脑Crh、垂体Pomc和Lhb基因表达均显著下调(P<0.05);雄性小鼠睾丸Star基因及StAR蛋白以及垂体Fshb和Lhb基因表达均显著下调(P<0.05)。与DDP建模组比较,补充DHEA后,雄性小鼠肾上腺Cyp17a1基因和睾丸Cyp17a1、Lhr、Fshr基因表达均显著下调(P<0.05);雌性小鼠肾上腺Cyp11a1基因表达显著下调(P<0.05),而肾上腺Hsd3b2基因和卵巢Star、Cyp11a1、Hsd3b2、Lhr基因,以及垂体Lhb基因表达均上调(P<0.05)。 结论 DDP间断给药可以抑制下丘脑-垂体-肾上腺皮质与性腺轴功能,并且雌性受抑制更显著;补充DHEA有助于调节体内类固醇激素水平的自身稳态。
中图分类号:
潘志强,农淄心,谢海纳,等. 顺铂对小鼠下丘脑-垂体-肾上腺/性腺轴功能的损伤作用及脱氢表雄酮的干预效应[J]. 实验动物与比较医学, 2023, 43(3): 229-242. DOI: 10.12300/j.issn.1674-5817.2022.182.
Zhiqiang PAN,Zixin NONG,Haina XIE,et al. Injurious Effect of Cisplatin on the Function of Hypothalamus-pituitary-adrenal/gonadal Axis in Mice and the Intervention Effect of Dehydroepiandrosterone[J]. Laboratory Animal and Comparative Medicine, 2023, 43(3): 229-242. DOI: 10.12300/j.issn.1674-5817.2022.182.
基因名称 Gene name | 引物序列 Primer sequence | 产物大小 Length/bp |
|---|---|---|
| β-actin | F:5'-TGTTACCAACTGGGACGACA-3'; R:5'-GGGGTGTTGAAGGTCTCAAA-3' | 165 |
| Star | F:5'-TTGGGCATACTCAACAACCA-3'; R:5'-GAAACACCTTGCCCACATCT-3' | 103 |
| Cyp11a1 | F:5'-ACTTCCGGTACTTGGGCTTT-3'; R:5'-GCTTGAGAGGCTGGAAGTTG-3' | 201 |
| Cyp11b1 | F:5'-GTATCGAGAGCTGGCAGAGG-3'; R:5'-GGGTTGATGTCGTGTCAGTG-3' | 140 |
| Cyp21a1 | F:5'-CTCCGGCTATGACATCCCTA-3'; R:5'-ACAGCCAAAGGATGGTGTTC-3' | 151 |
| Cyp17a1 | F:5'-TGGTCATATGCATGCCAACT-3'; R:5'-CCCTTCTTCACGAGCACTTC-3' | 132 |
| Hsd3b2 | F:5'-TCCAGCTCAGTTGATGTTGC-3'; R:5'-TGCCTTCTCAGCCATCTTTT-3' | 129 |
| Cyp19a1 | F:5'-CTTTCAGCCTTTTGGCTTTG-3'; R:5'-ATTTCCACAAGGTGCCTGTC-3' | 192 |
| Hsd17b1 | F:5'-GTTATGAGCAAGCCCTGAGC-3'; R:5'-AAGCGGTTCGTGGAGAAGTA-3' | 112 |
| Ar | F:5'-AAGCAGGTAGCTCTGGGACA-3'; R:5'-GAGCCAGCGGAAAGTTGTAG-3' | 117 |
| Fshr | F:5'-ATCACACATGCCATGCAACT-3'; R:5'-GTACGAGGAGGGCCATAACA-3' | 199 |
| Lhr | F:5'-CAATGGGACGACGCTAATCT-3'; R:5'-CTGGAGGGCAGAGTTTTCAG-3' | 204 |
| Esr1 | F:5'-TTACGAAGTGGGCATGATGA-3'; R:5'-CCTGAAGCACCCATTTCATT-3' | 117 |
| Esr2 | F:5'-GAAGCTGGCTGACAAGGAAC-3'; R:5'–AACGAGGTCTGGAGCAAAGA-3' | 187 |
| Gper1 | F:5'-GTCAACCTTGCAGCCTTCTC-3'; R:5'-AGCACTGCTGAACCTGACCT-3' | 198 |
| Pomc | F:5'-CATTAGGCTTGGAGCAGGTC-3'; R:5'-CTTCTCGGAGGTCATGAAGC-3' | 128 |
| Fshb | F:5'-TCAGCTTTCCCCAGAAGAGA-3'; R:5'-CCGAGCTGGGTCCTTATACA-3' | 249 |
| Lhb | F:5'-CTCAGCCAGTGTGCACCTAC-3'; R:5'-GACCCCCACAGTCAGAGCTA-3' | 150 |
| GnRH | F:5'-AGCACTGGTCCTATGGGTTG-3'; R:5'-GGGCCAGTGCATCTACATCT-3' | 221 |
| Crh | F:5'-TCTCACCTTCCACCTTCTGC-3'; R:5'-AAGCGCAACATTTCATTTCC-3' | 118 |
表1 实时荧光定量PCR引物序列
Table 1 Primer sequences in real-time fluorescent quantitative PCR
基因名称 Gene name | 引物序列 Primer sequence | 产物大小 Length/bp |
|---|---|---|
| β-actin | F:5'-TGTTACCAACTGGGACGACA-3'; R:5'-GGGGTGTTGAAGGTCTCAAA-3' | 165 |
| Star | F:5'-TTGGGCATACTCAACAACCA-3'; R:5'-GAAACACCTTGCCCACATCT-3' | 103 |
| Cyp11a1 | F:5'-ACTTCCGGTACTTGGGCTTT-3'; R:5'-GCTTGAGAGGCTGGAAGTTG-3' | 201 |
| Cyp11b1 | F:5'-GTATCGAGAGCTGGCAGAGG-3'; R:5'-GGGTTGATGTCGTGTCAGTG-3' | 140 |
| Cyp21a1 | F:5'-CTCCGGCTATGACATCCCTA-3'; R:5'-ACAGCCAAAGGATGGTGTTC-3' | 151 |
| Cyp17a1 | F:5'-TGGTCATATGCATGCCAACT-3'; R:5'-CCCTTCTTCACGAGCACTTC-3' | 132 |
| Hsd3b2 | F:5'-TCCAGCTCAGTTGATGTTGC-3'; R:5'-TGCCTTCTCAGCCATCTTTT-3' | 129 |
| Cyp19a1 | F:5'-CTTTCAGCCTTTTGGCTTTG-3'; R:5'-ATTTCCACAAGGTGCCTGTC-3' | 192 |
| Hsd17b1 | F:5'-GTTATGAGCAAGCCCTGAGC-3'; R:5'-AAGCGGTTCGTGGAGAAGTA-3' | 112 |
| Ar | F:5'-AAGCAGGTAGCTCTGGGACA-3'; R:5'-GAGCCAGCGGAAAGTTGTAG-3' | 117 |
| Fshr | F:5'-ATCACACATGCCATGCAACT-3'; R:5'-GTACGAGGAGGGCCATAACA-3' | 199 |
| Lhr | F:5'-CAATGGGACGACGCTAATCT-3'; R:5'-CTGGAGGGCAGAGTTTTCAG-3' | 204 |
| Esr1 | F:5'-TTACGAAGTGGGCATGATGA-3'; R:5'-CCTGAAGCACCCATTTCATT-3' | 117 |
| Esr2 | F:5'-GAAGCTGGCTGACAAGGAAC-3'; R:5'–AACGAGGTCTGGAGCAAAGA-3' | 187 |
| Gper1 | F:5'-GTCAACCTTGCAGCCTTCTC-3'; R:5'-AGCACTGCTGAACCTGACCT-3' | 198 |
| Pomc | F:5'-CATTAGGCTTGGAGCAGGTC-3'; R:5'-CTTCTCGGAGGTCATGAAGC-3' | 128 |
| Fshb | F:5'-TCAGCTTTCCCCAGAAGAGA-3'; R:5'-CCGAGCTGGGTCCTTATACA-3' | 249 |
| Lhb | F:5'-CTCAGCCAGTGTGCACCTAC-3'; R:5'-GACCCCCACAGTCAGAGCTA-3' | 150 |
| GnRH | F:5'-AGCACTGGTCCTATGGGTTG-3'; R:5'-GGGCCAGTGCATCTACATCT-3' | 221 |
| Crh | F:5'-TCTCACCTTCCACCTTCTGC-3'; R:5'-AAGCGCAACATTTCATTTCC-3' | 118 |
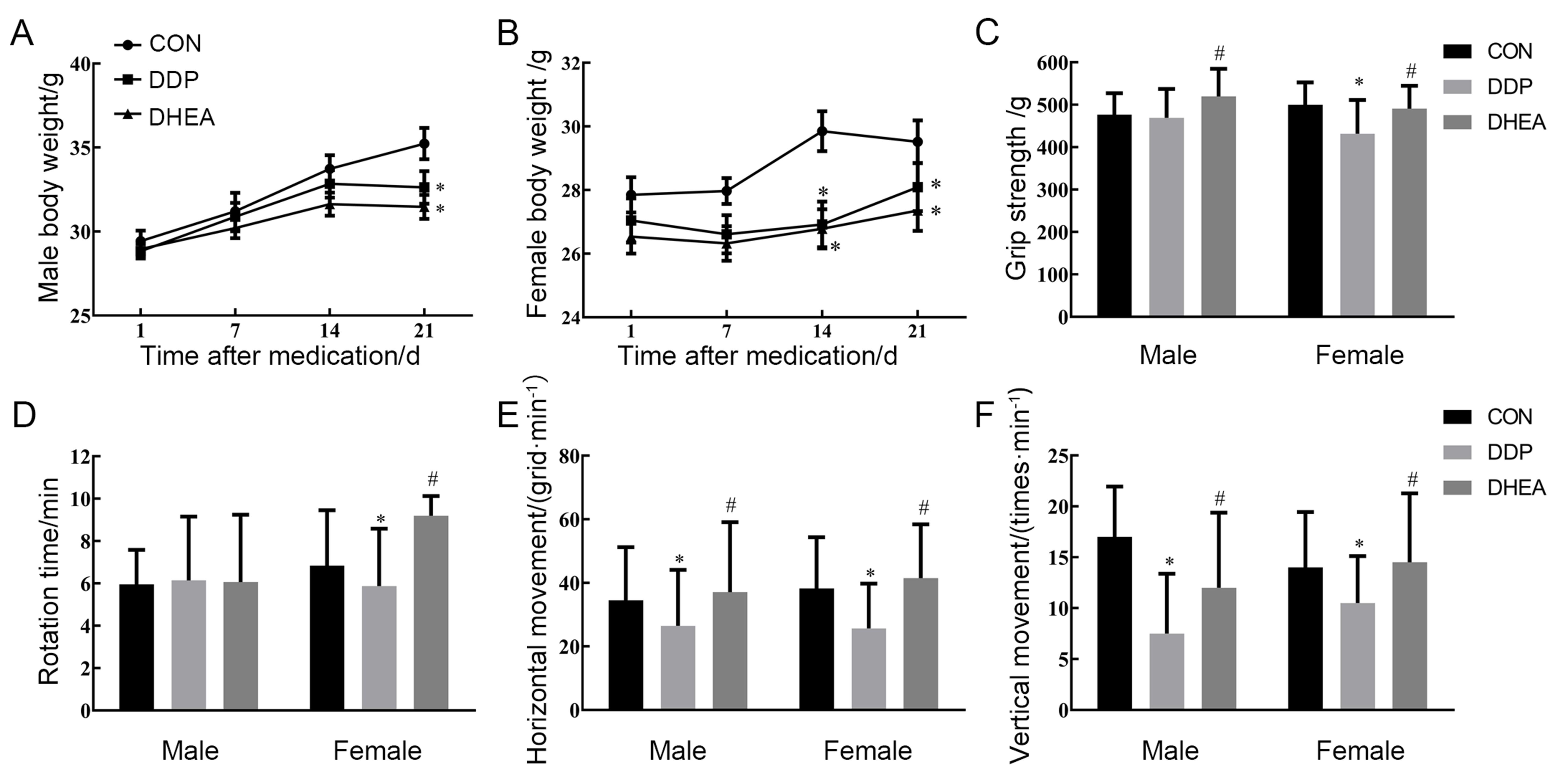
图1 DDP和DHEA处理后小鼠体重和疲劳指标的变化注:CON为对照组,DDP为DDP建模组,DHEA为脱氢表雄酮治疗组。与正常组比较,?P<0.05;与DDP建模组比较,#P<0.05。
Figure 1 Changes of body weight and fatigue indexes in mice after DDP and DHEA treatmentNote:CON indicates control group, DDP indicates cis-dichlorodiamineplatinum (cisplatin) modeling group, DHEA indicates dehydroepian-drosterone (DHEA) treatment after cisplatin modeling group. Compared with the control group, ?P<0.05; Compared with the cisplatin modeling group, #P<0.05.
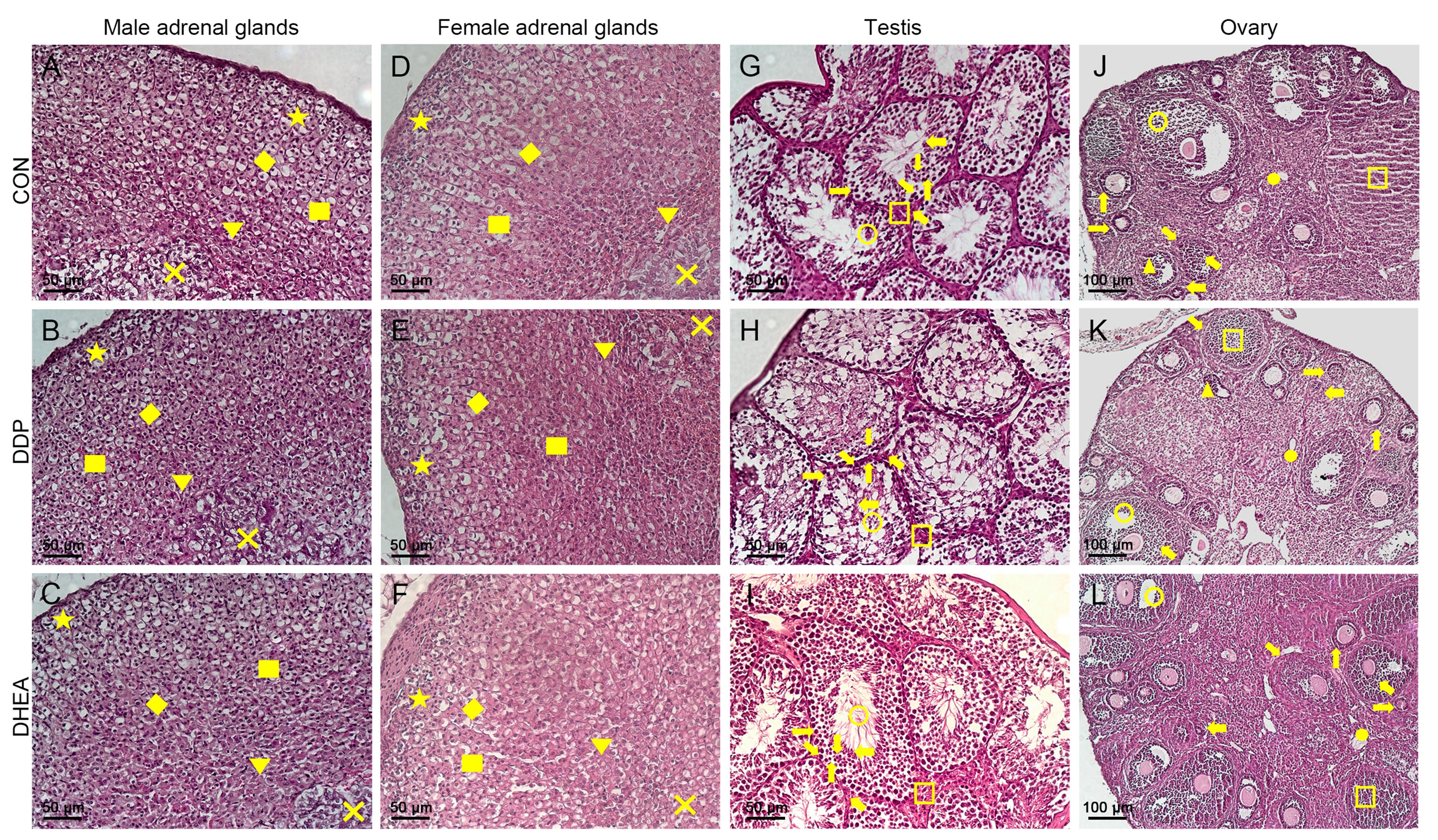
图2 小鼠肾上腺(×200)、睾丸(×200)、卵巢(×100)组织病理形态学观察(HE染色)注:CON为对照组,DDP为DDP建模组,DHEA为脱氢表雄酮治疗组。肾上腺组织(图A~F, 比例尺大小为50 μm)中,■表示肾上腺皮质;×表示肾上腺髓质;★表示球状带;◆表示束状带;▼表示网状带。睾丸组织(图G~I,比例尺大小为50 μm)中,○表示曲细精管;←表示精子细胞;→表示精原细胞;↑表示初级精母细胞;↓表示次级精母细胞;↘表示支持细胞;↖表示睾丸间质细胞。卵巢组织(图J~L,比例尺大小为100 μm)中,○表示卵泡腔;□表示黄体;▲表示闭锁卵泡;●表示白体;←表示原始卵泡;→表示初级卵泡;↑表示次级卵泡;↘表示卵泡膜层;↖表示颗粒细胞。
Figure 2 Pathological morphology of adrenal gland (×200), testis (×200) and ovary (×100) in mice (HE staining)Note:CON indicates control group, DDP indicates cisplatin modeling group, DHEA indicates dehydroepiandrosterone (DHEA) treatment after cisplatin modeling group. In Fig. A-F (scale bar size is 50 μm), ■ indicates adrenal cortex, × indicates adrenal medulla, ★ indicates zona glomerulosa, ◆indicates zona fasciculata, ▼ indicates zona reticularis. In Fig. G-I (scale bar size is 50 μm), ○ indicates seminiferous tubule, ← indicate spermatocyte, → indicates spermatogonia, ↑ indicates primary spermatocyte, ↓ indicates secondary spermatocyte, ↘indicates sertoli cells, ↖ indicates leydig cells. In Fig. J-L (scale bar size is 100 μm), ○ indicates follicular cavity, □ indicates corpus luteum, ▲ indicates atresia follicles, ● indicates corpus albicans of ovary, ← indicates primordial follicle, → indicates primary follicle, ↑ indicates secondary follicle, ↘ indicates follicle, ↖ indicates granulosa cells.
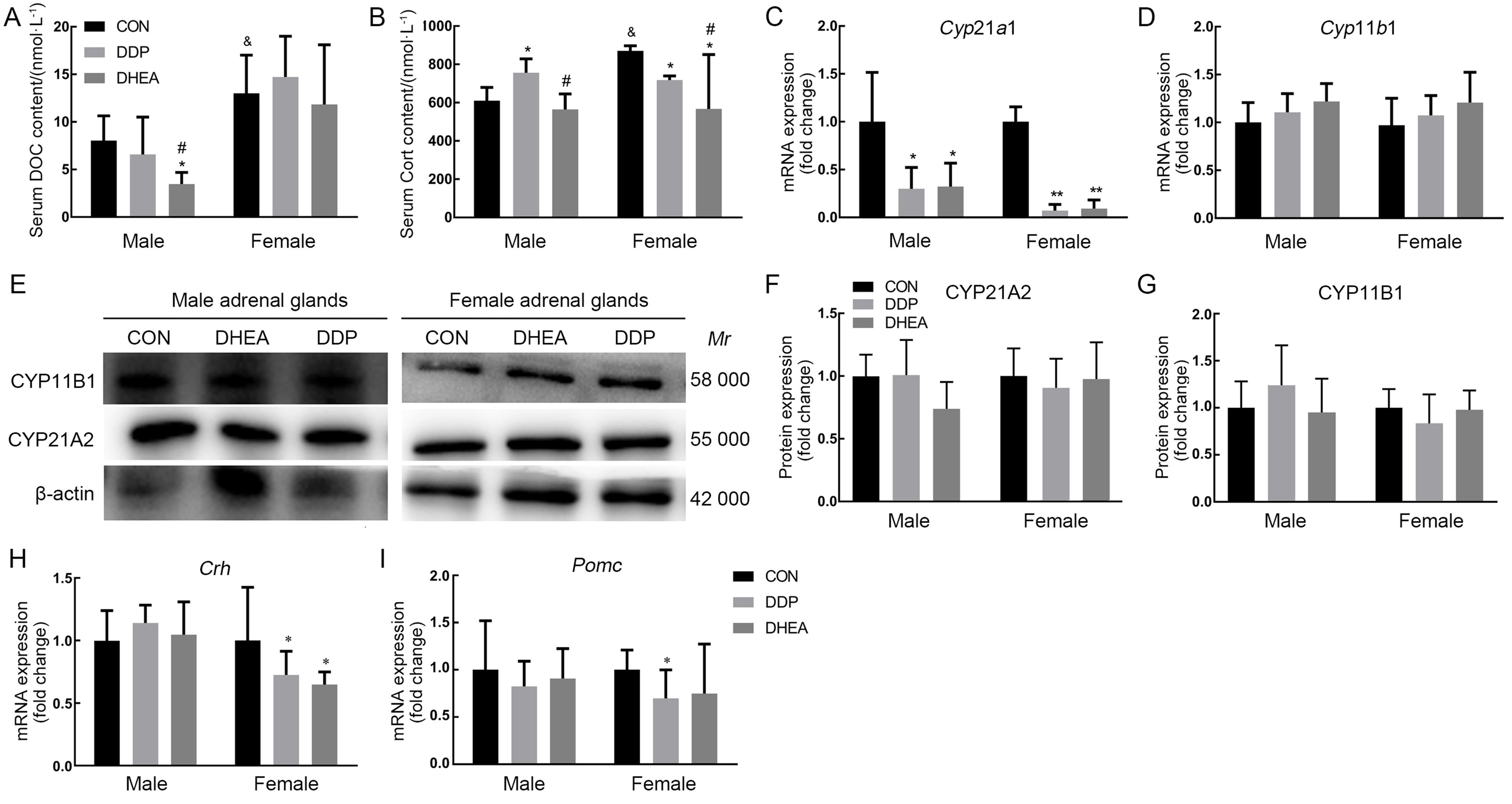
图3 小鼠血清皮质激素含量及肾上腺、下丘脑和垂体组织中皮质激素合成相关基因表达水平注:A~B,高效液相色谱串联质谱联用仪检测小鼠血清中11-脱氧皮质酮(DOC)和皮质酮(Cort)含量的结果;C~D,实时荧光定量PCR检测小鼠肾上腺组织中Cyp21a1和Cyp11b1 mRNA水平;E~G,蛋白质印迹法检测肾上腺组织中CYP11B1与CYP21A2蛋白表达及其相对表达量分析;H~I,实时荧光定量PCR检测小鼠下丘脑组织中促肾上腺皮质激素释放激素(Crh)和垂体组织中促肾上腺皮质激素(Pomc)mRNA水平。CON为对照组,DDP为DDP建模组,DHEA为脱氢表雄酮治疗组。与对照组比较,*P<0.05,**P<0.01;与DDP建模组比较,#P<0.05;与同组雄性小鼠比较, &P<0.05。
Figure 3 The content of serum corticosteroid and the expression levels of corticosteroid synthesis-related genes in adrenal, hypothalamic, and pituitary tissues of miceNote:A-B, The contents of 11-deoxycorticosterone (DOC) and corticosterone (Cort) in mouse serum were determined by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS); C-D, The expression levels of Cyp21a1and Cyp11b1 mRNA in adrenal tissue were determined by real-time fluorescent quantitative PCR (RT-qPCR); E-G, The expression levels of CYP11B1 and CYP21A2 protein in adrenal tissue were determined by Western blotting; H-I, The expression levels of corticotropin releasing hormone (Crh) mRNA in hypothalamus and pro-opiomelanocortin-alph (Pomc) mRNA in pituitary were determined by RT-qPCR. CON indicates control group, DDP indicates cisplatin modeling group, DHEA indicates dehydroepiandrosterone (DHEA) treatment after cisplatin modeling group. Compared with control group, *P<0.05, **P<0.01; Compared with the DDP group, #P<0.05; Compared with male mice in the same group, &P<0.05.
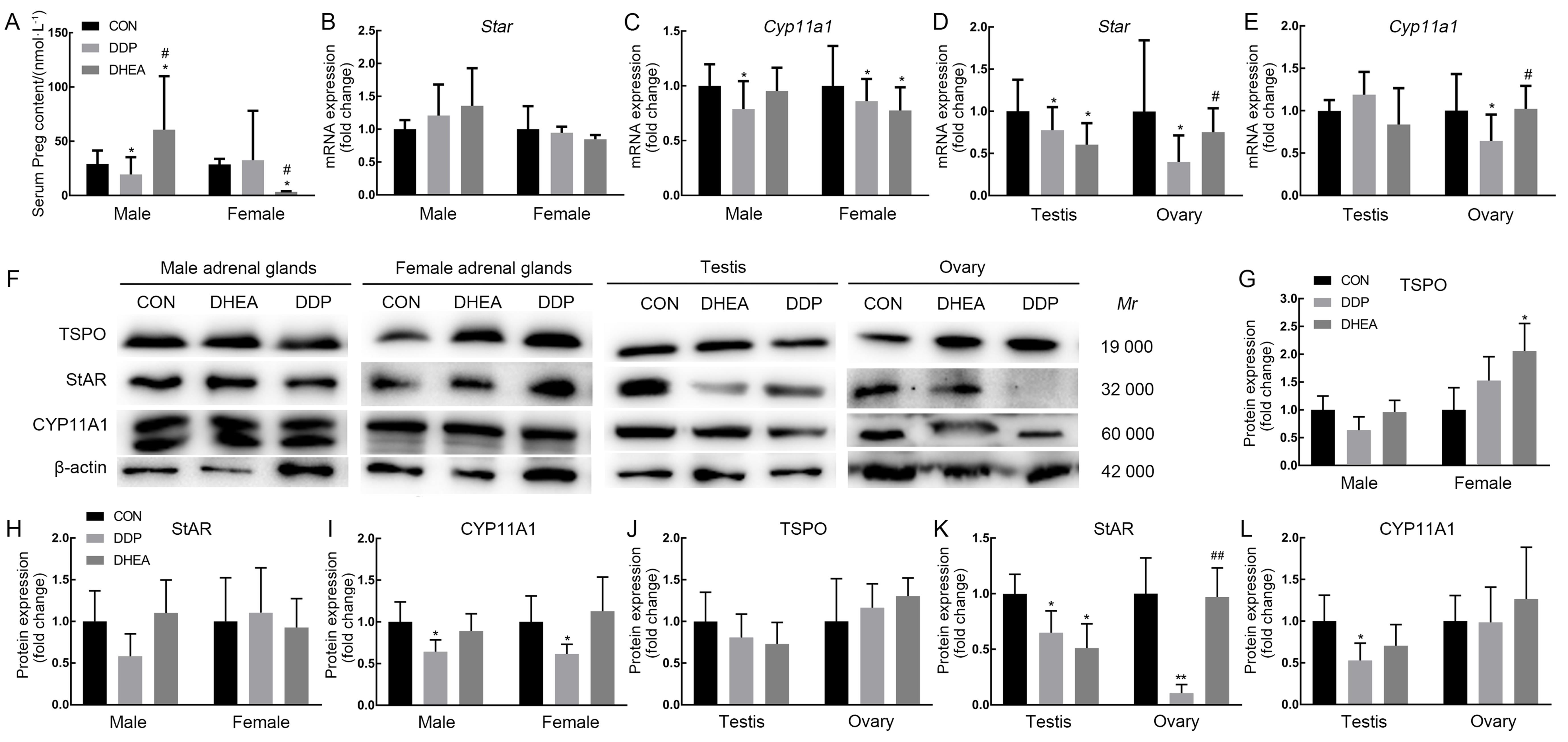
图4 小鼠血清孕烯醇酮含量及肾上腺、睾丸与卵巢组织中孕烯醇酮合成相关基因表达水平注:A,高效液相色谱串联质谱联用仪检测小鼠血清孕烯醇酮(Preg)含量的结果;B~C,实时荧光定量PCR检测小鼠肾上腺组织中类固醇激素急性调节蛋白(Star)和P450scc裂解酶编码基因Cyp11a1的mRNA转录水平;D~E,实时荧光定量PCR检测小鼠睾丸与卵巢组织中Star和Cyp11a1 mRNA水平;F~L,蛋白质印迹法检测小鼠肾上腺、睾丸与卵巢组织中18ku转位蛋白(TSPO)、StAR与CYP11A1蛋白及其相对表达量分析。CON为对照组,DDP为DDP建模组,DHEA为脱氢表雄酮治疗组。与对照组比较,*P<0.05,**P<0.01;与DDP建模组比较,#P<0.05,##P<0.01。
Figure 4 The content of serum pregnenolone and the expression levels of pregnenolone-synthesis-related genes in adrenal, testis and ovary tissues of miceNote:A, The contents of pregnenolone (Preg) in mouse serum was determined by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS); B-C, The expression levels of steroidogenic acute regulatory protein (Star) and Cyp11a1 mRNA in adrenal tissue were determined by real-time fluorescent quantitative PCR (RT-qPCR); D-E, The expression levels of Star and Cyp11a1 mRNA in testis and ovary were determined by RT-qPCR; F-L, The expression levels of translocatorprotein (TSPO), StAR and CYP11A1 proteins in adrenal, testis and ovary tissues were determined by Western blotting. CON indicates control group, DDP indicates cisplatin modeling group, DHEA indicates dehydroepiandrosterone (DHEA) treatment after cisplatin modeling group. Compared with control group, *P<0.05, **P<0.01; Compared with the DDP group, #P<0.05, ##P<0.01.
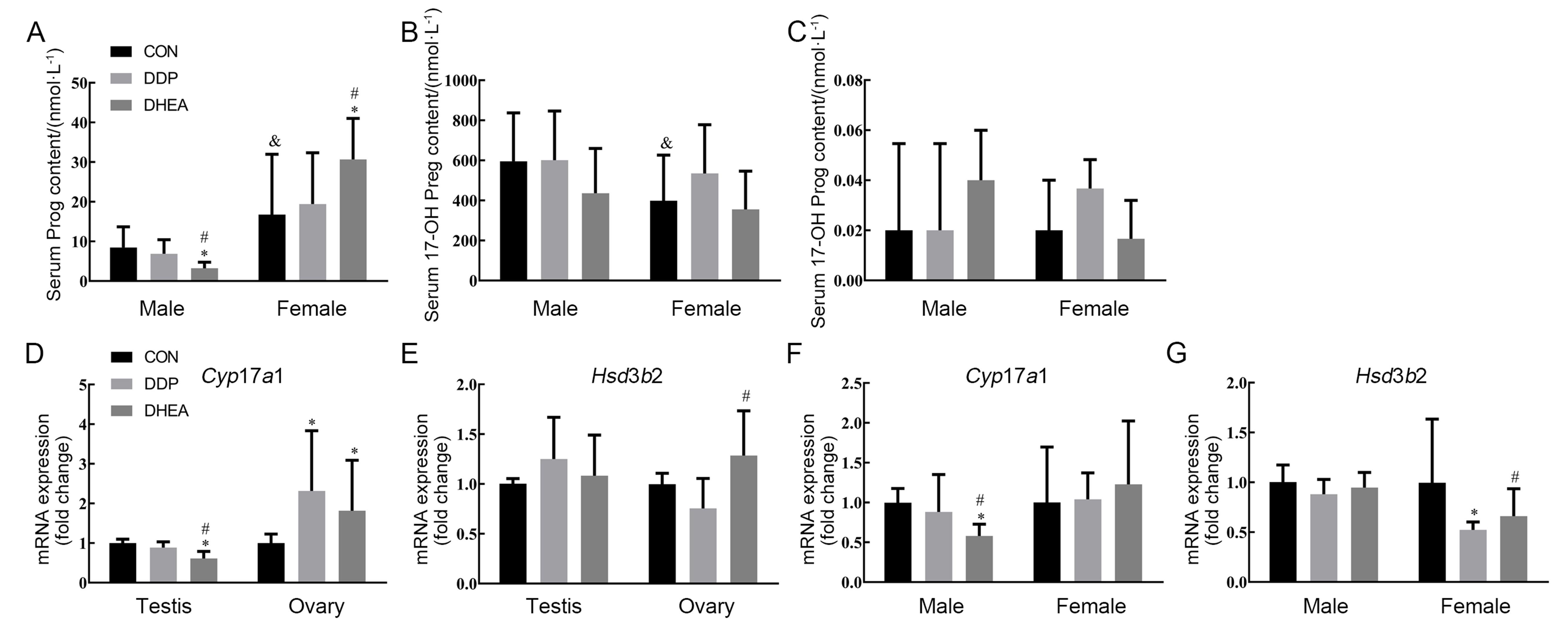
图5 小鼠血清孕酮、17-羟孕烯醇酮和17-羟孕酮含量以及肾上腺、睾丸与卵巢组织中合成相关基因表达注:A~C,高效液相色谱串联质谱联用仪检测小鼠血清孕酮(Prog)、17-羟孕烯醇酮(17-OH Preg)和17-羟孕酮(17-OH Prog)含量的结果;D~E,实时荧光定量PCR检测小鼠睾丸和卵巢组织中17α-羟化酶编码基因Cyp17a1和3β-羟基类固醇脱氢酶2(Hsd3b2)mRNA水平;F~G,实时荧光定量PCR检测小鼠肾上腺组织中Cyp17a1和Hsd3b2 mRNA水平。CON为对照组,DDP为DDP建模组,DHEA为脱氢表雄酮治疗组。与对照组比较,*P<0.05;与DDP建模组比较,#P<0.05;与同组雄性小鼠比较, &P<0.05。
Figure 5 The content of serum progesterone, 17-hydroxypregnenolone and 17-hydroxyprogesterone as well as the expression levels of their synthesis-related genes in adrenal, testis and ovary tissues of miceNote:A-C, The contents of progesterone (Prog), 17-hydroxypregnenolone (17-OH Preg) and 17-hydroxyprogesterone (17-OH Prog) in mouse serum were determined by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS); D-E, The expression levels of Cyp17a1 and 3-beta-hydroxysteroid dehydrogenase type 2 (Hsd3b2) mRNA in testis and ovary were determined by real-time fluorescent quantitative PCR (RT-qPCR); F-G, The expression levels of Cyp17a1 and Hsd3b2 mRNA in adrenal tissue were determined by RT-qPCR. CON indicates the control group, DDP indicates cisplatin modeling group, DHEA indicates dehydroepian-drosterone (DHEA) treatment after cisplatin modeling group. Compared with the control group, *P<0.05; Compared with the DDP group, #P<0.05; Compared with male mice in the same group, &P<0.05.
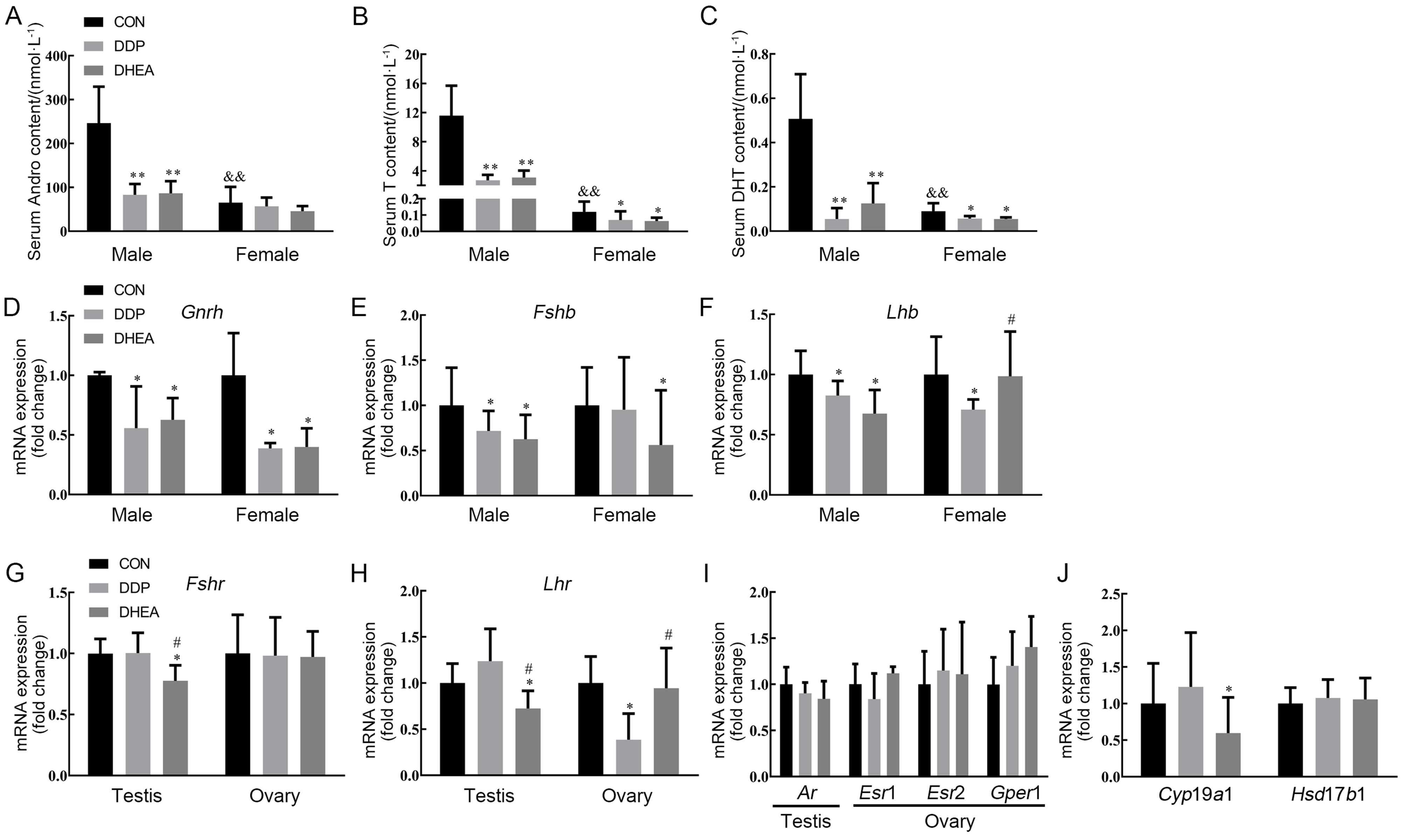
图6 小鼠血清雄激素水平及下丘脑-垂体-性腺轴相关基因表达水平注:A~C,高效液相色谱串联质谱联用仪检测小鼠血清雄烯二酮(Andro)、睾酮(T)和二氢睾酮(DHT)含量的结果;D~F, 实时荧光定量PCR检测小鼠下丘脑组织中促性腺激素释放激素(Gnrh)以及垂体组织中促卵泡刺激素β亚基(Fshb)、黄体生成素β亚基(Lhb)mRNA表达水平;G~I,实时荧光定量PCR检测小鼠睾丸和卵巢组织中促卵泡刺激素受体(Fshr)、黄体生成素受体(Lhr)、雄激素受体(Ar)、雌激素受体(Esr1、Esr2)和G蛋白偶联雌激素受体1(Gper1)mRNA表达水平;J,实时荧光定量PCR检测小鼠卵巢组织中芳香化酶编码基因Cyp19a1和17β-羟基类固醇脱氢酶1(Hsd17b1)mRNA表达水平。CON为对照组,DDP为DDP建模组,DHEA为脱氢表雄酮治疗组。与对照组比较,?P<0.05,??P<0.01;与DDP建模组比较,#P<0.05;与同组雄性小鼠比较, &&P<0.01。
Figure 6 The content of serum androgen and the expression levels of hypothalamus-pituitary-adrenal/gonadal axis-related genes in adrenal, testis and ovary tissues of miceNote:A-C, The contents of androstenedione (Andro), testosterone (T) and dihydrotestosterone (DHT) in mouse serum were determined by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS); D-F, The expression levels of gonadotropin-releasing hormone (Gnrh) mRNA in hypothalamus tissue, folicular stimulating hormone beta subunit (Fshb) and luteinizing hormone subunit beta (Lhb) mRNA in pituitary were determined by real-time fluorescent quantitative PCR (RT-qPCR); G-I, The expression levels of folicular stimulating hormone receptor (Fshr), luteinizing hormone receptor (Lhr), androgen receptor (Ar), estrogen receptor (Esr)1/2 and G-protein coupled estrogen receptor 1 (Gper1) mRNA in testis and ovary were determined by RT-qPCR; L, The expression levels of Cyp19a1 and 17-beta-hydroxysteroid dehydrogenase type 1 (Hsd7b1) mRNA in mouse ovarian tissue by RT-qPCR. CON indicates control group, DDP indicates cisplatin modeling group, DHEA indicates dehydroepiandrosterone (DHEA) treatment after cisplatin modeling group. Compared with control group, ?P<0.05, ??P<0.01; Compared with the DDP group, #P<0.05; Compared with male mice in the same group, &&P<0.01.
| 1 | 邓成艳, 黄荣丽, 连利娟, 等. 顺铂的毒副作用及其防治[J]. 中华妇产科杂志, 1995, 30(6): 376-379. |
| DENG C Y, HUANG R L, LIAN L J, et al. Toxic and side effects of cisplatin and its prevention and treatment[J]. Chin J Obstet Gynecol, 1995, 30(6): 376-379. | |
| 2 | 董若曦, 朱小丹, 樊伯珍, 等. 不同剂量顺铂腹腔注射建立大鼠化疗损伤性卵巢早衰模型[J]. 实验动物与比较医学, 2020, 40(2): 104-109. DOI: 10.3969/j.issn.1674-5817.2020.02.003 . |
| DONG R X, ZHU X D, FAN B Z, et al. Intraperitoneal injection in different dosages of cisplatin to establish a rat model of premature ovarian failure induced by chemotherapy[J]. Lab Anim Comp Med, 2020, 40(2): 104-109. DOI: 10.3969/j.issn.1674-5817.2020.02.003 . | |
| 3 | 农淄心, 邓琳, 贺健祥, 等. 顺铂短期给药诱发小鼠药源性虚证模型的评价研究[J]. 上海中医药大学学报, 2021, 35(4):35-44. DOI: 10.16306/j.1008-861x.2021.04.006 . |
| NONG Z X, DENG L, HE J X, et al. Evaluation on drug-induced deficiency syndrome model by short-term administration of cisplatin in mice[J]. Acad J Shanghai Univ Tradit Chin Med, 2021, 35(4):35-44. DOI: 10.16306/j.1008-861x.2021.04.006 . | |
| 4 | 潘志强. "药源性证候"的新生学术问题与思考[J]. 上海中医药杂志, 2018, 52(6):5-8. DOI: 10.16305/j.1007-1334.2018.06.002 . |
| PAN Z Q. New academic problems and thinking of drug-induced syndromes[J]. Shanghai J Tradit Chin Med, 2018, 52(6):5-8. DOI: 10.16305/j.1007-1334.2018.06.002 . | |
| 5 | 陈晓. 神经内分泌免疫网络在虚证实验动物中的变化[J]. 上海中医药大学学报, 2002, 16(2):61-65. DOI: 10.16306/j.1008-861x.2002.02.021 . |
| CHEN X. Change of neuroendocrine immune network in experimental animals with deficient syndrome[J]. Acta Univ Tradit Med Sin Pharmacol Shanghai, 2002, 16(2):61-65. DOI: 10.16306/j.1008-861x.2002.02.021 . | |
| 6 | 沈自尹, 黄建华, 林伟, 等. 从整体论到系统生物学进行肾虚和衰老的研究[J]. 中国中西医结合杂志, 2009, 29(6):548-550. DOI: 10.3321/j.issn: 1003-5370.2005.03.017 . |
| SHEN Z Y, HUANG J H, LIN W, et al. Transition of studying on Shen deficiency syndrome and aging from holistic concept to systematic biologic viewpoint[J]. Chin J Integr Tradit West Med, 2009, 29(6):548-550. DOI: 10.3321/j.issn: 1003-5370.2005.03.017 . | |
| 7 | 韩咏梅, 朱永廉. 减轻顺铂毒性的研究进展[J]. 国外医学(肿瘤学分册), 1999, 26(2): 86-89. |
| HAN Y M, ZHU Y L. Research progress on reducing the toxicity of cisplatin[J]. Foreign Med Sci Cancer Sect, 1999, 26(2): 86-89. | |
| 8 | 涂钰莹, 易英, 涂玉梅, 等. 地塞米松对顺铂致斑马鱼毛细胞损伤的保护作用研究[J]. 听力学及言语疾病杂志, 2022, 30(1): 64-68. DOI: 10.3969/j10.3969/j.issn.1006-7299.2022.01.015 . |
| TU Y Y, YI Y, TU Y M, et al. Protective effects of dexamethasone on cisplatin-induced hair cell injury in zebrafsih[J]. J Audiol Speech Pathol, 2022, 30(1): 64-68. DOI: 10.3969/j10.3969/j.issn.1006-7299.2022.01.015 . | |
| 9 | 孔庆志, 黄涛, 费雁, 等. 黄芪对大剂量顺铂所致肾毒性防护的实验研究[J]. 中国药学杂志, 1999, 34(7): 447-450. DOI: 10.3321/j.issn: 1001-2494.1999.07.006 . |
| KONG Q Z, HUANG T, FEI Y, et al. Experimental study on protective effect of astragalus membranaceus on nephrotoxicity induced by high dose cisplatin[J]. Chin Pharm J, 1999, 34(7):447-450. DOI: 10.3321/j.issn: 1001-2494.1999.07.006 . | |
| 10 | 孙美娟, 费梅, 祝皓. 参芪水煎液防治顺铂化疗所致肾损伤临床研究[J]. 南京中医药大学学报, 2021, 37(1):21-24. DOI: 10.14148/j.issn.1672-0482.2021.0021 . |
| SUN M J, FEI M, ZHU H. Clinical study on the prevention and treatment of renal injury induced by cisplatin chemotherapy with Shenqi Decoction[J]. J Nanjing Univ Tradit Chin Med, 2021, 37(1):21-24. DOI: 10.14148/j.issn.1672-0482.2021.0021 . | |
| 11 | 张慧琼, 李刚敏, 陈俊仁, 等. 西洋参总皂苷联合顺铂对Luc-A549肺癌小鼠瘤体生长的影响[J]. 中药药理与临床, 2021, 37(3):50-55. DOI: 10.13412/j.cnki.zyyl.2021.03.010 . |
| ZHANG H Q, LI G M, CHEN J R, et al. Effect of total saponins in panacis quinquefolii Radix combined with cisplatin on tumor growth in mice bearing luc-A549 cells[J]. Pharmacol Clin Chin Mater Med, 2021, 37(3):50-55. DOI: 10.13412/j.cnki.zyyl.2021.03.010 . | |
| 12 | 廖英俊, 汤浩, 金亚平. 抗癌药顺铂对小鼠的耳、肾和肝毒性及其机制的研究[J]. 中国药理学通报, 2004, 20(1):82-85. DOI: 10.3321/j.issn: 1001-1978.2004.01.023 . |
| LIAO Y J, TANG H, JIN Y P. Study of toxic effects on hearing, kidney and liver of mice induced by anticancer agent of cisplatin and their mechanisms[J]. Chin Pharmacol Bull, 2004, 20(1):82-85. DOI: 10.3321/j.issn: 1001-1978.2004.01.023 . | |
| 13 | 刘崇斌, 黄柳维, 李彩珍, 等. 阻断pannexin-1可减轻肾脏组织炎症细胞浸润和缓解顺铂诱导的急性肾损伤[J]. 南方医科大学学报, 2019, 39(5): 508-514. DOI: 10.12122/j.issn.1673-4254.2019.05.02 . |
| LIU C B, HUANG L W, LI C Z, et al. Blocking pannexin-1 alleviates cisplatin-induced acute kidney injury in mice by reducing renal inflammatory cell infiltration[J]. J South Med Univ, 2019, 39(5): 508-514. DOI: 10.12122/j.issn.1673-4254.2019.05.02 . | |
| 14 | 舍雅莉, 闫德祺, 刘永琦, 等. 当归贝母苦参丸对顺铂化疗H22荷瘤小鼠肿瘤及肝脏、肾脏和胸腺组织病理形态的影响[J]. 中国中医药信息杂志, 2014, 21(5): 56-60. DOI: 10.3969/j.issn.1005-5304.2014.05.018 . |
| SHE Y L, YAN D Q, LIU Y Q, et al. Effect of Danggui Beimu Kushen pill on pathological morphology of tumor, liver, kidney and Thymus of H22 tumor-bearing mice treated with cisplatin[J]. Chin J Inf Tradit Chin Med, 2014, 21(5): 56-60. DOI: 10.3969/j.issn.1005-5304.2014.05.018 . | |
| 15 | 孙铱钒, 关朕, 孙应彪, 等. 顺铂染毒大鼠睾丸定量组织学分析及睾丸酶活力的变化[J]. 毒理学杂志, 2015, 29(3):172-176. DOI: 10.16421/j.cnki.1002-3127.2015.03.003 . |
| SUN Y F, GUAN Z, SUN Y B, et al. Quantitative histological analysis of testes and effect on testicular enzymes induced by cisplatin in rats[J]. J Toxicol, 2015, 29(3):172-176. DOI: 10.16421/j.cnki.1002-3127.2015.03.003 . | |
| 16 | ERFANI MAJD N, TABANDEH M R, HOSSEINIFAR S H, et al. Protective effect of Aloe vera gel against cisplatin-induced testicular damage, sperm alteration and oxidative stress in rats[J]. Int J Fertil Steril, 2021, 15(3):210-218. DOI: 10.22074/IJFS.2020.134691 . |
| 17 | 齐聪. 顺铂腹腔内给药对大鼠卵巢的毒性作用[J]. 上海医科大学学报, 2000, 27(4): 324-325. DOI: 10.3969/j.issn.1672-8467.2000.04.031 . |
| QI C. Observation of ovarian toxicity following intraperitoneal administration of cisplatin in mice[J]. J Shanghai Med Univ, 2000, 27(4): 324-325. DOI: 10.3969/j.issn.1672-8467.2000.04.031 . | |
| 18 | NILSSON M E, VANDENPUT L, TIVESTEN Å, et al. Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry[J]. Endocrinology, 2015, 156(7):2492-2502. DOI: 10.1210/en.2014-1890 . |
| 19 | SCHIFFER L, BARNARD L, BARANOWSKI E S, et al. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: a comprehensive review[J]. J Steroid Biochem Mol Biol, 2019, 194:105439. DOI: 10.1016/j.jsbmb.2019.105439 . |
| 20 | CUTLER G B Jr, GLENN M, BUSH M, et al. Adrenarche: a survey of rodents, domestic animals, and Primates[J]. Endocrinology, 1978, 103(6):2112-2118. |
| 21 | 欧阳可育, 胡卫列, 聂奇伟. 辛二酰苯胺异羟肟酸联合顺铂对裸鼠肾上腺皮质癌的实验研究[J]. 临床泌尿外科杂志, 2014, 29(4):342-345. DOI: 10.13201/j.issn.1001-1420.2014.04.021 . |
| OUYANG K Y, HU W L, NIE Q W. Effects of SAHA combined with cisplatin on adrenocortical carcinoma in nude mice model[J]. J Clin Urol, 2014, 29(4):342-345. DOI: 10.13201/j.issn.1001-1420.2014.04.021 . | |
| 22 | 王泽先, 郭菲菲, 公衍玲, 等. 顺铂化疗对下丘脑、血浆ghrelin和orexin表达的影响[J]. 现代生物医学进展, 2016, 16(31):6023-6027, 6041. DOI: 10.13241/j.cnki.pmb.2016.31.006 . |
| WANG Z X, GUO F F, GONG Y L, et al. Effects of orexin on the cisplatin-induced Pica in rats[J]. Prog Mod Biomed, 2016, 16(31):6023-6027, 6041. DOI: 10.13241/j.cnki.pmb.2016.31.006 . |
| [1] | 贡磊磊, 王晓霞, 封学伟, 李心蕾, 赵涵, 张雪艳, 冯欣. 不同浓度环磷酰胺诱导早发性卵巢功能不全小鼠模型及作用机制研究[J]. 实验动物与比较医学, 2025, 45(4): 403-410. |
| [2] | 姜娟, 宋宁, 连文博, 邵丛丛, 顾文文, 石燕. 两种浓度乙醇溶液灌注建立小鼠宫腔粘连模型的组织病理和分子病理表型比较[J]. 实验动物与比较医学, 2025, 45(4): 393-402. |
| [3] | 刘月琴, 薛卫国, 王淑友, 申耀华, 贾术永, 王广军, 宋晓晶. 探头式激光共聚焦成像技术用于小鼠消化道组织形态特征分析[J]. 实验动物与比较医学, 2025, 45(4): 457-465. |
| [4] | 罗莲莲, 袁艳春, 王俊岭, 时广森. 肌萎缩侧索硬化症小鼠模型研究进展[J]. 实验动物与比较医学, 2025, 45(3): 290-299. |
| [5] | 孔志豪, 魏晓锋, 于灵芝, 冯丽萍, 朱琦, 施国君, 王晨. 鳞状皮屑裸小鼠木糖葡萄球菌的分离鉴定[J]. 实验动物与比较医学, 2025, 45(3): 368-375. |
| [6] | 许秋雨, 严国锋, 付丽, 范文华, 周晶, 朱莲, 仇淑雯, 张洁, 吴铃. 来曲唑缓释片皮下给药构建小鼠多囊卵巢综合征模型及其肝脏转录组学分析[J]. 实验动物与比较医学, 2025, 45(2): 119-129. |
| [7] | 潘钱家, 葛峻沂, 胡楠, 华飞, 顾敏. 基于16S rRNA测序的2型糖尿病db/db小鼠模型口腔菌群差异分析[J]. 实验动物与比较医学, 2025, 45(2): 147-157. |
| [8] | 肖文娴, 吕龙宝. 非人灵长类实验动物用于人类卵巢衰老研究进展[J]. 实验动物与比较医学, 2025, 45(1): 47-54. |
| [9] | 刘荣乐, 程灏, 尚付生, 常书福, 徐平. SHJH hr 小鼠的心脏衰老表型研究[J]. 实验动物与比较医学, 2025, 45(1): 13-20. |
| [10] | 吴志浩, 曹舒扬, 周正宇. 肝螺杆菌感染引起VDR-/-小鼠炎性肠病相关肠纤维化模型的建立及机制探讨[J]. 实验动物与比较医学, 2025, 45(1): 37-46. |
| [11] | 张楠, 李怀银, 连晓娣, 魏娟鹏, 高明. 不同光照时长对NIH小鼠体重和学习记忆能力的影响[J]. 实验动物与比较医学, 2025, 45(1): 73-78. |
| [12] | 王芊芊, 陶斯珏, 卫振, 金晖晖, 刘平, 汪洌. 利用辅助生殖技术挽救基因修饰小鼠的实例分析[J]. 实验动物与比较医学, 2025, 45(1): 79-86. |
| [13] | 赵赫, 张帆, 肖宇宙, 安学芳, 张涛, 李丽. 一例干扰素受体缺失小鼠自发未成熟型睾丸畸胎瘤诊断[J]. 实验动物与比较医学, 2024, 44(6): 691-694. |
| [14] | 赵小娜, 王鹏, 叶茂青, 曲新凯. 应用Triacsin C构建新型高血糖肥胖小鼠心功能减退模型[J]. 实验动物与比较医学, 2024, 44(6): 605-612. |
| [15] | 谭贺, 杨晓辉, 张大秀, 王贵成. 不同发育时期小鼠代谢笼实验的最佳适应期探讨[J]. 实验动物与比较医学, 2024, 44(5): 502-510. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||