













实验动物与比较医学 ›› 2025, Vol. 45 ›› Issue (4): 449-456.DOI: 10.12300/j.issn.1674-5817.2024.177
收稿日期:2024-11-26
修回日期:2025-04-10
出版日期:2025-08-25
发布日期:2025-09-01
通讯作者:
作者简介:刘 鹍(1996—),男,硕士,研究方向:预防兽医学,分子生物学。E-mail:Kuniooo@163.com
LIU Kun, LAN Qing, YI Bing, XIE Xiaojie( )(
)( )
)
Received:2024-11-26
Revised:2025-04-10
Published:2025-08-25
Online:2025-09-01
Contact:
XIE Xiaojie (ORCID: 0009-0000-3025-769X), E-mail: xjxie@glpcd.com摘要:
药物非临床生殖毒性试验多选用大鼠、家兔和食蟹猴等哺乳动物,而动物妊娠是目前影响非临床生殖毒性试验的关键环节,本文主要针对动物妊娠环节中存在的难点及应对方法进行讨论。大鼠可应用于生育力评估与早期胚胎发育毒性试验(Ⅰ段)、胚胎-胎仔发育毒性试验(Ⅱ段)和围产期毒性试验(Ⅲ段),可以通过阴道涂片法确定雌性大鼠的动情周期。在雌、雄大鼠按1∶1比例合笼过夜后,次日通过阴道栓检查来确认大鼠是否发生交配行为。家兔一般应用于胚胎-胎仔发育毒性试验(Ⅱ段),可通过观察雄性家兔和处于发情期的雌性家兔合笼后是否发生交配行为来确定受孕时间。家兔在生殖毒性动物试验中常出现雌兔发情不典型、不同批次间家兔发情情况差异显著、交配失败等问题,可通过延长光照时间、增加蛋白质摄入、选择合适的交配方式、改变饲养环境(雌兔和雄兔邻近饲养)等方式改善。非人灵长类动物一般应用于围产期毒性试验(Ⅲ段),准确判断雄性非人灵长类动物是否性成熟是其药物非临床生殖毒性试验的难点之一,可通过综合评估动物年龄、体重和睾丸体积等指标来判断它们是否性成熟。一般雄性猕猴年龄在4.5岁以上,体重大于4.5 kg,单侧睾丸体积超过10 mL,双侧睾丸总体积大于20 mL则表示性成熟。关于雌性非人灵长类动物是否妊娠,可通过超声检查是否能观察到明显孕囊进行判定,但这需要经验丰富的临床兽医制定完善的B超检查方法。综上所述,在非临床生殖毒性动物研究中,应根据不同种属动物的生殖系统解剖学特性,选择适合的检测/观察方法和结果判定标准,以确保动物成功交配,使非临床生殖毒性试验顺利开展。
中图分类号:
刘鹍,兰青,易兵,等. 药物非临床生殖毒性试验中动物妊娠的主要难点及应对方法[J]. 实验动物与比较医学, 2025, 45(4): 449-456. DOI: 10.12300/j.issn.1674-5817.2024.177.
LIU Kun,LAN Qing,YI Bing,et al. Key Challenges and Mitigation Strategies for Animal Pregnancy in Non-clinical Reproductive Toxicity Testing of Drugs[J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 449-456. DOI: 10.12300/j.issn.1674-5817.2024.177.
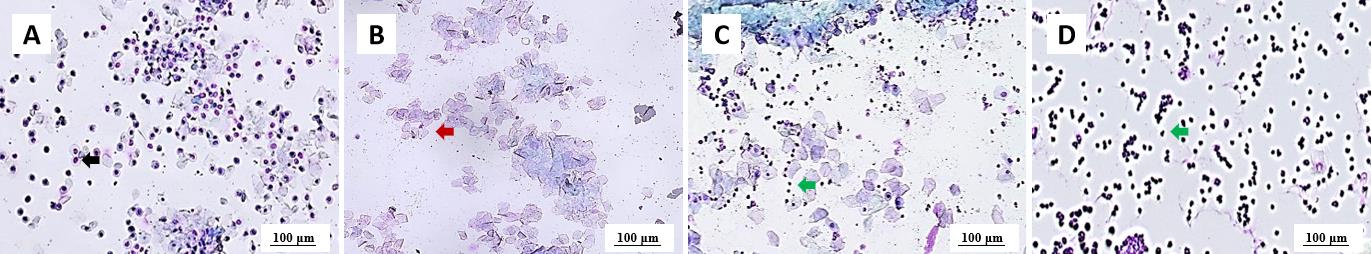
图1 成年雌性大鼠动情周期各阶段的典型阴道组织涂片注:A,动情前期;B,动情期;C,动情后期;D,间情期。黑色箭头所指的是有核上皮细胞;红色箭头所指的是无核上皮细胞;绿色箭头所指的是白细胞。涂片的染色方法为HE染色。图像的放大倍数为40,标尺为100 μm。
Figure 1 Typical vaginal smear characteristics from adult female rats at each stage of estrous cycleNote:A, proestrus; B, estrus; C, metestrus; D, diestrus. The black arrow indicates nucleated epithelial cells; the red arrow indicates anucleated epithelial cells; the green arrows indicate leukocytes. The staining method of the smear was hematoxylin and eosin (HE) staining. The magnification of the images is 40 and the scale bars are 100 μm.
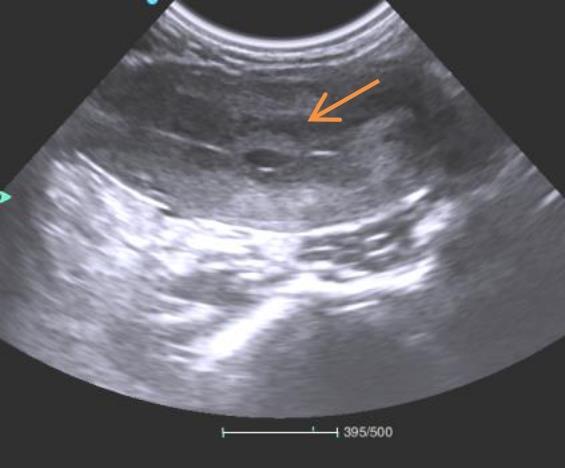
图2 妊娠初期雌猴孕囊B超切面注:图中箭头所指区域可见成型的孕囊结构。
Figure 2 Ultrasound cross-sectional view of gestational sac in a female monkey during early pregnancyNote:The area indicated by the arrow in the figure shows a well-formed gestational sac structure.
| [1] | 黄冬妍, 吴建辉. 生殖毒理学研究动物模型的建立方法及应用评价[J]. 实验动物与比较医学, 2024, 44(5):550-559. DOI:10.12300/j.issn.1674-5817.2024.028 . |
| HUANG D Y, WU J H. Establishment methods and application evaluation of animal models in reproductive toxicology research[J]. Lab Anim Comp Med, 2024, 44(5):550-559. DOI:10.12300/j.issn.1674-5817.2024.028 . | |
| [2] | 林蔚, 张荣标, 陈润. SD大鼠毒性试验生殖指标参考值建立及不同体重大鼠孕后生殖指标分析[J]. 海峡预防医学杂志, 2023, 29(4):60-62. |
| LIN W, ZHANG R B, CHEN R. Establishment of reference value for reproductive index in SD rats toxicity test and analysis of post-pregnancy reproductive index in rats of different body weights[J]. Strait J Prev Med, 2023, 29(4):60-62. | |
| [3] | 刘晓宇, 王娇, 钱欣, 等. 围绝经期大鼠动情周期变化的研究[J]. 哈尔滨医科大学学报, 2014, 48(5):358-360. DOI:CNKI:SUN:HYDX.0.2014-05-003 . |
| LIU X Y, WANG J, QIAN X, et al. Changes of estrous cycle of perimenopausal rats[J]. J Harbin Med Univ, 2014, 48(5):358-360. DOI:CNKI:SUN:HYDX.0.2014-05-003 . | |
| [4] | 陈思充, 陈子豪, 高青华, 等. 不同阴道涂片方法的比较及对大鼠动情周期的判断[J]. 解剖科学进展, 2023, 29(1):93-95. DOI: 10.16695/j.cnki.1006-2947.2023.01.025 . |
| CHEN S C, CHEN Z H, GAO Q H, et al. Comparison of different vaginal smears and judgment of estrous cycle in rats[J]. Prog Anat Sci, 2023, 29(1):93-95. DOI: 10.16695/j.cnki.1006-2947.2023.01.025 . | |
| [5] | 闫婧, 赵季宇, 马梦娜, 等. 大鼠阴道细胞涂片技术及其HE染色方法的研究[J]. 山西中医药大学学报, 2023, 24(1):45-51. DOI: 10.19763/j.cnki.2096-7403.2023.01.07 . |
| YAN J, ZHAO J Y, MA M N, et al. Study on smear technique and HE staining of rat vaginal cells[J]. J Shanxi Univ Chin Med, 2023, 24(1):45-51. DOI: 10.19763/j.cnki.2096-7403.2023.01.07 . | |
| [6] | 于农淇, 梁莎莎, 黄健, 等. 阴道分泌物在动物发情鉴定中的研究进展[J]. 中国牛业科学, 2022, 48(5):66-70. DOI:10.3969/j.issn.1001-9111.2022.05.015 . |
| YU N Q, LIANG S S, HUANG J, et al. Research progress of vaginal secretions in animal estrus identification[J]. China Cattle Sci, 2022, 48(5):66-70. DOI:10.3969/j.issn.1001-9111.2022.05.015 . | |
| [7] | 罗成龙, 程皓洋, 张琪, 等. 雄性大鼠性动机的检测方法与评价指标研究进展[J]. 中华男科学杂志, 2024, 30(6):569-573. DOI: 10.13263/j.cnki.nja.2024.06.012 . |
| LUO C L, CHENG H Y, ZHANG Q, et al. Detection methods and evaluation indexes of sexual motivation in male rats: an update[J]. Natl J Androl, 2024, 30(6):569-573. DOI: 10.13263/j.cnki.nja.2024.06.012 . | |
| [8] | 王洁, 吴素慧, 尚海霞. 性成熟雌性SD大鼠动情周期的观察[J]. 当代医学, 2013, 19(28):25-26. DOI:10.3969/j.issn.1009-4393.2013.28.015 . |
| WANG J, WU S H, SHANG H X. Observation of the estrous cycle in sexually mature female sd rats[J]. Contemp Med, 2013, 19(28):25-26. DOI:10.3969/j.issn.1009-4393.2013.28.015 . | |
| [9] | 张森, 王新, 韦旭斌, 等. 大鼠发情周期各阶段的阴道细胞变化观察[J]. 动物医学进展, 2006, 27(2):69-72. DOI: 10.16437/j.cnki.1007-5038.2006.02.018 . |
| ZHANG S, WANG X, WEI X B, et al. Observation on the vaginal smear photographs for each stage of estrus cycle in rats[J]. Prog Vet Med, 2006, 27(2):69-72. DOI: 10.16437/j.cnki.1007-5038.2006.02.018 . | |
| [10] | 谢晓婕, 易兵, 杨刚坤. 兔生殖毒性评价试验中动物的选择及饲养管理要求[C]. 第九届药物毒理学年会——新时代·新技术·新策略·新健康论文集. 武汉: 2019: 2. DOI: 10.26914/c.cnkihy.2019.072236 . |
| XIE X J, YI B, YANG G K. Animal selection and husbandry requirements in rabbit reproductive toxicity evaluation tests [C]. Proceedings of the 9th Annual Conference on Drug Toxicology - New Era, New Technologies, New Strategies, New Health. Wuhan: 2019: 2. DOI:10.26914/c.cnkihy.2019.072236 . | |
| [11] | 黄雪如, 黄博定, 付丽萍, 等. 家兔生殖生理特点与提高繁殖力措施[J]. 中国养兔, 2020(3):42-43, 36. DOI:10.3969/j.issn.1005-6327.2020.03.015 . |
| HUANG X R, HUANG B D, FU L P, et al. Reproductive physiological characteristics of rabbits and measures to improve their fertility[J]. Chin J Rabbit Farming, 2020(3):42-43, 36. DOI:10.3969/j.issn.1005-6327.2020.03.015 . | |
| [12] | 李召英. 临床促进杂交母兔发情受孕实用技巧[J]. 中国养兔杂志, 2023(2):30-32, 35. DOI: 10.3969/j.issn.1005-6327.2021.06.009 . |
| LI Z Y. Practical skills of promoting estrus pregnancy of hybrid female rabbits in clinic[J]. Chin J Rabbit Farming, 2023(2):30-32, 35. DOI: 10.3969/j.issn.1005-6327.2021.06.009 . | |
| [13] | 孙世坤, 桑雷, 王锦祥, 等. 不同光源对闽西南黑母兔发情及激素GnRH水平影响[J]. 中国畜禽种业, 2021, 17(1):42-43. DOI: 10.3969/j.issn.1673-4556.2021.01.022 . |
| SUN S K, SANG L, WANG J X, et al. Effect of different light sources on estrus and GnRH level of female black rabbit in southwest Fujian[J]. Chin Livest Poult Breed, 2021, 17(1):42-43. DOI: 10.3969/j.issn.1673-4556.2021.01.022 . | |
| [14] | 王玉军, 张晓茜, 方有贵, 等. 高原鼠兔发情周期性激素与性器官变化特征[J]. 兽类学报, 2024, 44(3):351-359. DOI: 10.16829/j.slxb.150872 . |
| WANG Y J, ZHANG X Q, FANG Y G, et al. Characteristics of sex hormone level and sex organ histology in estrus cycle of plateau pika(Ochotona curzoniae)[J]. Acta Theriol Sin, 2024, 44(3):351-359. DOI: 10.16829/j.slxb.150872 . | |
| [15] | 潘孝青, 王杏龙, 杨杰, 等. LED单色光对兔行为及同期发情影响的机理研究[J]. 浙江农业学报, 2020, 32(12):2128-2137. DOI:10.3969/j.issn.1004-1524.2020.12.03 . |
| PAN X Q, WANG X L, YANG J, et al. Effects of different LED light colors on estrus synchronization of rabbits and their molecular regulation mechanism through retina-pineal gland pathway[J]. Acta Agric Zhejiangensis, 2020, 32(12):2128-2137. DOI: 10.3969/j.issn.1004-1524.2020.12.03 . | |
| [16] | LIN H H, KUANG M C, HOSSAIN I, et al. A nutrient-specific gut hormone arbitrates between courtship and feeding[J]. Nature, 2022, 602(7898):632-638. DOI:10.1038/s41586-022-04408-7 . |
| [17] | 刘鹍, 卫澜欣, 管晓琳, 等. 实验用新西兰兔压力水平的探究[J]. 现代畜牧科技, 2022(12):14-17. DOI: 10.19369/j.cnki.2095-9737.2022.12.005 . |
| LIU K, WEI L X, GUAN X L, et al. Preliminary exploration of pressure level in experimental New Zealand rabbits[J]. Mod Anim Husb Sci Technol, 2022(12):14-17. DOI: 10.19369/j.cnki.2095-9737.2022.12.005 . | |
| [18] | 周墨. 秋季种母兔管理要点[J]. 农家致富, 2024(19):39. |
| ZHOU M. Key management points for breeding rabbits in autumn[J]. Farmhouse Fortune, 2024(19):39. | |
| [19] | 孙祖越, 周莉, 闫晗, 等. 如何成功开展药物非临床生殖毒性试验[J]. 中国新药杂志, 2011, 20(22):2195-2204. DOI: CNKI:SUN:ZXYZ.0.2011-22-006 . |
| SUN Z Y, ZHOU L, YAN H, et al. How to successfully carry out non-clinical reproductive toxicity study on new drugs[J]. Chin J New Drugs, 2011, 20(22):2195-2204. DOI: CNKI:SUN:ZXYZ.0.2011-22-006 . | |
| [20] | 伶俐. 人工授精对母兔繁殖性能的影响[J]. 吉林畜牧兽医, 2022, 43(4):89-90. DOI: 10.3969/j.issn.1004-4264.2012.08.026 . |
| LING L. Effect of artificial insemination on reproductive performance of female rabbits[J]. Jilin Anim Husb Vet Med, 2022, 43(4):89-90. DOI: 10.3969/j.issn.1004-4264.2012.08.026 . | |
| [21] | 王家洁, 杨祺颖, 石宏. 非人灵长类动物模型在女性不孕症研究中的运用[J]. 生命科学, 2022, 34(11):1456-1464. DOI: 10.13376/j.cbls/2022159 . |
| WANG J J, YANG Q Y, SHI H. The use of non-human primate models in female infertility research[J]. Chin Bull Life Sci, 2022, 34(11):1456-1464. DOI: 10.13376/j.cbls/2022159 . | |
| [22] | 杜永洪, 赖国旗, 邹建中, 等. 重庆地区雌性恒河猴生殖生理的季节性变化[J]. 四川动物, 2008, 27(2):197-200. DOI: CNKI:SUN:SCDW.0.2008-02-010 . |
| DU Y H, LAI G Q, ZOU J Z, et al. Seasonal changes of reproductive physiology in female Rhesus monkey in Chongqing[J]. Sichuan J Zool, 2008, 27(2):197-200. DOI: CNKI:SUN:SCDW.0.2008-02-010 . | |
| [23] | LUETJENS C M, WEINBAUER G F. Functional assessment of sexual maturity in male macaques (Macaca fascicularis)[J]. Regul Toxicol Pharmacol, 2012, 63(3):391-400. DOI:10.1016/j.yrtph.2012.05.003 . |
| [24] | KU W W, PAGLIUSI F, FOLEY G, et al. A simple orchidometric method for the preliminary assessment of maturity status in male Cynomolgus monkeys (Macaca fascicularis) used for nonclinical safety studies[J]. J Pharmacol Toxicol Methods, 2010, 61(1):32-37. DOI:10.1016/j.vascn.2009.10.006 . |
| [25] | PLANT T M. Neurobiological bases underlying the control of the onset of puberty in the Rhesus monkey: a representative higher primate[J]. Front Neuroendocrinol, 2001, 22(2):107-139. DOI:10.1006/frne.2001.0211 . |
| [26] | PLANT T M, RAMASWAMY S. Kisspeptin and the regulation of the hypothalamic–pituitary–gonadal axis in the Rhesus monkey (Macaca mulatta)[J]. Peptides, 2009, 30(1):67-75. DOI:10.1016/j.peptides.2008.06.029 . |
| [27] | 袁芳, 潘晓靓, 林海霞, 等. 非人灵长类模型在生殖发育毒性评价中的应用[J]. 中国新药杂志, 2012, 21(9):989-993, 1006. DOI: CNKI:SUN:ZXYZ.0.2012-09-012 . |
| YUAN F, PAN X L, LIN H X, et al. Nonhuman Primates as animal models for developmental and reproductive toxicity study[J]. Chin J New Drugs, 2012, 21(9):989-993, 1006. DOI: CNKI:SUN:ZXYZ.0.2012-09-012 . | |
| [28] | NIEHOFF M O, BERGMANN M, WEINBAUER G F. Effects of social housing of sexually mature male Cynomolgus monkeys during general and reproductive toxicity evaluation[J]. Reprod Toxicol, 2010, 29(1):57-67. DOI:10.1016/j.reprotox. 2009.09.007 . |
| [29] | LIU X, LIU X Y, WANG X Q, et al. Multi-omics analysis reveals changes in tryptophan and cholesterol metabolism before and after sexual maturation in captive macaques[J]. BMC Genomics, 2023, 24(1):308. DOI:10.1186/s12864-023-09404-3 . |
| [30] | ANTJE F, EBERHARD B, F G W. Embryo fetal development studies in nonhuman primates[J]. Methods Mol Biol, 2013, (947)169-183.DOI: 10.1007/978-1-62703-131-8_14 . |
| [31] | BUSE E, HABERMANN G, OSTERBURG I, et al. Reproductive/developmental toxicity and immunotoxicity assessment in the nonhuman primate model[J]. Toxicology, 2003, 185(3):221-227. DOI:10.1016/s0300-483x(02)00614-5 . |
| [32] | 花秀春, 时彦胜, 孙兆增, 等. 人工饲养恒河猴、食蟹猴的繁殖性能初报[J]. 中国实验动物学报, 2009, 17(3):219-221. DOI: 10.3969/j.issn.1005-4847.2009.03.015 . |
| HUA X C, SHI Y S, SUN Z Z, et al. Preliminary study on the reproductive characteristics of Rhesus and Cynomolgus monkeys bred in captivity in Beijing area[J]. Acta Lab Animalis Sci Sin, 2009, 17(3):219-221. DOI: 10.3969/j.issn.1005-4847.2009.03.015 . | |
| [33] | 王双棋, 韦秋奖, 江海天, 等. 昆明地区食蟹猴种群繁殖规律与繁殖率的分析[J]. 现代畜牧兽医, 2024(3):64-67. DOI: 10.20154/j.cnki.issn1672-9692.2024.03.015 . |
| WANG S Q, WEI Q J, JIANG H T, et al. Analysis of reproductive rule and reproductive rate of Cynomolgus monkey population in Kunming area[J]. Mod J Anim Husb Vet Med, 2024(3):64-67. DOI: 10.20154/j.cnki.issn1672-9692.2024.03.015 . |
| [1] | 王晗玥, 陈嘉玮, 高湘滨, 罗威, 刘素宁. 模式生物黑腹果蝇心侧体功能的研究概述[J]. 实验动物与比较医学, 2025, (): 1-14. |
| [2] | 孙强. 非动物实验替代知多少[J]. 实验动物与比较医学, 2025, 45(4): 508-514. |
| [3] | 刘月琴, 薛卫国, 王淑友, 申耀华, 贾术永, 王广军, 宋晓晶. 探头式激光共聚焦成像技术用于小鼠消化道组织形态特征分析[J]. 实验动物与比较医学, 2025, 45(4): 457-465. |
| [4] | 郑卿勇, 杨冬华, 马智超, 周姿余, 陆洋, 王晶宇, 邢丽娜, 康迎英, 杜莉, 赵春香, 狄宝山, 田金徽. 动物实验系统评价与Meta分析报告的规范撰写建议[J]. 实验动物与比较医学, 2025, 45(4): 496-507. |
| [5] | 王庭君, 罗浩, 陈琦. 基于人工智能的实验动物中心信息化升级及应用实践[J]. 实验动物与比较医学, 2025, 45(4): 473-482. |
| [6] | 王娇祥, 张璐, 陈姝含, 角德灵, 赵恒, 魏太云, 郭建雄, 徐凯祥, 魏红江. GTKO/hCD55基因编辑异种器官移植供体猪的构建及功能验证[J]. 实验动物与比较医学, 2025, 45(4): 379-392. |
| [7] | 秦超, 李双星, 赵婷婷, 蒋晨晨, 赵晶, 杨艳伟, 林志, 王三龙, 文海若. 药物安全评价用SD大鼠90 d喂养试验的背景数据研究[J]. 实验动物与比较医学, 2025, 45(4): 439-448. |
| [8] | 焦青贞, 吴桂华, 唐雯, 樊帆, 冯凯, 杨春响, 乔建, 邓素芳. 暖通系统暂停送风下实验动物设施氨浓度的动态监测与分析[J]. 实验动物与比较医学, 2025, 45(4): 490-495. |
| [9] | 刘文涛, 罗艳红, 龙永霞, 罗启慧, 陈正礼, 刘丽达. 四川省实验动物设施常见环境问题及检测经验[J]. 实验动物与比较医学, 2025, 45(4): 483-489. |
| [10] | 赵鑫, 王晨曦, 石文清, 娄月芬. 斑马鱼在炎症性肠病机制及药物研究中的应用进展[J]. 实验动物与比较医学, 2025, 45(4): 422-431. |
| [11] | 贡磊磊, 王晓霞, 封学伟, 李心蕾, 赵涵, 张雪艳, 冯欣. 不同浓度环磷酰胺诱导早发性卵巢功能不全小鼠模型及作用机制研究[J]. 实验动物与比较医学, 2025, 45(4): 403-410. |
| [12] | 林振华, 褚祥宇, 魏振西, 董传俊, 赵增琳, 孙晓霞, 李庆雨, 张琪. 椎体成形术用于实验猪体内骨水泥安全性及有效性评价[J]. 实验动物与比较医学, 2025, 45(4): 466-472. |
| [13] | 姜娟, 宋宁, 连文博, 邵丛丛, 顾文文, 石燕. 两种浓度乙醇溶液灌注建立小鼠宫腔粘连模型的组织病理和分子病理表型比较[J]. 实验动物与比较医学, 2025, 45(4): 393-402. |
| [14] | 陈子宜, 孙红燕, 康品方, 武文娟. 肺动脉高压动物实验模型的研究进展[J]. 实验动物与比较医学, 2025, (): 1-12. |
| [15] | 徐英韬, 王蒙蒙, 林平, 迟海涛, 王怡, 白鹰. 外泌体通过NRF2/SLC7A11/GPX4通路调控铁死亡治疗小鼠缺血性脑卒中[J]. 实验动物与比较医学, 2025, (): 1-11. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||