












实验动物与比较医学 ›› 2022, Vol. 42 ›› Issue (5): 384-392.DOI: 10.12300/j.issn.1674-5817.2022.050
收稿日期:2022-04-10
修回日期:2022-07-16
出版日期:2022-10-25
发布日期:2022-10-25
作者简介:高晨(1977—),男,博士,副主任医师,研究生导师,主要从事发作性神经系统疾病相关研究。E-mail: gc2006418@163.com。ORCID:0000-0002-4699-099X
基金资助:
Chen GAO( )(
)( ), Chunling FAN, Yurong LI, Wenjuan PEI, Caiping GUAN
), Chunling FAN, Yurong LI, Wenjuan PEI, Caiping GUAN
Received:2022-04-10
Revised:2022-07-16
Published:2022-10-25
Online:2022-10-25
Contact:
GAO Chen (ORCID: 0000-0002-4699-099X), E-mail: gc2006418@163.com摘要:
目的 探讨高原低氧环境下运动疲劳适应性的产生与大脑皮层乳酸转运及代谢状态的关联性。 方法 63只清洁级SD大鼠随机分为对照组、常规运动组、急进高原组、高原习服3 d组、高原习服1周组、高原习服2周组及给予乳酸转运体抑制剂组(简称高原抑制剂组)。除对照组外,各组大鼠分别在常压常氧或模拟高原低压低氧条件下建立跑台力竭运动疲劳模型。以平均力竭时间确定大鼠运动疲劳的变化趋势。采用免疫印迹和免疫组织化学法检测运动区皮层单羧酸转运蛋白2(monocarboxylate transporters 2,MCT2)和MCT4的表达。采用尼氏染色进行大脑皮层迟发性神经元病理改变评价,并测定鼠脑乳酸含量。 结果 急进高原组和高原抑制剂组大鼠平均力竭时间分别为(61.00±6.55)min和(71.25±9.59)min,显著低于常规运动组[(124.75±9.36)min]和高原习服2周组[(100.25±9.74)min,P<0.05)]。免疫印迹检测提示,高原习服2周组及高原抑制剂组鼠脑运动区皮层MCT2表达水平较对照组明显升高,分别为120.6%和164.4%(P<0.05);MCT4的表达分别为174.6%和168.8%(P<0.05)。免疫组织化学检测印证了免疫印迹结果。模拟高原环境下,各组鼠脑乳酸平均含量较常规运动组[(0.175±0.021)mmoL/g]均明显升高(P<0.05)。病理学评价提示,急进高原组和高原抑制剂组鼠脑皮层平均神经元密度值(每个高倍视野的神经元数量)分别为46.75±8.65和63.50±7.65,较对照组(135.88±8.59)明显降低(P<0.05);高原习服2周组(121.75±16.00)与对照组差异无统计学意义(P >0.05)。 结论 高原习服后机体对运动疲劳的适应性与脑内MCT2和MCT4表达变化相关联,可作为运动疲劳医学干预的靶点。
中图分类号:
高晨,凡春玲,李玉荣,等. 模拟高原低氧环境下运动疲劳大鼠脑皮层单羧酸转运蛋白的变化及其意义[J]. 实验动物与比较医学, 2022, 42(5): 384-392. DOI: 10.12300/j.issn.1674-5817.2022.050.
Chen GAO,Chunling FAN,Yurong LI,et al. Changes in Expression of Monocarboxylate Transporters in the Rat Cerebral Cortex after Exercise-induced Fatigue Under Simulated High-altitude Hypoxia and its Significance[J]. Laboratory Animal and Comparative Medicine, 2022, 42(5): 384-392. DOI: 10.12300/j.issn.1674-5817.2022.050.
分组 Group | 平均运动力竭时间 Average exhaustion time /min |
|---|---|
| 常规运动组 NE group | 124.75±9.36 #△ |
| 急进高原组 REIA group | 61.00±6.55* |
| 高原习服3 d组 3 d AA group | 66.38±4.72* |
| 高原习服1周组 1 week AA group | 73.13±7.02* |
| 高原习服2周组 2 weeks AA group | 100.25±9.74#△ |
| 高原抑制剂组 MTI group | 71.25±9.59* |
表1 各组运动疲劳模型大鼠平均力竭时间 (xˉ±s, n=9)
Table 1 Average time–to–exhaustion in rats under exercise-induced fatigue conditions
分组 Group | 平均运动力竭时间 Average exhaustion time /min |
|---|---|
| 常规运动组 NE group | 124.75±9.36 #△ |
| 急进高原组 REIA group | 61.00±6.55* |
| 高原习服3 d组 3 d AA group | 66.38±4.72* |
| 高原习服1周组 1 week AA group | 73.13±7.02* |
| 高原习服2周组 2 weeks AA group | 100.25±9.74#△ |
| 高原抑制剂组 MTI group | 71.25±9.59* |
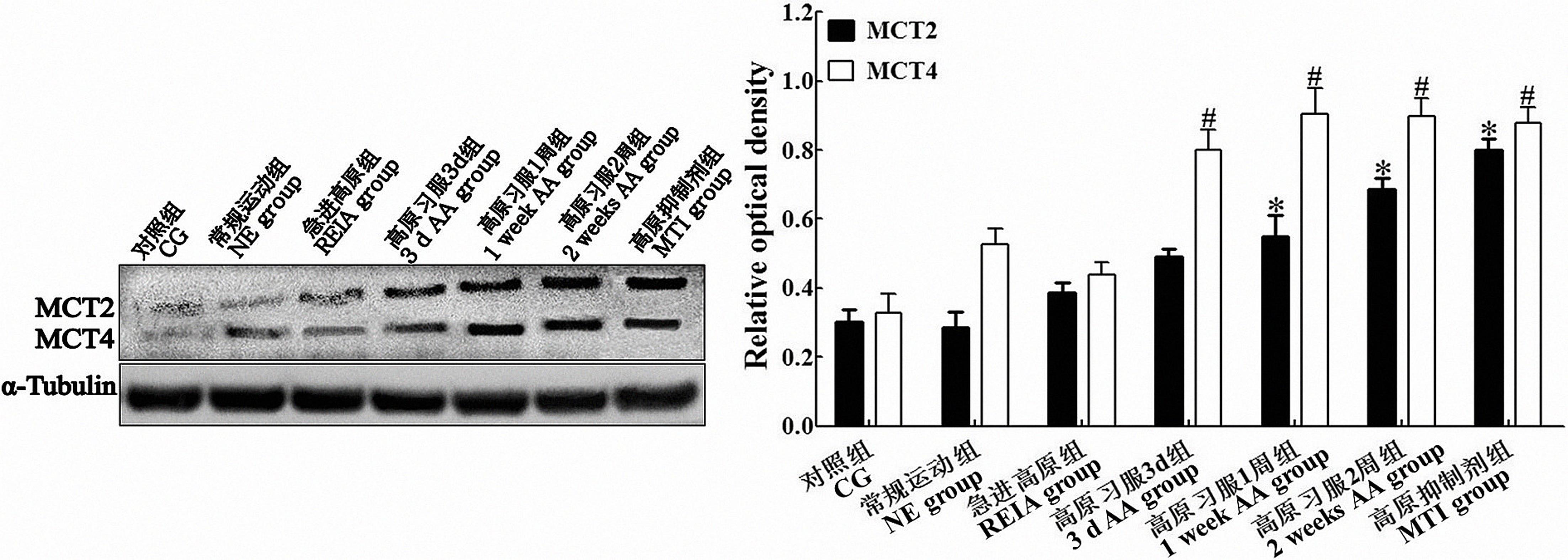
图1 免疫印迹检测各组运动疲劳模型大鼠脑运动皮层中MCT2和MCT4的表达注:MCT2与对照组比较,*P<0.05;MCT4与对照组比较,#P<0.05。单因素方差分析采信Bonferroni法结果,n=3。
Figure 1 Western blotting analysis of MCT2 and MCT4 expressions in the cerebral motor cortex of rats in each groupNote:CG: Control group; NE group: Normal exercise group; REIA group: Rush-entry-into-altitude group; 3 d AA group: 3-day-altitude acclimatization group; 1 week AA group: 1-week-altitude acclimatization group; 2 weeks AA group: 2-week-altitude acclimatization group; MTI group: Monocarboxylate transporter inhibitor group. *P<0.05, versus CG group for MCT2 quantitative analysis; #P<0.05, versus CG group for MCT4 quantitative analysis, ANOVA with post hoc Bonferroni correction. n=3 for each group.
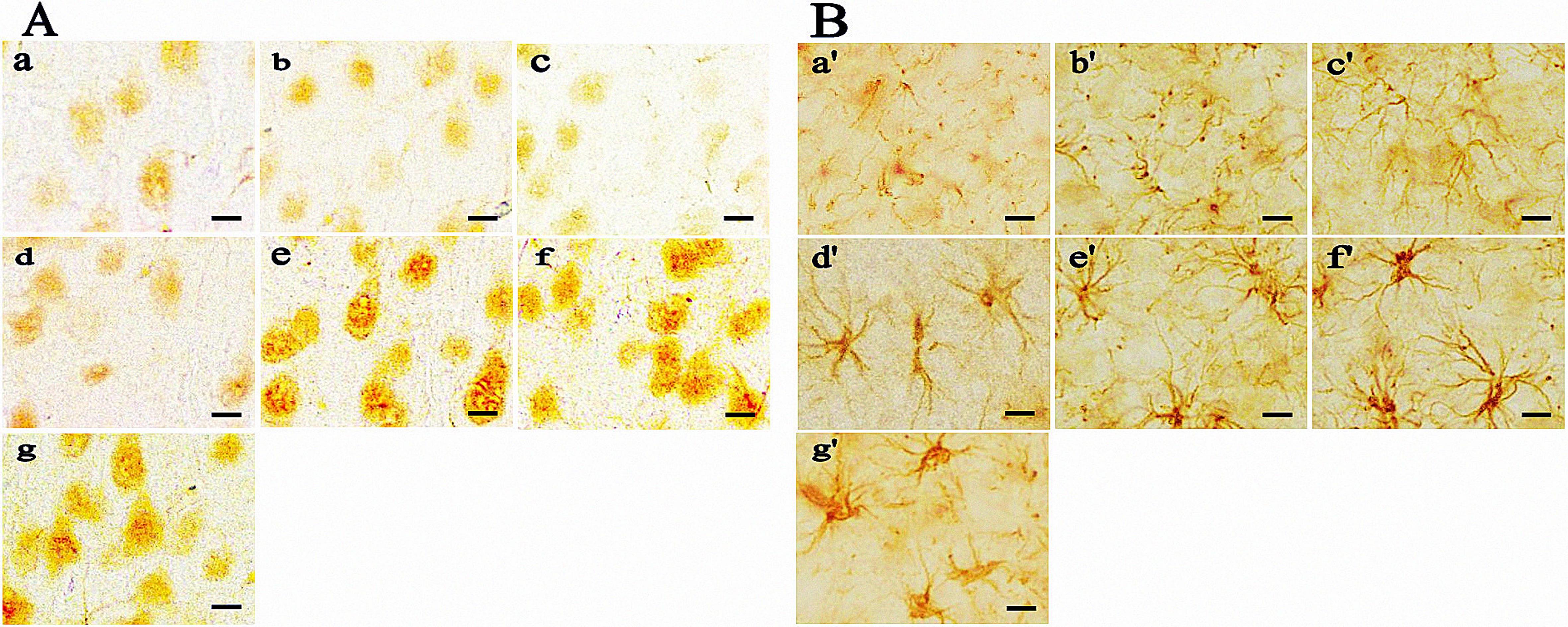
图2 免疫组织化学检测各组运动疲劳模型鼠脑运动皮层中MCT2(A)和MCT4(B)表达(DAB染色法,×400)注:A为大鼠脑运动皮层神经元MCT2表达;B为大鼠脑运动皮层星形胶质细胞MCT4表达。a和a’为对照组;b和b’为常规运动组;c和c’为急进高原组;d和d’为高原习服3 d组;e和e’为高原习服1周组;f和f’为高原习服2周组;g和g’为高原抑制剂组。比例尺大小为25 μm。
Figure 2 Immunohistochemical detection of MCT2 (A) and MCT4 (B) in the cerebral motor cortex of rats in each group (DAB staining, ×400)Note: A, MCT2 expression of neurons in the cerebral motor cortex of rats; B, MCT4 expression in astrocytes in the cerebral motor cortex of rats. a and a’, control group; b and b’, normal exercise group; c and c’, rush-entry-into-altitude group; d and d’, 3-day-altitude acclimatization group; e and e’, 1-week-altitude acclimatization group; f and f’, 2-week-altitude acclimatization group; g and g’, mono-carboxylate transporter inhibitor group. Scale bar = 25 μm.
分组 Group | 神经元组织学分级 Neuronal histological grade (count) | 平均神经元密度值 Average neuronal density/(cells·HPF-1) | |||
|---|---|---|---|---|---|
0级 0 grade | Ⅰ级 Ⅰ grade | Ⅱ级 Ⅱ grade | Ⅲ级 Ⅲ grade | ||
| 对照组 CG | 3 | 0 | 0 | 0 | 135.88±8.59 |
| 常规运动组 NE group | 2 | 1 | 0 | 0 | 123.88±6.71 |
| 急进高原组 REIA group | 0 | 0 | 2 | 1 | 46.75±8.65* |
| 高原习服3 d组 3 d AA group | 0 | 0 | 3 | 0 | 54.13±11.33* |
| 高原习服1周组 1 week AA group | 0 | 2 | 1 | 0 | 119.50±6.99* |
| 高原习服2周组 2 weeks AA group | 0 | 3 | 0 | 0 | 121.75±16.00 |
| 高原抑制剂组 MTI group | 0 | 1 | 2 | 0 | 63.50±7.65* |
表2 各组运动疲劳模型鼠脑运动皮层神经元组织学分级数量和平均神经元密度值 (xˉ±s, n=3)
Table 2 Histological grade and average neuronal density in the cerebral motor cortex of rats under exercise-induced fatigue conditions
分组 Group | 神经元组织学分级 Neuronal histological grade (count) | 平均神经元密度值 Average neuronal density/(cells·HPF-1) | |||
|---|---|---|---|---|---|
0级 0 grade | Ⅰ级 Ⅰ grade | Ⅱ级 Ⅱ grade | Ⅲ级 Ⅲ grade | ||
| 对照组 CG | 3 | 0 | 0 | 0 | 135.88±8.59 |
| 常规运动组 NE group | 2 | 1 | 0 | 0 | 123.88±6.71 |
| 急进高原组 REIA group | 0 | 0 | 2 | 1 | 46.75±8.65* |
| 高原习服3 d组 3 d AA group | 0 | 0 | 3 | 0 | 54.13±11.33* |
| 高原习服1周组 1 week AA group | 0 | 2 | 1 | 0 | 119.50±6.99* |
| 高原习服2周组 2 weeks AA group | 0 | 3 | 0 | 0 | 121.75±16.00 |
| 高原抑制剂组 MTI group | 0 | 1 | 2 | 0 | 63.50±7.65* |
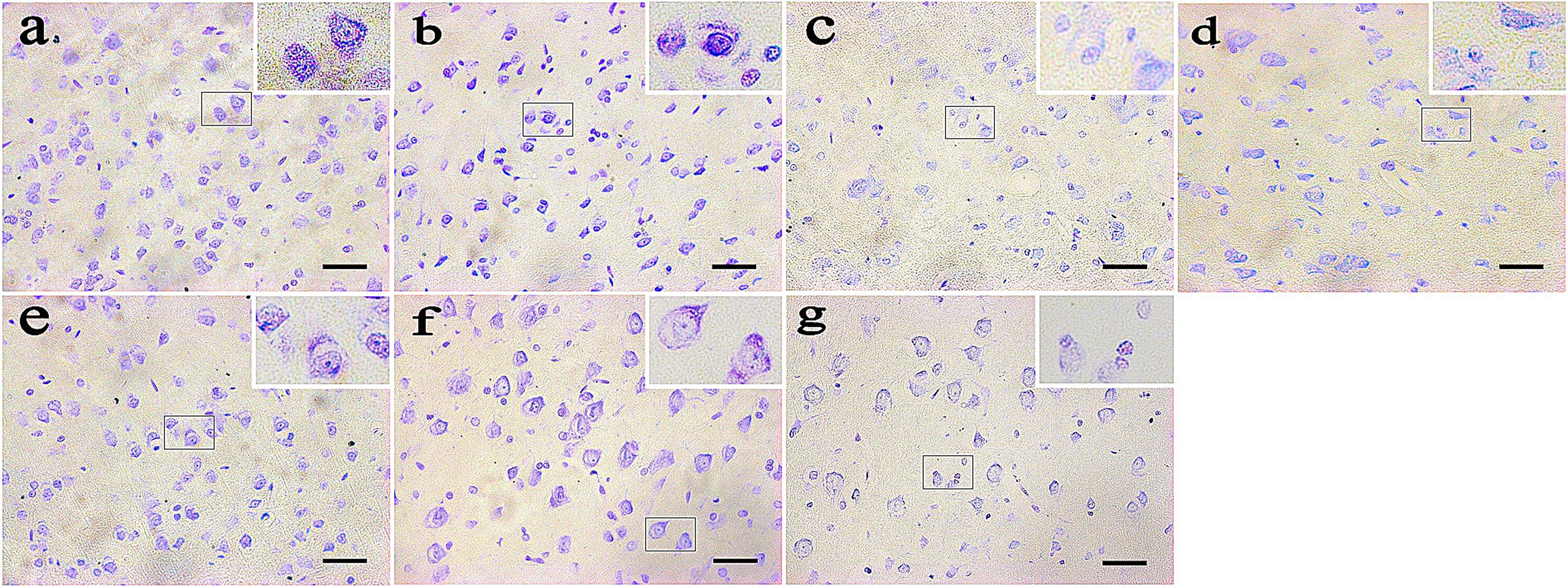
图3 各组运动疲劳模型鼠脑运动皮层神经元尼氏染色(×200,局部放大×400)注:a为对照组;b为常规运动组;c为急进高原组;d为高原习服3 d组;e为高原习服1周组;f为高原习服2周组;g为高原抑制剂组。比例尺大小为50 μm。
Figure 3 Nissl staining in the cerebral motor cortex of rats under exercise-induced fatigue conditions (×200,local enlarged image×400 )Note:a, control group; b, normal exercise group; c, rush-entry-into-altitude group; d, 3-day-altitude acclimatization group; e, 1-week-altitude acclimatization group; f, 2-week-altitude acclimatization group; g, monocarboxylate transporter inhibitor group. Scale bar = 50 μm.
分组 Group | 脑组织乳酸含量 Average brain lactate content/(mmol·g-1) |
|---|---|
| 对照组 CG | 0.163±0.011#△ |
| 常规运动组 NE group | 0.175±0.021#△ |
| 急进高原组 REIA group | 0.239±0.017*△ |
| 高原习服3 d组 3 d AA group | 0.247±0.012*△ |
| 高原习服1周组 1 week AA group | 0.397±0.017*#△ |
| 高原习服2周组 2 weeks AA group | 0.412±0.024*#△ |
| 高原抑制剂组 MTI group | 0.537±0.011*# |
表3 各组运动疲劳模型大鼠平均脑组织乳酸含量 (xˉ±s, n=3)
Table 3 Average brain lactate content in rats under exercise-induced fatigue conditions
分组 Group | 脑组织乳酸含量 Average brain lactate content/(mmol·g-1) |
|---|---|
| 对照组 CG | 0.163±0.011#△ |
| 常规运动组 NE group | 0.175±0.021#△ |
| 急进高原组 REIA group | 0.239±0.017*△ |
| 高原习服3 d组 3 d AA group | 0.247±0.012*△ |
| 高原习服1周组 1 week AA group | 0.397±0.017*#△ |
| 高原习服2周组 2 weeks AA group | 0.412±0.024*#△ |
| 高原抑制剂组 MTI group | 0.537±0.011*# |
| 1 | 邓树勋. 运动生理学[M]. 北京: 高等教育出版社, 1999. |
| DENG S X. Exercise physiology[M]. Beijing: Higher Education Press, 1999. | |
| 2 | 高强. 疲劳及其测定(上)[J]. 中国运动医学杂志, 1985, 4(3):179-183. DOI:10.16038/j.1000-6710.1985.03.019 . |
| GAO Q. Fatigue and its fatigue measurement (part one)[J]. Chin J Sports Med, 1985, 4(3):179-183. DOI:10.16038/j.1000-6710.1985.03.019 . | |
| 3 | 鲁建清, 满维祥, 罗荣保. 5-羟色胺与中枢运动疲劳的分析研究[J]. 湖南科技学院学报, 2010, 31(4):79-82. DOI:10.3969/j.issn.1673-2219.2010.04.023 . |
| LU J Q, MAN W X, LUO R B. An analyze 5-HT, fatigue and their relationships[J]. J Hunan Univ Sci Eng, 2010, 31(4):79-82. DOI:10.3969/j.issn.1673-2219.2010.04.023 . | |
| 4 | GIRARD O, MILLET G P. Neuromuscular fatigue in racquet sports [J]. Neurol Clin, 2008, 26(1):181-194. DOI: 10.1016/j.ncl. 2007.11.011 . |
| 5 | AMANN M, DEMPSEY J A. Ensemble input of group III/IV muscle afferents to CNS: a limiting factor of central motor drive during endurance exercise from normoxia to moderate hypoxia[J]. Adv Exp Med Biol, 2016, 903:325-342. DOI:10.1007/978-1-4899-7678-9_22 . |
| 6 | DAVIS J M, ALDERSON N L, WELSH R S. Serotonin and central nervous system fatigue: nutritional considerations[J]. Am J Clin Nutr, 2000, 72(2):573S-578S. DOI:10.1093/ajcn/72.2.573S . |
| 7 | 刘红平, 杨国愉, 张晶轩, 等. 急进高原驻训军人躯体-脑力疲劳追踪研究[J]. 西北国防医学杂志, 2017, 38(9):565-569. DOI:10.16021/j.cnki.1007-8622.2017.09.002 . |
| LIU H P, YANG G Y, ZHANG J X, et al. Investigation of physical-mental fatigue on armymen acutely entering in plateau[J]. Med J Natl Defending Forces Northwest China, 2017, 38(9):565-569. DOI:10.16021/j.cnki.1007-8622.2017.09.002 . | |
| 8 | 陶文迪, 田秀玉, 李茂星, 等. 黄芪水提取物对高原缺氧大鼠运动能力的影响[J]. 解放军医药杂志, 2019, 31(12):12-18. DOI:10.3969/j.issn.2095-140X.2019.12.003 . |
| TAO W D, TIAN X Y, LI M X, et al. Effect of Astragalus membranaceus aqueous extract on ability of plateau hypoxia exercise in rats[J]. Med & Pharm J Chin People's Liberation Army, 2019, 31(12):12-18. DOI:10.3969/j.issn.2095-140X.2019.12.003 . | |
| 9 | DHILLON H S, CARMAN H M, ZHANG D, et al. Severity of experimental brain injury on lactate and free fatty acid accumulation and Evans blue extravasation in the rat cortex and hippocampus[J]. J Neurotrauma, 1999, 16(6):455-469. DOI:10.1089/neu.1999.16.455 . |
| 10 | 王静, 刘洪涛. 脑乳酸对运动性中枢疲劳的作用及影响[J]. 中国临床康复, 2004, 8(22):4572-4573. DOI:10.3321/j.issn: 1673-8225.2004.22.102 . |
| WANG J, LIU H T. Effect of brain lactic acid on exercise-induced central fatigue[J]. Chin J Clin Rehabil, 2004, 8(22):4572-4573. DOI:10.3321/j.issn: 1673-8225.2004.22.102 . | |
| 11 | 杨东升, 刘晓莉, 乔德才. "乳酸穿梭"背景下的运动性疲劳中枢机制研究新进展[J]. 中国康复医学杂志, 2012,27(3):285-288. DOI:10.3969/j.issn.1001-1242.2012.03.024 . |
| YANG D S, LIU X L, QIAO D C. New progress of research on central mechanism of exercise-induced fatigue under the background of lactic acid shuttle[J]. Chin J Rehabil Med, 2012, 27(3):285-288. DOI:10.3969/j.issn.1001-1242.2012.03.024 . | |
| 12 | PELLERIN L, MAGISTRETTI P J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization[J]. Proc Natl Acad Sci USA, 1994, 91(22):10625-10629. DOI:10.1073/pnas. 91.22.10625 . |
| 13 | SCHURR A. Glycolysis paradigm shift dictates a reevaluation of glucose and oxygen metabolic rates of activated neural tissue[J]. Front Neurosci, 2018, 12:700. DOI:10.3389/fnins. 2018.00700 . |
| 14 | GAO C, ZHOU L Y, ZHU W X, et al. Monocarboxylate transporter-dependent mechanism confers resistance to oxygen- and glucose-deprivation injury in astrocyte-neuron co-cultures[J]. Neurosci Lett, 2015, 594:99-104. DOI:10.1016/j.neulet.2015.03.062 . |
| 15 | HALESTRAP A P. The SLC16 gene family - Structure, role and regulation in health and disease[J]. Mol Aspects Med, 2013, 34(2-3):337-349. DOI:10.1016/j.mam.2012.05.003 . |
| 16 | GAO C, ZHU W X, TIAN L Z, et al. MCT4-Mediated Expression of EAAT1 is Involved in the Resistance to Hypoxia Injury in Astrocyte-Neuron co-Cultures[J]. Neurochem Res, 2015, 40(4):818-828. DOI:10.1007/s11064-015-1532-2 . |
| 17 | BEDFORD T G, TIPTON C M, WILSON N C, et al. Maximum oxygen consumption of rats and its changes with various experimental procedures[J]. J Appl Physiol Respir Environ Exerc Physiol, 1979, 47(6):1278-1283. DOI:10.1152/jappl.1979.47.6.1278 . |
| 18 | 胡琰茹, 乔德才, 刘晓莉. 力竭运动过程中大鼠苍白球内侧部对皮层的调控作用[J]. 中国运动医学杂志, 2013, 32(5):420-425, 419. DOI:10.16038/j.1000-6710.2013.05.001 . |
| HU Y R, QIAO D C, LIU X L. Modulatory effect of the internal segment of globus pallidus on motor cortex activity during exhaustive exercise[J]. Chin J Sports Med, 2013, 32(5):420-425, 419. DOI:10.16038/j.1000-6710.2013.05.001 . | |
| 19 | SCHURR A, PAYNE R S, MILLER J J, et al. Blockade of lactate transport exacerbates delayed neuronal damage in a rat model of cerebral ischemia[J]. Brain Res, 2001, 895(1-2):268-272. DOI:10.1016/S0006-8993(01)02082-0 . |
| 20 | GAO C, WANG C, LIU B, et al. Intermittent hypoxia preconditioning-induced epileptic tolerance by upregulation of monocarboxylate transporter 4 expression in rat hippocampal astrocytes[J]. Neurochem Res, 2014, 39(11):2160-2169. DOI:10.1007/s11064-014-1411-2 . |
| 21 | LI D Y, LIU X Y, LIU T M, et al. Neurochemical regulation of the expression and function of glial fibrillary acidic protein in astrocytes[J]. Glia, 2020, 68(5):878-897. DOI:10.1002/glia.23734 . |
| 22 | GAO C, LI Z Y, BAI J, et al. Involvement of monocarboxylate transporters in the cross-tolerance between epilepsy and cerebral infarction: a promising choice towards new treatments[J]. Neurosci Lett, 2019, 707:134305. DOI:10.1016/j.neulet.2019.134305 . |
| 23 | 高晨, 白洁, 雷鹏, 等. 低氧预处理对氯化锂-匹鲁卡品致痫大鼠的保护作用[J]. 中华实验外科杂志, 2018, 35(1):111-115. DOI:10.3760/cma.j.issn.1001-9030.2018.01.037 . |
| GAO C, BAI J, LEI P, et al. The protective effect of hypoxia preconditioning on epilepsy induced by lithium-pilocarpine in rats[J]. Chin J Exp Surg, 2018, 35(1):111-115. DOI:10.3760/cma.j.issn.1001-9030.2018.01.037 . | |
| 24 | ZHANG L S, LI J, LIN A N. Assessment of neurodegeneration and neuronal loss in aged 5XFAD mice[J]. STAR Protoc, 2021, 2(4):100915. DOI:10.1016/j.xpro.2021.100915 . |
| 25 | 武柠子, 马慧萍, 王昕, 等. 模拟高原缺氧环境对大鼠心、脑组织损伤的研究[J]. 药学实践杂志, 2018, 36(3):250-254. DOI:10.3969/j.issn.1006-0111.2018.03.013 . |
| WU N Z, MA H P, WANG X, et al. Study on myocardium and brain damage in rats by simulating high altitude[J]. J Pharm Pract, 2018, 36(3):250-254. DOI:10.3969/j.issn.1006-0111.2018.03.013 . | |
| 26 | 高钰琪. 高原军事医学[M]. 重庆: 重庆出版社, 2005: 180-220. |
| GAO Y Q. High altitude military medicine[M]. Chongqing: Chongqing Publishing House, 2005: 180-220. | |
| 27 | 程泽鹏, 冯钰, 史仍飞. 运动过程中单羧酸转运蛋白(MCTs)作用的研究进展[J]. 军事体育学报, 2017, 36(3):89-94. DOI:10.3969/j.issn.1671-1300.2017.03.025 . |
| CHENG Z P, FENG Y, SHI R F. Research on MCTs function during the exercise[J]. J Mil Phys Educ Sports, 2017, 36(3):89-94. DOI:10.3969/j.issn.1671-1300.2017.03.025 . | |
| 28 | 高晨, 王菀, 李玉荣, 等. 运动疲劳状态下大鼠脑皮层单羧酸转运蛋白表达的变化及意义[J]. 实验动物与比较医学, 2022, 42(1):42-47. DOI:10.12300/j.issn.1674-5817.2021-053 . |
| GAO C, WANG W, LI Y R, et al. Expression and significance of monocarboxylate transporters in cortex of rats after exercise-induced fatigue[J]. Lab Animal Comp Med, 2022, 42(1):42-47. DOI:10.12300/j.issn.1674-5817.2021-053 . | |
| 29 | ÓRDENES P, VILLAR P S, TARIFEÑO-SALDIVIA E, et al. Lactate activates hypothalamic POMC neurons by intercellular signaling[J]. Sci Rep, 2021, 11:21644. DOI:10.1038/s41598-021-00947-7 . |
| 31 | GUAN X W, MORRIS M E. In vitro and In vivo efficacy of AZD3965 and alpha-cyano-4-hydroxycinnamic acid in the murine 4T1 breast tumor model[J]. AAPS J, 2020, 22(4):84. DOI:10.1208/s12248-020-00466-9 . |
| 32 | YU J T, WEI Z X, LI Q, et al. Advanced cancer starvation therapy by simultaneous deprivation of lactate and glucose using a MOF nanoplatform[J]. Adv Sci (Weinh), 2021, 8(19): e2101467. DOI:10.1002/advs.202101467 . |
| 33 | GARCIA C K, BROWN M S, PATHAK R K, et al. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1[J]. J Biol Chem, 1995, 270(4):1843-1849. DOI:10.1074/jbc.270.4.1843 . |
| 34 | BRÖER S, BRÖER A, SCHNEIDER H P, et al. Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes[J]. Biochem J, 1999, 341(Pt 3):529-535. DOI:10.1042/0264-6021:3410529 . |
| 35 | ERLICHMAN J S, HEWITT A, DAMON T L, et al. Inhibition of monocarboxylate transporter 2 in the retrotrapezoid nucleus in rats: a test of the astrocyte-neuron lactate-shuttle hypothesis[J]. J Neurosci, 2008, 28(19):4888-4896. DOI:10.1523/JNEUROSCI.5430-07.2008 . |
| 36 | PARKIN G M, UDAWELA M, GIBBONS A, et al. Glutamate transporters, EAAT1 and EAAT2, are potentially important in the pathophysiology and treatment of schizophrenia and affective disorders[J]. World J Psychiatry, 2018, 8(2):51-63. DOI:10.5498/wjp.v8.i2.51 . |
| [1] | 秦超, 李双星, 赵婷婷, 蒋晨晨, 赵晶, 杨艳伟, 林志, 王三龙, 文海若. 药物安全评价用SD大鼠90 d喂养试验的背景数据研究[J]. 实验动物与比较医学, 2025, 45(4): 439-448. |
| [2] | 刘力瑜, 嵇波, 刘小玄, 方洋, 张玲, 郭亭廷, 全烨, 李鹤文, 刘翼天. 大鼠胎儿期肺组织固定方法的探索[J]. 实验动物与比较医学, 2025, 45(4): 432-438. |
| [3] | 潘颐聪, 蒋汶洪, 胡明, 覃晓. 慢性肾脏病大鼠主动脉钙化模型的术式优化及效果评价[J]. 实验动物与比较医学, 2025, 45(3): 279-289. |
| [4] | 刘智伟, 杨然, 连浩, 张玉, 金立伦. 秦皮素对碘乙酸钠诱导骨关节炎模型大鼠的软骨保护与抗炎作用[J]. 实验动物与比较医学, 2025, 45(3): 259-268. |
| [5] | 姜萌, 郝淑兰, 仝立国, 仲启明, 高振飞, 王永辉, 王晞星, 吉海杰. 长春瑞滨诱导大鼠足背静脉炎模型的动态评价[J]. 实验动物与比较医学, 2025, 45(3): 251-258. |
| [6] | 肖林林, 杨逸萱, 黎珊杉, 罗兰诗雨, 尹思威, 孙俊铭, 施维, 欧阳轶强, 李习艺. 利用脑立体定位技术将人源三突变APP基因导入海马区构建阿尔茨海默病大鼠模型[J]. 实验动物与比较医学, 2025, 45(3): 269-278. |
| [7] | 连辉, 姜艳玲, 刘佳, 张玉立, 谢伟, 薛晓鸥, 李健. 异常子宫出血大鼠模型的构建与评价[J]. 实验动物与比较医学, 2025, 45(2): 130-146. |
| [8] | 何雨昕, 白振忠, 薛华, 郭子旭, 曹学锋. 基于UHPLC-QE-MS的高原鼠兔肾脏差异代谢物及低氧适应机制分析[J]. 实验动物与比较医学, 2025, 45(1): 3-12. |
| [9] | 孙效容, 苏丹, 贵文娟, 陈玥. 手术诱导大鼠中重度膝骨关节炎模型的建立与评价[J]. 实验动物与比较医学, 2024, 44(6): 597-604. |
| [10] | 殷玉莲, 马丽娜, 屠思远, 陈玲, 叶媚娜, 陈红风. 非哺乳期乳腺炎大鼠模型的建立及评价[J]. 实验动物与比较医学, 2024, 44(6): 587-596. |
| [11] | 杨劲, 俞诗雅, 林楠, 方永超, 赵虎, 邱锦维, 林鸿铭, 陈惠燕, 王瑜, 吴伟航. 改良型十二指肠旷置术对2型糖尿病大鼠糖代谢的影响[J]. 实验动物与比较医学, 2024, 44(5): 523-530. |
| [12] | 戚龙菊, 陈世园, 廖泽华, 石袁虎, 孙郁雨, 王庆华. 经血干细胞移植联合运动训练促进大鼠脊髓损伤康复的转录组学分析[J]. 实验动物与比较医学, 2024, 44(5): 531-542. |
| [13] | 张乃群, 袁飘漂, 曹琳茸, 应娜, 杨涛涛. PNR检测在糖尿病肾脏疾病模型大鼠诊断及药效评价中的应用[J]. 实验动物与比较医学, 2024, 44(5): 543-549. |
| [14] | 郑艺清, 邓亚胜, 范燕萍, 梁天薇, 黄慧, 刘永辉, 倪召兵, 林江. 基于数据挖掘的盆腔炎性疾病动物模型应用分析[J]. 实验动物与比较医学, 2024, 44(4): 405-418. |
| [15] | 肖攀, 王红义, 陆璐, 张梅, 陈克明, 申栋帅, 牛廷献. 低氧敏感和低氧耐受型Wistar大鼠筛选及其G1代的低氧敏感性初探[J]. 实验动物与比较医学, 2024, 44(4): 374-383. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||