













实验动物与比较医学 ›› 2024, Vol. 44 ›› Issue (5): 531-542.DOI: 10.12300/j.issn.1674-5817.2024.031
戚龙菊1( ), 陈世园1,3, 廖泽华1,3, 石袁虎1,3, 孙郁雨1, 王庆华2(
), 陈世园1,3, 廖泽华1,3, 石袁虎1,3, 孙郁雨1, 王庆华2( )(
)( )
)
收稿日期:2024-02-27
修回日期:2024-08-06
出版日期:2024-10-25
发布日期:2024-10-25
通讯作者:
王庆华(1978—),男,博士,讲师,研究方向为脊髓损伤后功能康复。E-mail: wangqh@ntu.edu.cn。ORCID: 0000-0003-3661-6408作者简介:戚龙菊(1985—),女,硕士,副主任护师,研究方向为脊髓损伤后功能康复。E-mail: qilongjunt@163.com
基金资助:
QI Longju1( ), CHEN Shiyuan1,3, LIAO Zehua1,3, SHI Yuanhu1,3, SUN Yuyu1, WANG Qinghua2(
), CHEN Shiyuan1,3, LIAO Zehua1,3, SHI Yuanhu1,3, SUN Yuyu1, WANG Qinghua2( )(
)( )
)
Received:2024-02-27
Revised:2024-08-06
Published:2024-10-25
Online:2024-10-25
Contact:
WANG Qinghua (ORCID: 0000-0003-3661-6408), E-mail: wangqh@ntu.edu.cn摘要:
目的 通过转录组测序分析探讨经血干细胞(menstrual blood-derived stem cells,MenSCs)移植联合运动训练治疗脊髓损伤(spinal cord injury,SCI)大鼠的潜在干预靶点和分子机制。 方法 选用SPF级2月龄雌性SD大鼠,采用第十胸椎(T10)处半切方式构建SCI模型后,分为SCI后MenSCs移植联合运动训练组[简称细胞与跑步机训练(cell and treadmill training,CTMT)组]和SCI组(作为对照),每组12只大鼠。其中,CTMT组大鼠在建模后1周于损伤局部显微注射MenSCs 1×105个,随后进行为期2周的减重有氧运动训练。选取损伤处的脊髓组织进行转录组测序分析,获得SCI组和CTMT组大鼠的脊髓组织中mRNA表达数据,进行基因表达差异分析、GO功能富集分析、KEGG通路富集分析和蛋白质互作网络分析。同时,采用BBB评分评估两组大鼠的运动功能康复情况,苏木精-伊红(hematoxylin and eosin,HE)染色评估两组大鼠损伤局部的组织病理学改善程度,采用实时荧光定量PCR法和蛋白质印迹法对差异基因表达进行验证。 结果 转录组测序分析差异基因的表达数据显示,与SCI组相比,CTMT组有247个上调基因及174个下调基因,其中Bdnf、Hmox1、Sd4、Mmp3和Cd163等基因显著上调[|log2(FoldChange)|≥0.66,P<0.05]。KEGG通路富集分析与GO功能富集分析提示,这些差异基因主要参与了生长发育、代谢反应及免疫炎症过程,例如轴突生长、电子传递链等。其中,Bdnf基因富集在PI3K-Akt信号通路。BBB评分显示,MenSCs移植联合运动训练显著提高SCI大鼠的运动能力。HE染色提示治疗组大鼠的损伤局部病理变化程度显著减轻。实时荧光定量PCR法和蛋白质印迹法证明,CTMT组脊髓组织中脑源性神经营养因子(brain-derived neurotrophic factor,BDNF)mRNA和蛋白表达水平均显著高于SCI组(P<0.001)。 结论 MenSCs移植联合运动训练治疗可能通过上调BDNF表达促进SCI大鼠运动功能的恢复,这为SCI的临床康复治疗提供了一个新思路。
中图分类号:
戚龙菊,陈世园,廖泽华,等. 经血干细胞移植联合运动训练促进大鼠脊髓损伤康复的转录组学分析[J]. 实验动物与比较医学, 2024, 44(5): 531-542. DOI: 10.12300/j.issn.1674-5817.2024.031.
QI Longju,CHEN Shiyuan,LIAO Zehua,et al. Transcriptomic Analysis of Menstrual Blood-Derived Stem Cells Transplantation Combined with Exercise Training in Promoting Spinal Cord Injury Recovery in Rats[J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 531-542. DOI: 10.12300/j.issn.1674-5817.2024.031.
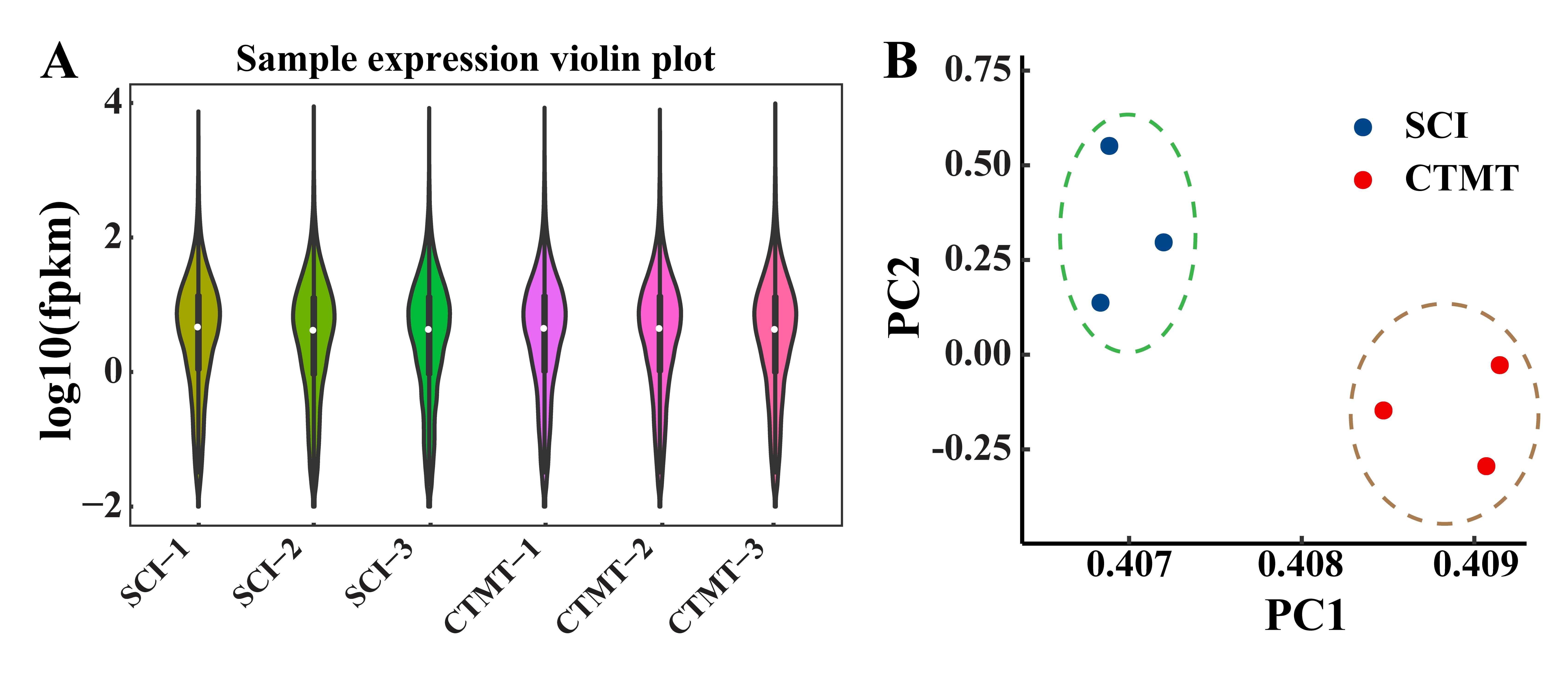
图1 两组大鼠的脊髓组织样本间基因表达量分布情况和主成分分析注:A为样本表达量分布情况(FPKM,每千个碱基的转录每百万映射读取的片段),可见SCI组(即单纯脊髓损伤手术组,作为对照,n=3)和CTMT组(即SCI手术后经血干细胞移植联合运动训练组,n=3)各样本的中位数、四分位数范围和极值均较为接近,表明测序结果在组内均一性较好,测序数据质量可靠。B为主成分分析(PC1,第一主成分;PC2,第二主成分),可见同组样品聚类在比较接近的空间内,而样本间差异主要来源于组间差异。
Figure 1 Distribution of gene expression and principal component analysis of spinal cord tissue samples in the two groups of ratsNote:A showed the distribution of sample expression levels (FPKM, fragments per kilobase of exon model per million mapped fragments); in the figure, the median, interquartile range and extreme values of the samples in SCI (spinal cord injury, as the control, n=3) group and the CTMT (the transplantation of menstrual blood-derived stem cells combined with treadmill training after SCI surgery, n=3) group were relatively close, which indicated that the sequencing results were more homogeneous within the group and the sequencing data quality was high. B showed the principal components analysis (PC1, principal component 1; PC2, principal component 2), and the samples from the same group were clustered together with close proximity, and the differences between samples mainly arose from intergroup differences.
表达上调基因 Up-regulated gene | log2(差异倍数) log2 (FoldChange) | t值 t value | P值 P value | 表达下调基因 Down-regulated gene | log2(差异倍数) log2 (FoldChange) | t值 t value | P值 P value | |
|---|---|---|---|---|---|---|---|---|
| Psme3 | 2.542 363 895 | 2.672 366 157 | 0.040 381 267 | Ptpdc1 | -0.941 169 616 | -2.823 151 331 | 0.033 418 310 | |
| Fgd2 | 1.870 409 798 | 5.277 683 881 | 0.002 476 179 | RT1-N2 | -0.961 937 408 | -3.508 946 559 | 0.014 746 432 | |
| Sdc4 | 1.591 559 898 | 2.530 079 050 | 0.048 412 378 | Cd79al | -1.007 349 648 | -3.292 831 274 | 0.018 934 378 | |
| Cthrc1 | 1.259 963 833 | 3.177 793 692 | 0.021 693 595 | Six4 | -1.071 889 332 | -15.482 205 520 | 0.000 009 930 | |
| RGD1302996 | 1.205 943 515 | 12.811 980 370 | 0.000 027 300 | Matn2 | -1.137 472 728 | -3.927 529 073 | 0.009 277 287 | |
| Rabepk | 1.201 118 420 | 2.962 815 910 | 0.028 125 989 | Clec4a3 | -1.273 541 156 | -2.512 270 495 | 0.049 532 946 | |
| Bdnf | 1.141 863 432 | 3.525 110 815 | 0.001 447 754 | AABR07051551.2 | -1.308 692 094 | -2.825 531 707 | 0.033 319 465 | |
| Pkig | 1.036 827 364 | 6.255 767 217 | 0.001 095 780 | Cmtm3 | -1.324 596 882 | -5.371 453 966 | 0.002 279 345 | |
| AABR07065925.1 | 0.970 924 295 | 6.151 591 228 | 0.001 189 396 | Dpy19l3 | -1.623 739 520 | -3.419 084 313 | 0.016 347 174 | |
| Ehmt1 | 0.944 670 184 | 6.578 534 619 | 0.000 855 702 | Cd74 | -2.034 312 055 | -2.712 776 259 | 0.038 372 308 | |
| AABR07062152.1 | 0.939 321 669 | 6.344 499 009 | 0.001 022 741 | Cxcl13 | -2.697 259 120 | -3.378 916 162 | 0.017 124 825 | |
| Ctbp1 | 0.898 198 041 | 5.961 511 123 | 0.001 385 227 | RGD1563231 | -3.151 695 504 | -2.603 665 774 | 0.044 063 014 | |
| Akr1b10 | 0.859 136 478 | 3.868 507 287 | 0.009 887 528 | Jchain | -3.390 915 739 | -2.827 200 269 | 0.033 250 368 | |
| Ssh1 | 0.818 982 469 | 5.813 203 431 | 0.001 564 263 | Ighm | -3.673 986 054 | -3.369 414 954 | 0.017 314 754 | |
| Piga | 0.785 438 853 | 3.303 450 635 | 0.018 700 031 | AABR07060872.1 | -5.052 108 514 | -3.355 872 330 | 0.017 589 546 |
表1 经血干细胞移植联合运动训练治疗后脊髓损伤大鼠的脊髓组织中显著上调和下调基因
Table 1 Significantly up-regulated and down-regulated genes in spinal cord tissues of spinal cord injury rats after transplantation of menstrual blood-derived stem cells combined with exercise training
表达上调基因 Up-regulated gene | log2(差异倍数) log2 (FoldChange) | t值 t value | P值 P value | 表达下调基因 Down-regulated gene | log2(差异倍数) log2 (FoldChange) | t值 t value | P值 P value | |
|---|---|---|---|---|---|---|---|---|
| Psme3 | 2.542 363 895 | 2.672 366 157 | 0.040 381 267 | Ptpdc1 | -0.941 169 616 | -2.823 151 331 | 0.033 418 310 | |
| Fgd2 | 1.870 409 798 | 5.277 683 881 | 0.002 476 179 | RT1-N2 | -0.961 937 408 | -3.508 946 559 | 0.014 746 432 | |
| Sdc4 | 1.591 559 898 | 2.530 079 050 | 0.048 412 378 | Cd79al | -1.007 349 648 | -3.292 831 274 | 0.018 934 378 | |
| Cthrc1 | 1.259 963 833 | 3.177 793 692 | 0.021 693 595 | Six4 | -1.071 889 332 | -15.482 205 520 | 0.000 009 930 | |
| RGD1302996 | 1.205 943 515 | 12.811 980 370 | 0.000 027 300 | Matn2 | -1.137 472 728 | -3.927 529 073 | 0.009 277 287 | |
| Rabepk | 1.201 118 420 | 2.962 815 910 | 0.028 125 989 | Clec4a3 | -1.273 541 156 | -2.512 270 495 | 0.049 532 946 | |
| Bdnf | 1.141 863 432 | 3.525 110 815 | 0.001 447 754 | AABR07051551.2 | -1.308 692 094 | -2.825 531 707 | 0.033 319 465 | |
| Pkig | 1.036 827 364 | 6.255 767 217 | 0.001 095 780 | Cmtm3 | -1.324 596 882 | -5.371 453 966 | 0.002 279 345 | |
| AABR07065925.1 | 0.970 924 295 | 6.151 591 228 | 0.001 189 396 | Dpy19l3 | -1.623 739 520 | -3.419 084 313 | 0.016 347 174 | |
| Ehmt1 | 0.944 670 184 | 6.578 534 619 | 0.000 855 702 | Cd74 | -2.034 312 055 | -2.712 776 259 | 0.038 372 308 | |
| AABR07062152.1 | 0.939 321 669 | 6.344 499 009 | 0.001 022 741 | Cxcl13 | -2.697 259 120 | -3.378 916 162 | 0.017 124 825 | |
| Ctbp1 | 0.898 198 041 | 5.961 511 123 | 0.001 385 227 | RGD1563231 | -3.151 695 504 | -2.603 665 774 | 0.044 063 014 | |
| Akr1b10 | 0.859 136 478 | 3.868 507 287 | 0.009 887 528 | Jchain | -3.390 915 739 | -2.827 200 269 | 0.033 250 368 | |
| Ssh1 | 0.818 982 469 | 5.813 203 431 | 0.001 564 263 | Ighm | -3.673 986 054 | -3.369 414 954 | 0.017 314 754 | |
| Piga | 0.785 438 853 | 3.303 450 635 | 0.018 700 031 | AABR07060872.1 | -5.052 108 514 | -3.355 872 330 | 0.017 589 546 |
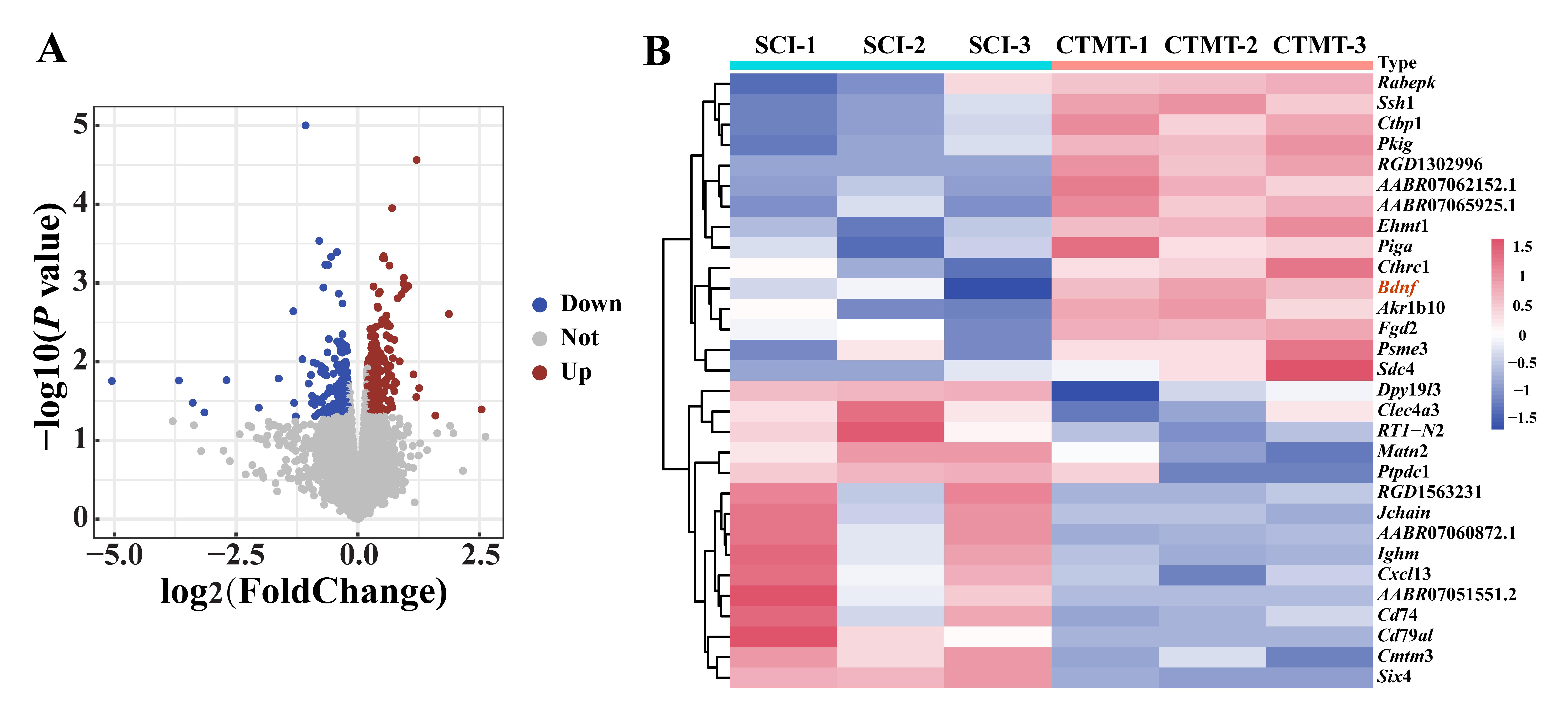
图2 两组大鼠的脊髓组织间差异表达基因火山图和聚类热图注:A,火山图中,横坐标为基因表达变化倍数,纵坐标表示差异基因表达量的统计显著性,上调和下调的基因分别用红色和蓝色实心圆点表示,灰色圆点表示表达无差异的基因。B,聚类热图中,横坐标为样品名称,纵坐标为基因名称,粉色表示高表达基因,蓝色表示低表达基因。SCI为对照组(即单纯脊髓损伤手术组,作为对照,n=3),CTMT为联合治疗组(即SCI手术后经血干细胞移植联合运动训练组,n=3)。
Figure 2 Volcano plot and clustered heatmap of differentially expressed genes in spinal cord tissues between the two groups of ratsNote: A, in volcano plot, horizontal axis indicated the fold change in the gene expression, the vertical axis indicated the statistical significance of differential gene expression profile, the red and blue dots indicated the upregulated and downregulated genes, respectively, and the gray dots indicated the non-DEGs; B, in clustered heatmap, the horizontal axis indicated the sample names, the vertical axis indicated gene names, the pink indicated highly expressed genes, and the blue indicated low expressed genes. SCI was the control group (spinal cord injury, as the control, n=3), and CTMT was the cell and treadmill training group (the transplantation of menstrual blood-derived stem cells combined with treadmill training after SCI surgery, n=3).
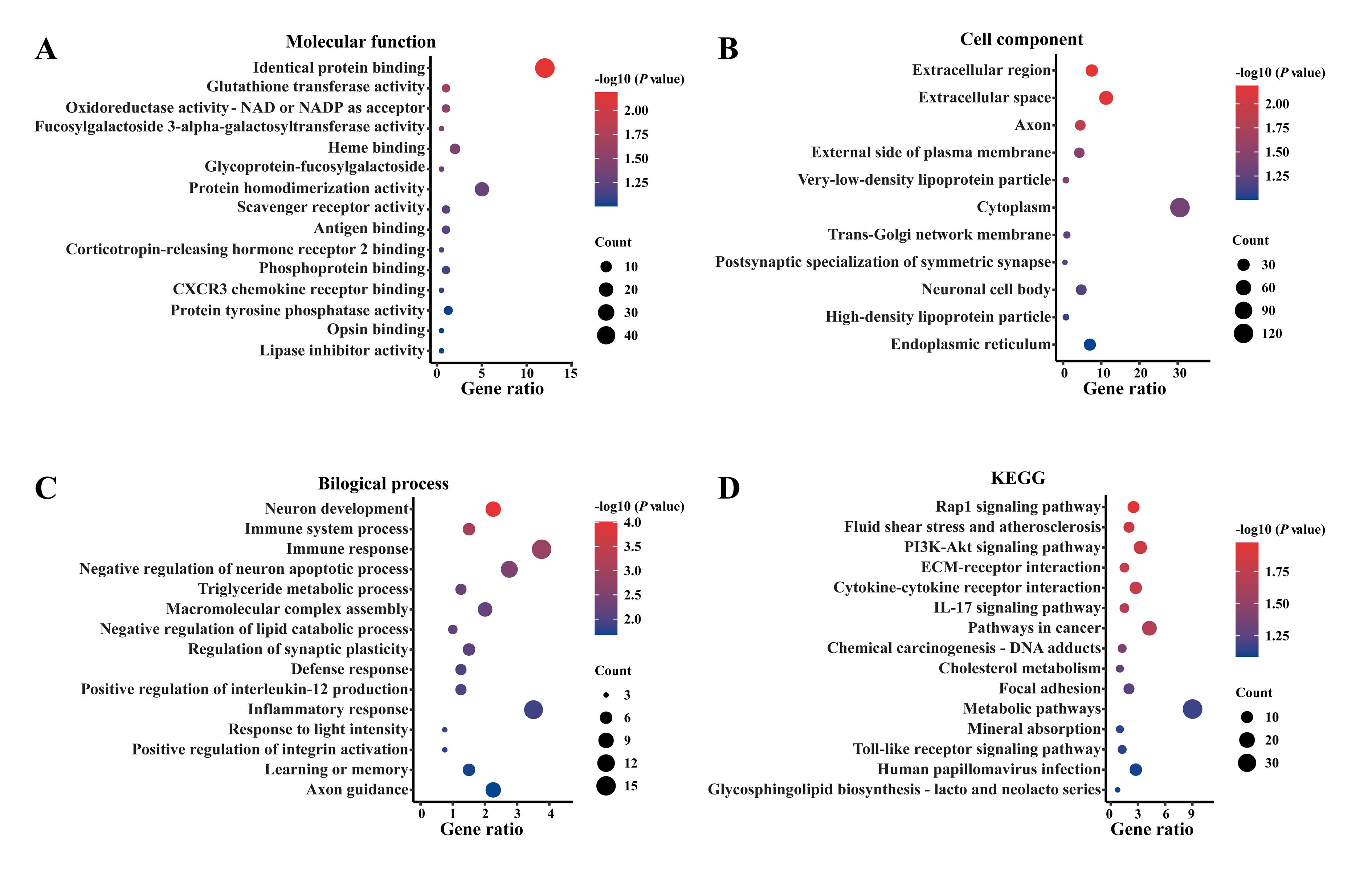
图3 两组大鼠脊髓组织中差异表达基因的GO富集分析和KEGG通路富集分析注:A~C分别为GO富集分析中的3个本体,即分子功能(A)、细胞组分(B)与生物过程(C);D为KEGG通路富集分析。富集系数列在X轴,富集条目列在Y轴,气泡大小表示富集在各条目上的基因数量,面积愈大表示富集基因数量愈多,而颜色呈现的是反映差异显著性的P值大小,红色愈深代表其差异性愈显著。
Figure 3 GO enrichment and KEGG pathway enrichment analyses of differentially expressed genes in spinal cord tissues of the two groups of ratsNote:A-C indicated the 3 analysis ontologies for Gene Ontology (GO) enrichment analysis, including molecular function (A), cellular components (B) and biological processes (C), respectively. D represented the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. The X-axis represented the fold enrichment, the Y-axis represented the enrichment items, and the bubble size indicated the number of genes enriched on each item. The larger the bubble, the greater the number of enriched genes. The color corresponded to P-value. The darker the red color, the more significant the difference.
信号通路名 Term of signal pathway | 计数 Count | 富集系数 Gene ratio | P值 P value | 基因名 Genes |
|---|---|---|---|---|
| Rap1 signaling pathway | 10 | 2.506 265 664 | 0.010 546 665 | Angpt1, Rassf5、Vegfb、Lpar1、Rapgef6、Efna4、Met、Bcar1、Rap1gap、Adcy6 |
Fluid shear stress and atherosclerosis | 8 | 2.005 012 531 | 0.014 625 259 | Prkaa1、Mef2c、Sdc4、Gsta4、Il1r2、Gstt3、Hmox1 |
PI3K-Akt signaling pathway | 13 | 3.258 145 363 | 0.014 714 234 | Prkaa1、Itga4、Angpt1、Bdnf、 Lama4、Lpar1、Vegfb、Ppp2r3b、Efna4、Gng7、Spp1、Col9a3、Met |
ECM-receptor interaction | 6 | 1.503 759 398 | 0.016 252 600 | Sdc4、Itga4、Rgd1565355、Lama4、Spp1、Col9a3 |
Cytokine-cytokine receptor interaction | 11 | 2.756 892 231 | 0.016 582 561 | Cxcl10、Cntf、Il25、Tnfsf15、Tnfrsf9、Il1r2、Il21r、Ccr7、Ltb、Cxcl13、Ccl17 |
| IL-17 signaling pathway | 6 | 1.503 759 398 | 0.020 227 194 | Cxcl10、Il25、Traf3、Mmp3、Lcn2、Ccl17 |
| Pathways in cancer | 17 | 4.260 651 629 | 0.020 758 687 | Ctbp1、Lama4、Lpar1、Vegfb、Gstt3、Adcy6、Dll4、Gsta4、Peg12、Traf3、Gng7、Rassf5、Ccdc6、Hmox1、E2f3、Met |
Chemical carcinogenesis- DNA adducts | 5 | 1.253 132 832 | 0.040 451 734 | Cyp3a9、Hpgds、Gsta4、Gstt3 |
| Cholesterol metabolism | 4 | 1.002 506 266 | 0.053 168 933 | Rgd1565355、Apoh、Apoc1、Apoc3 |
| Focal adhesion | 8 | 2.005 012 531 | 0.054 767 437 | Itga4、Lama4、Vegfb、Spp1、Col9a3、 Pip5k1c、Met、Bcar1 |
Metabolic pathway | 36 | 9.022 556 391 | 0.068 256 357 | Mtmr2、Gldc、Mtmr14、Pde3b、Gstt3、Hexd、Ehmt1、Adcy6、Abo、Selenbp1、Cyp3a9、Hpgds、Neu4、Spp1、Hmox1、St3gal6、Ca8、Pip5k1c、Csgalnact1、Car1、Uros、Qrsl1、 Coq7、Sirt3、Nt5c3b、Akr1b10、 Cyp2u1、Gsta4、Akr1c15、P4ha3、Piga、Cat、Acot1、Idnk |
| Mineral absorption | 4 | 1.002 506 266 | 0.072 932 376 | Atp7b、Slc40a1、Hmox1、Slc39a4 |
Toll-like receptor signaling pathway | 5 | 1.253 132 832 | 0.074 076 217 | Cxcl10、Irf3、Traf3、Spp1、Tlr3 |
Human papillomavirus infection | 11 | 2.756 892 231 | 0.079 092 324 | Irf3、Itga4、Traf3、Rt1-N2、Lama4、Spp1、Col9a3、Ppp2r3b、Rt1-T24-1、Tlr3、Llgl1 |
Glycosphingolipid biosynthesis | 3 | 0.751 879 699 | 0.081 761 102 | St3gal6、Abo |
表2 两组大鼠脊髓组织中差异表达基因的KEGG信号通路富集分析结果
Table 2 Results of KEGG pathway enrichment analysis of differentially expressed genes in spinal cord tissues of the two groups of rats
信号通路名 Term of signal pathway | 计数 Count | 富集系数 Gene ratio | P值 P value | 基因名 Genes |
|---|---|---|---|---|
| Rap1 signaling pathway | 10 | 2.506 265 664 | 0.010 546 665 | Angpt1, Rassf5、Vegfb、Lpar1、Rapgef6、Efna4、Met、Bcar1、Rap1gap、Adcy6 |
Fluid shear stress and atherosclerosis | 8 | 2.005 012 531 | 0.014 625 259 | Prkaa1、Mef2c、Sdc4、Gsta4、Il1r2、Gstt3、Hmox1 |
PI3K-Akt signaling pathway | 13 | 3.258 145 363 | 0.014 714 234 | Prkaa1、Itga4、Angpt1、Bdnf、 Lama4、Lpar1、Vegfb、Ppp2r3b、Efna4、Gng7、Spp1、Col9a3、Met |
ECM-receptor interaction | 6 | 1.503 759 398 | 0.016 252 600 | Sdc4、Itga4、Rgd1565355、Lama4、Spp1、Col9a3 |
Cytokine-cytokine receptor interaction | 11 | 2.756 892 231 | 0.016 582 561 | Cxcl10、Cntf、Il25、Tnfsf15、Tnfrsf9、Il1r2、Il21r、Ccr7、Ltb、Cxcl13、Ccl17 |
| IL-17 signaling pathway | 6 | 1.503 759 398 | 0.020 227 194 | Cxcl10、Il25、Traf3、Mmp3、Lcn2、Ccl17 |
| Pathways in cancer | 17 | 4.260 651 629 | 0.020 758 687 | Ctbp1、Lama4、Lpar1、Vegfb、Gstt3、Adcy6、Dll4、Gsta4、Peg12、Traf3、Gng7、Rassf5、Ccdc6、Hmox1、E2f3、Met |
Chemical carcinogenesis- DNA adducts | 5 | 1.253 132 832 | 0.040 451 734 | Cyp3a9、Hpgds、Gsta4、Gstt3 |
| Cholesterol metabolism | 4 | 1.002 506 266 | 0.053 168 933 | Rgd1565355、Apoh、Apoc1、Apoc3 |
| Focal adhesion | 8 | 2.005 012 531 | 0.054 767 437 | Itga4、Lama4、Vegfb、Spp1、Col9a3、 Pip5k1c、Met、Bcar1 |
Metabolic pathway | 36 | 9.022 556 391 | 0.068 256 357 | Mtmr2、Gldc、Mtmr14、Pde3b、Gstt3、Hexd、Ehmt1、Adcy6、Abo、Selenbp1、Cyp3a9、Hpgds、Neu4、Spp1、Hmox1、St3gal6、Ca8、Pip5k1c、Csgalnact1、Car1、Uros、Qrsl1、 Coq7、Sirt3、Nt5c3b、Akr1b10、 Cyp2u1、Gsta4、Akr1c15、P4ha3、Piga、Cat、Acot1、Idnk |
| Mineral absorption | 4 | 1.002 506 266 | 0.072 932 376 | Atp7b、Slc40a1、Hmox1、Slc39a4 |
Toll-like receptor signaling pathway | 5 | 1.253 132 832 | 0.074 076 217 | Cxcl10、Irf3、Traf3、Spp1、Tlr3 |
Human papillomavirus infection | 11 | 2.756 892 231 | 0.079 092 324 | Irf3、Itga4、Traf3、Rt1-N2、Lama4、Spp1、Col9a3、Ppp2r3b、Rt1-T24-1、Tlr3、Llgl1 |
Glycosphingolipid biosynthesis | 3 | 0.751 879 699 | 0.081 761 102 | St3gal6、Abo |
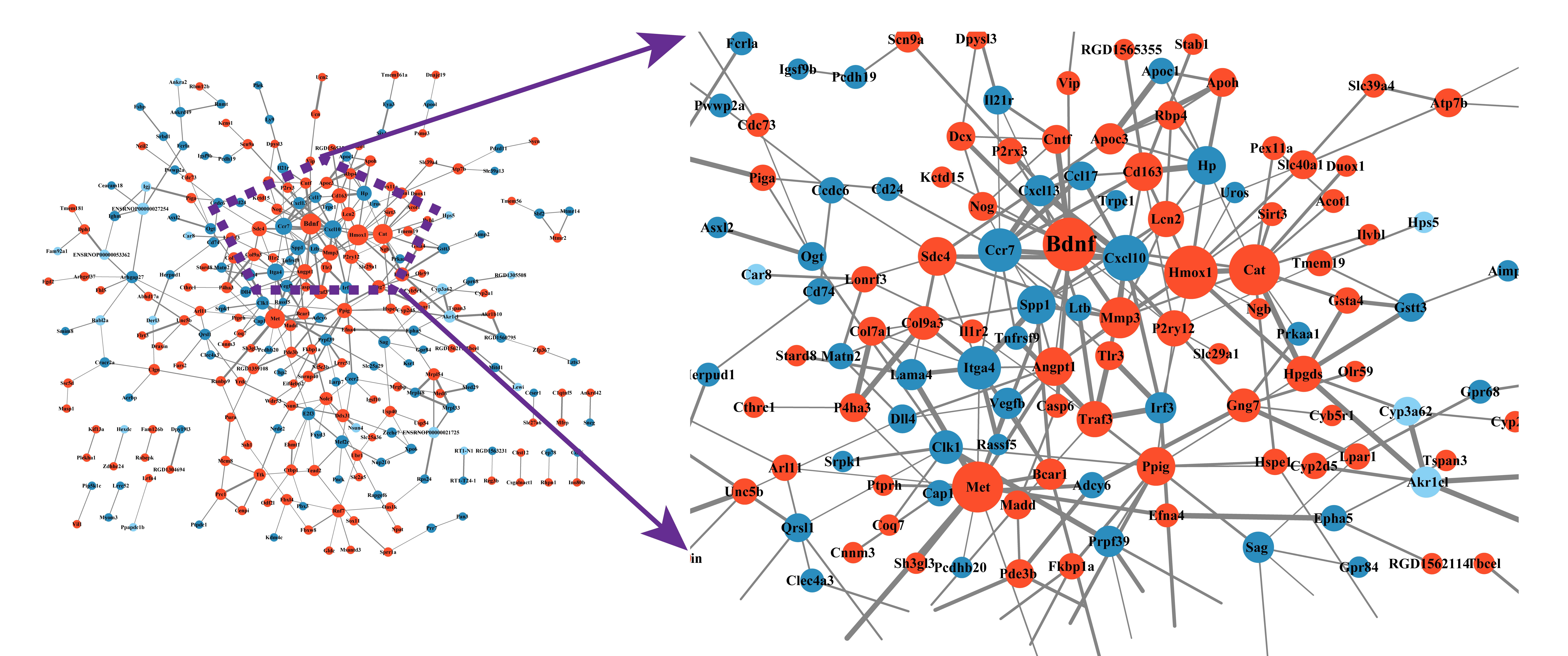
图4 两组大鼠脊髓组织中差异表达基因的蛋白互作网络图注:圆点及其大小分别代表节点(node)和度(degree),与此节点连接的基因数目越多,节点越大;点内填充红色代表该节点的基因表达量上调,蓝色代表该节点的基因表达量下调。
Figure 4 Protein-protein interaction network of differentially expressed genes in spinal cord tissues of the two groups of ratsNote:The size of the dots represented the node and degree, the node with more connections had a larger size; the red color in the dots represented the up-regulation of gene expression in this node, and the blue color represented the down-regulation of gene expression.
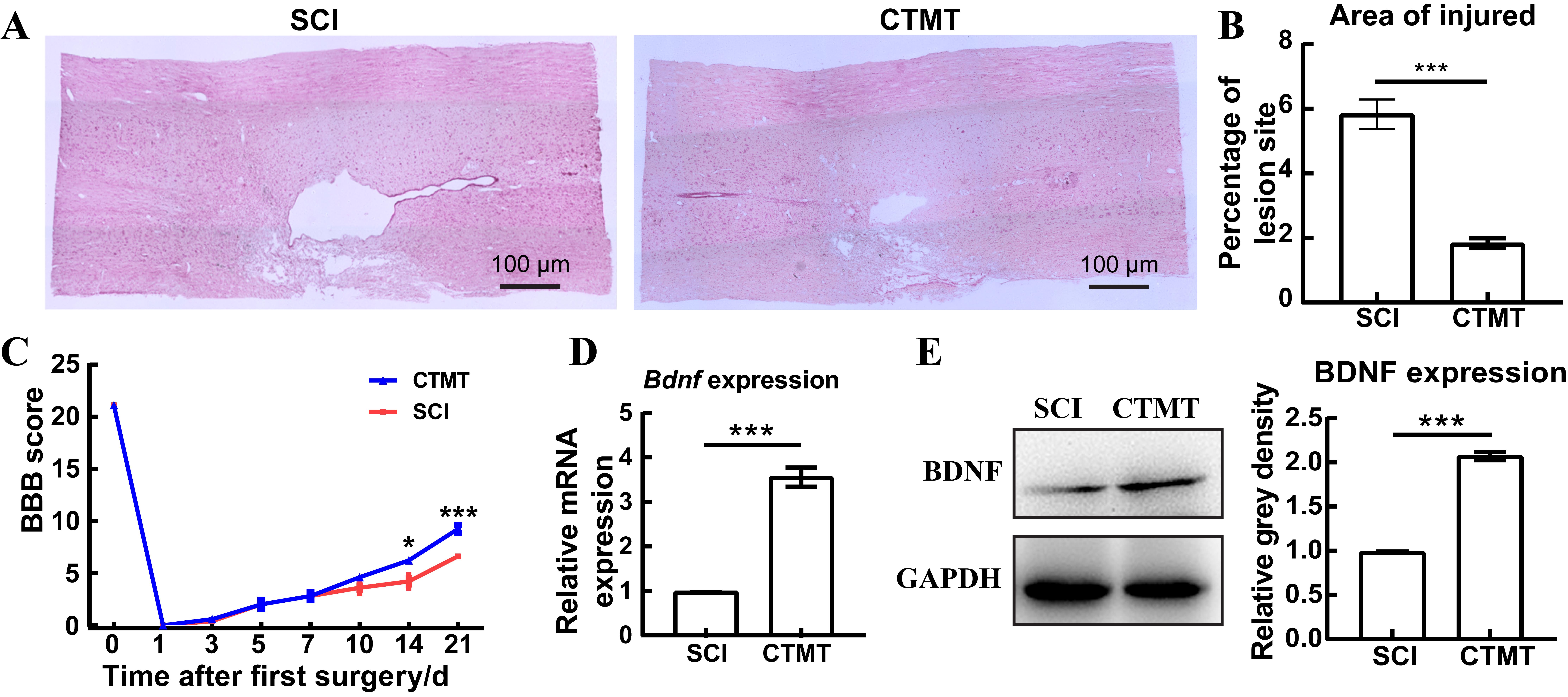
图5 经血干细胞移植联合运动训练治疗可促进脊髓损伤大鼠的运动功能恢复及BDNF表达注:A为苏木精-伊红染色观察SCI组(即单纯脊髓损伤手术组,作为对照,n=3)和CTMT组(即SCI手术后经血干细胞移植联合运动训练组,n=3)大鼠的脊髓损伤局部结构病理变化情况(放大40倍)。B,A图中损伤区域面积的统计结果(***P<0.001,n=3)。C,2组大鼠的BBB运动评分比较,分数越高说明运动功能恢复越好。D,实时荧光定量PCR检测两组大鼠损伤脊髓组织中Bdnf的转录水平差异(***P<0.001,n=5)。E,蛋白质印迹法检测两组大鼠损伤脊髓组织中BDNF蛋白水平差异(***P<0.001,n=5)。
Figure 5 Menstrual blood-derived stem cells transplantation combined with treadmill training promotes motor function recovery and BDNF expression in spinal cord injury ratsNote:A showed the local structural pathological changes of the spinal cord injury in the SCI group (spinal cord injury, as the control, n=3) and the CTMT group (the transplantation of menstrual blood-derived stem cells combined with treadmill training after SCI surgery, n=3), observed by hematoxylin-eosin staining (magnified 40 times). B, statistical results of the lesion area in Figure A (***P<0.001, n=3). C, BBB motor score, a higher score indicated a better recovery of motor function. D, Real-time fluorescence quantitative PCR was performed to detect the changes in the transcriptional level of Bdnf gene in the injured spinal cord in rats of SCI and CTMT groups (***P<0.001, n=5). E, Western blotting analysis was performed to detect the changes in the protein level of BDNF in SCI and CTMT groups (***P<0.001, n=5).
| 1 | COURTINE G, SOFRONIEW M V. Spinal cord repair: advances in biology and technology[J]. Nat Med, 2019, 25(6):898-908. DOI: 10.1038/s41591-019-0475-6 . |
| 2 | O'SHEA T M, BURDA J E, SOFRONIEW M V. Cell biology of spinal cord injury and repair[J]. J Clin Invest, 2017, 127(9):3259-3270. DOI: 10.1172/JCI90608 . |
| 3 | ZHOU X, WAHANE S, FRIEDL M S, et al. Microglia and macrophages promote corralling, wound compaction and recovery after spinal cord injury via Plexin-B2[J]. Nat Neurosci, 2020, 23(3):337-350. DOI: 10.1038/s41593-020-0597-7 . |
| 4 | ASSINCK P, DUNCAN G J, HILTON B J, et al. Cell transplantation therapy for spinal cord injury[J]. Nat Neurosci, 2017, 20(5):637-647. DOI: 10.1038/nn.4541 . |
| 5 | JONES I, NOVIKOVA L N, NOVIKOV L N, et al. Regenerative effects of human embryonic stem cell-derived neural crest cells for treatment of peripheral nerve injury[J]. J Tissue Eng Regen Med, 2018, 12(4): e2099-e2109. DOI: 10.1002/term.2642 . |
| 6 | CAO S X, MA J, ZHANG J Y, et al. Reprogramming of one human induced pluripotent stem cell line from healthy donor[J]. Stem Cell Res, 2021, 57:102613. DOI: 10.1016/j.scr. 2021.102613 . |
| 7 | FENG P H, LI P P, TAN J C. Human menstrual blood-derived stromal cells promote recovery of premature ovarian insufficiency via regulating the ECM-dependent FAK/AKT signaling[J]. Stem Cell Rev Rep, 2019, 15(2):241-255. DOI: 10.1007/s12015-018-9867-0 . |
| 8 | LI Y, LI X N, ZHAO H X, et al. Efficient induction of pluripotent stem cells from menstrual blood[J]. Stem Cells Dev, 2013, 22(7):1147-1158. DOI: 10.1089/scd.2012.0428 . |
| 9 | SHIRIAN S, EBRAHIMI-BAROUGH S, SABERI H, et al. Comparison of capability of human bone marrow mesenchymal stem cells and endometrial stem cells to differentiate into motor neurons on electrospun poly(ε-caprolactone) scaffold[J]. Mol Neurobiol, 2016, 53(8):5278-5287. DOI: 10.1007/s12035-015-9442-5 . |
| 10 | ZEMEL'KO V I, KOZHUKHAROVA I V, KOVALEVA Z V, et al. [BDNF secretion in human mesenchymal stem cells isolated from bone marrow, endometrium and adipose tissue].[J]. Tsitologiia, 2014, 56(3):204-211. DOI: 10.1134/S1990519X1404 0129 . |
| 11 | WU Q F, WANG Q H, LI Z J, et al. Human menstrual blood-derived stem cells promote functional recovery in a rat spinal cord hemisection model[J]. Cell Death Dis, 2018, 9(9):882. DOI: 10.1038/s41419-018-0847-8 . |
| 12 | RONG Y L, LIU W, WANG J X, et al. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy[J]. Cell Death Dis, 2019, 10(5):340. DOI: 10.1038/s41419-019-1571-8 . |
| 13 | SHARIF K, WATAD A, BRAGAZZI N L, et al. Physical activity and autoimmune diseases: get moving and manage the disease[J]. Autoimmun Rev, 2018, 17(1):53-72. DOI: 10.1016/j.autrev.2017.11.010 . |
| 14 | BOOTH F W, ROBERTS C K, LAYE M J. Lack of exercise is a major cause of chronic diseases[J]. Compr Physiol, 2012, 2(2):1143-1211. DOI: 10.1002/cphy.c110025 . |
| 15 | ZBOGAR D, ENG J J, MILLER W C, et al. Physical activity outside of structured therapy during inpatient spinal cord injury rehabilitation[J]. J Neuroeng Rehabil, 2016, 13(1):99. DOI: 10.1186/s12984-016-0208-8 . |
| 16 | BEHRMAN A L, ARDOLINO E M, HARKEMA S J. Activity-based therapy: from basic science to clinical application for recovery after spinal cord injury[J]. J Neurol Phys Ther, 2017, 41 Suppl 3(Suppl 3 IV STEP Spec Iss): S39-S45. DOI: 10.1097/NPT.0000000000000184 . |
| 17 | UNGERLEIDER L G, DOYON J, KARNI A. Imaging brain plasticity during motor skill learning[J]. Neurobiol Learn Mem, 2002, 78(3):553-564. DOI: 10.1006/nlme.2002.4091 . |
| 18 | SUN T S, YE C Q, WU J, et al. Treadmill step training promotes spinal cord neural plasticity after incomplete spinal cord injury[J]. Neural Regen Res, 2013, 8(27):2540-2547. DOI: 10.3969/j.issn.1673-5374.2013.27.005 . |
| 19 | WANG H X, LIU N K, ZHANG Y P, et al. Treadmill training induced lumbar motoneuron dendritic plasticity and behavior recovery in adult rats after a thoracic contusive spinal cord injury[J]. Exp Neurol, 2015, 271:368-378. DOI: 10.1016/j.expneurol.2015.07.004 . |
| 20 | 殷睿安, 王双燕, 王培, 等. 重复经颅磁刺激叠加运动训练对脊髓损伤大鼠运动功能和神经元可塑性的影响[J]. 中国康复医学杂志, 2021, 36(7):774-778, 792. DOI: 10.3969/j.issn.1001-1242.2021.07.002 . |
| YIN R A, WANG S Y, WANG P, et al. Effects of combined rTMS and exercise training on locomotor function and neuronal plasticity in rats with spinal cord injury[J]. Chin J Rehabil Med, 2021, 36(7):774-778, 792. DOI: 10.3969/j.issn.1001-1242.2021.07.002 . | |
| 21 | HE W H, ZHANG X X, LI X Z, et al. A decellularized spinal cord extracellular matrix-gel/GelMA hydrogel three-dimensional composite scaffold promotes recovery from spinal cord injury via synergism with human menstrual blood-derived stem cells[J]. J Mater Chem B, 2022, 10(30):5753-5764. DOI: 10.1039/d2tb00792d . |
| 22 | HWANG D H, SHIN H Y, KWON M J, et al. Survival of neural stem cell grafts in the lesioned spinal cord is enhanced by a combination of treadmill locomotor training via insulin-like growth factor-1 signaling[J]. J Neurosci, 2014, 34(38):12788-12800. DOI: 10.1523/JNEUROSCI.5359-13.2014 . |
| 23 | DE MIRANDA L, LEVI R, DIVANOGLOU A. Tapping into the unimpossible: Philosophical health in lives with spinal cord injury[J]. J Eval Clin Pract, 2023, 29(7):1203-1210. DOI: 10.1111/jep.13874 . |
| 24 | WANG M H, CORDELL H J, VAN STEEN K. Statistical methods for genome-wide association studies[J]. Semin Cancer Biol, 2019, 55:53-60. DOI: 10.1016/j.semcancer. 2018.04.008 . |
| 25 | LI Y, CHEN Y, LI X, et al. RNA sequencing screening of differentially expressed genes after spinal cord injury[J]. Neural Regen Res, 2019, 14(9):1583-1593. DOI: 10.4103/1673-5374.255994 . |
| 26 | 唐丹, 王先斌, 杨香莲, 等. 跑台运动训练对脊髓损伤后大鼠肺损伤及HMGB1/TLR4/NF-κB信号通路表达的影响[J]. 中国康复医学杂志, 2023, 38(2):159-166. DOI: 10.3969/j.issn.1001-1242.2023.02.004 . |
| TANG D, WANG X B, YANG X L, et al. Effects of treadmill training on lung injury and HMGB1/TLR-4/NF-κB signaling pathway after spinal cord injury in rats[J]. Chin J Rehabil Med, 2023, 38(2):159-166. DOI: 10.3969/j.issn.1001-1242.2023.02.004 . | |
| 27 | MITRE M, MARIGA A, CHAO M V. Neurotrophin signalling: novel insights into mechanisms and pathophysiology[J]. Clin Sci (Lond), 2017, 131(1):13-23. DOI: 10.1042/CS20160044 . |
| 28 | WEISHAUPT N, BLESCH A, FOUAD K. BDNF: the career of a multifaceted neurotrophin in spinal cord injury[J]. Exp Neurol, 2012, 238(2):254-264. DOI: 10.1016/j.expneurol.2012.09.001 . |
| 29 | KEEFE K M, SHEIKH I S, SMITH G M. Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury[J]. Int J Mol Sci, 2017, 18(3):548. DOI: 10.3390/ijms18030548 . |
| 30 | WU Q F, CAO Y N, DONG C M, et al. Neuromuscular interaction is required for neurotrophins-mediated locomotor recovery following treadmill training in rat spinal cord injury[J]. PeerJ, 2016, 4: e2025. DOI: 10.7717/peerj.2025 . |
| 31 | HERNANDEZ-TORRES V, GRANSEE H M, MANTILLA C B, et al. BDNF effects on functional recovery across motor behaviors after cervical spinal cord injury[J]. J Neurophysiol, 2017, 117(2):537-544. DOI: 10.1152/jn.00654.2016 . |
| 32 | SAHELI M, KHORAMIPOUR K, VOSOUGH M, et al. Athletes' mesenchymal stem cells could be the best choice for cell therapy in Omicron-infected patients[J]. Cells, 2022, 11(12):1926. DOI: 10.3390/cells11121926 . |
| 33 | TAKAHASHI A, NAKAJIMA H, KUBOTA A, et al. Adipose-derived mesenchymal stromal cell transplantation for severe spinal cord injury: functional improvement supported by angiogenesis and neuroprotection[J]. Cells, 2023, 12(11):1470. DOI: 10.3390/cells12111470 . |
| 34 | WU R, GUO Y P, ZHANG L Y, et al. Physical exercise promotes integration of grafted cells and functional recovery in an acute stroke rat model[J]. Stem Cell Reports, 2022, 17(2):276-288. DOI: 10.1016/j.stemcr.2021.12.006 . |
| 35 | SUN X, HUANG L Y, PAN H X, et al. Bone marrow mesenchymal stem cells and exercise restore motor function following spinal cord injury by activating PI3K/AKT/mTOR pathway[J]. Neural Regen Res, 2023, 18(5):1067-1075. DOI: 10.4103/1673-5374.355762 . |
| [1] | 秦超, 李双星, 赵婷婷, 蒋晨晨, 赵晶, 杨艳伟, 林志, 王三龙, 文海若. 药物安全评价用SD大鼠90 d喂养试验的背景数据研究[J]. 实验动物与比较医学, 2025, 45(4): 439-448. |
| [2] | 刘力瑜, 嵇波, 刘小玄, 方洋, 张玲, 郭亭廷, 全烨, 李鹤文, 刘翼天. 大鼠胎儿期肺组织固定方法的探索[J]. 实验动物与比较医学, 2025, 45(4): 432-438. |
| [3] | 潘颐聪, 蒋汶洪, 胡明, 覃晓. 慢性肾脏病大鼠主动脉钙化模型的术式优化及效果评价[J]. 实验动物与比较医学, 2025, 45(3): 279-289. |
| [4] | 刘智伟, 杨然, 连浩, 张玉, 金立伦. 秦皮素对碘乙酸钠诱导骨关节炎模型大鼠的软骨保护与抗炎作用[J]. 实验动物与比较医学, 2025, 45(3): 259-268. |
| [5] | 姜萌, 郝淑兰, 仝立国, 仲启明, 高振飞, 王永辉, 王晞星, 吉海杰. 长春瑞滨诱导大鼠足背静脉炎模型的动态评价[J]. 实验动物与比较医学, 2025, 45(3): 251-258. |
| [6] | 肖林林, 杨逸萱, 黎珊杉, 罗兰诗雨, 尹思威, 孙俊铭, 施维, 欧阳轶强, 李习艺. 利用脑立体定位技术将人源三突变APP基因导入海马区构建阿尔茨海默病大鼠模型[J]. 实验动物与比较医学, 2025, 45(3): 269-278. |
| [7] | 许秋雨, 严国锋, 付丽, 范文华, 周晶, 朱莲, 仇淑雯, 张洁, 吴铃. 来曲唑缓释片皮下给药构建小鼠多囊卵巢综合征模型及其肝脏转录组学分析[J]. 实验动物与比较医学, 2025, 45(2): 119-129. |
| [8] | 连辉, 姜艳玲, 刘佳, 张玉立, 谢伟, 薛晓鸥, 李健. 异常子宫出血大鼠模型的构建与评价[J]. 实验动物与比较医学, 2025, 45(2): 130-146. |
| [9] | 孙效容, 苏丹, 贵文娟, 陈玥. 手术诱导大鼠中重度膝骨关节炎模型的建立与评价[J]. 实验动物与比较医学, 2024, 44(6): 597-604. |
| [10] | 殷玉莲, 马丽娜, 屠思远, 陈玲, 叶媚娜, 陈红风. 非哺乳期乳腺炎大鼠模型的建立及评价[J]. 实验动物与比较医学, 2024, 44(6): 587-596. |
| [11] | 杨劲, 俞诗雅, 林楠, 方永超, 赵虎, 邱锦维, 林鸿铭, 陈惠燕, 王瑜, 吴伟航. 改良型十二指肠旷置术对2型糖尿病大鼠糖代谢的影响[J]. 实验动物与比较医学, 2024, 44(5): 523-530. |
| [12] | 张乃群, 袁飘漂, 曹琳茸, 应娜, 杨涛涛. PNR检测在糖尿病肾脏疾病模型大鼠诊断及药效评价中的应用[J]. 实验动物与比较医学, 2024, 44(5): 543-549. |
| [13] | 郑艺清, 邓亚胜, 范燕萍, 梁天薇, 黄慧, 刘永辉, 倪召兵, 林江. 基于数据挖掘的盆腔炎性疾病动物模型应用分析[J]. 实验动物与比较医学, 2024, 44(4): 405-418. |
| [14] | 肖攀, 王红义, 陆璐, 张梅, 陈克明, 申栋帅, 牛廷献. 低氧敏感和低氧耐受型Wistar大鼠筛选及其G1代的低氧敏感性初探[J]. 实验动物与比较医学, 2024, 44(4): 374-383. |
| [15] | 朱晓雨, 袁韩涛, 李四波. 微RNA-887-3p能抑制大鼠椎间盘纤维环细胞中 MDM4表达和细胞增殖并促进细胞凋亡[J]. 实验动物与比较医学, 2024, 44(3): 270-278. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||