













实验动物与比较医学 ›› 2025, Vol. 45 ›› Issue (3): 259-268.DOI: 10.12300/j.issn.1674-5817.2024.165
收稿日期:2024-11-08
修回日期:2025-02-07
出版日期:2025-07-07
发布日期:2025-06-25
通讯作者:
金立伦(1967—),男,硕士,主任医师,研究方向:膝骨关节炎的发病机制与中药干预。E-mail:jinlilun@xinhuamed.com.cn。ORCID:0000-0001-9840-836X作者简介:刘智伟(1997—),男,硕士研究生,研究方向:膝骨关节炎的发病机制与中药干预。E-mail:rickyliu13@163.com
基金资助:
LIU Zhiwei, YANG Ran, LIAN Hao, ZHANG Yu, JIN Lilun( )(
)( )
)
Received:2024-11-08
Revised:2025-02-07
Published:2025-06-25
Online:2025-07-07
Contact:
JIN Lilun (ORCID: 0000-0001-9840-836X), E-mail: jinlilun@xinhuamed.com.cn摘要:
目的 建立大鼠骨关节炎模型,研究秦皮素对骨关节炎大鼠的抗炎作用及机制。 方法 18只8周龄雄性SPF级SD大鼠随机分为3组:空白组大鼠右膝关节腔注射50 μL生理盐水并连续1周;模型组和干预组大鼠右膝关节腔注射碘乙酸钠(monosodium iodoacetate,MIA)造模,干预组再注射秦皮素(5 mg·kg-1·d-1)并连续干预1周。药物干预后4周,采集腹主动脉血,安乐死动物后采集膝关节软骨。采用苏木精-伊红染色、番红O-固绿染色和甲苯胺蓝染色进行膝关节软骨的组织病理学观察以及Mankin和OARSI评分,并用micro-CT扫描系统比较分析每组膝关节的骨体积分数、骨表面积密度和骨小梁数目。采用酶联免疫吸附试验法检测各组大鼠血清中多种炎症因子[肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)、白细胞介素1β(interleukin-1β,IL-1β)、白细胞介素6(interleukin-6,IL-6)]以及软骨寡聚基质蛋白(cartilage oligomeric matrix protein,COMP)的表达。采用蛋白质印迹法测定膝关节软骨中丝裂原活化蛋白激酶p38(mitogen-activated protein kinase p38,p38 MAPK)、磷酸化丝裂原活化蛋白激酶p38(phosphorylation-p38 MAPK,p-p38 MAPK)、c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK)、磷酸化JNK氨基末端激酶(phosphorylation-JNK,p-JNK)的表达。 结果 大鼠膝关节软骨切片染色显示,模型组的关节面缺损严重,干预组的软骨破坏相对减轻。micro-CT显示,干预组的骨体积分数、骨表面积密度和骨小梁数目均明显高于模型组(P<0.05);模型组的Mankin评分明显高于空白组(P<0.05),干预组的Mankin评分明显低于模型组(P<0.05);而干预组的OARSI评分明显低于模型组(P<0.05)。酶联免疫吸附试验结果显示,模型组大鼠血清中TNF-α、IL-1β、IL-6和COMP含量均明显高于空白组(均P<0.05),而干预组含量明显低于模型组(均P<0.05)。蛋白质印迹结果显示,干预组膝关节软骨组织中p-p38 MAPK和p-JNK表达水平明显低于模型组(均P<0.05),而模型组的p-p38 MAPK和p-JNK蛋白表达水平明显高于空白组(均P<0.05)。 结论 秦皮素药物干预可能通过p38 MAPK通路对碘乙酸钠诱导的骨关节炎模型大鼠发挥治疗作用。
中图分类号:
刘智伟,杨然,连浩,等. 秦皮素对碘乙酸钠诱导骨关节炎模型大鼠的软骨保护与抗炎作用[J]. 实验动物与比较医学, 2025, 45(3): 259-268. DOI: 10.12300/j.issn.1674-5817.2024.165.
LIU Zhiwei,YANG Ran,LIAN Hao,et al. Cartilage Protection and Anti-Inflammatory Effects of Fraxetin on Monosodium Iodoacetate-Induced Rat Model of Osteoarthritis[J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 259-268. DOI: 10.12300/j.issn.1674-5817.2024.165.
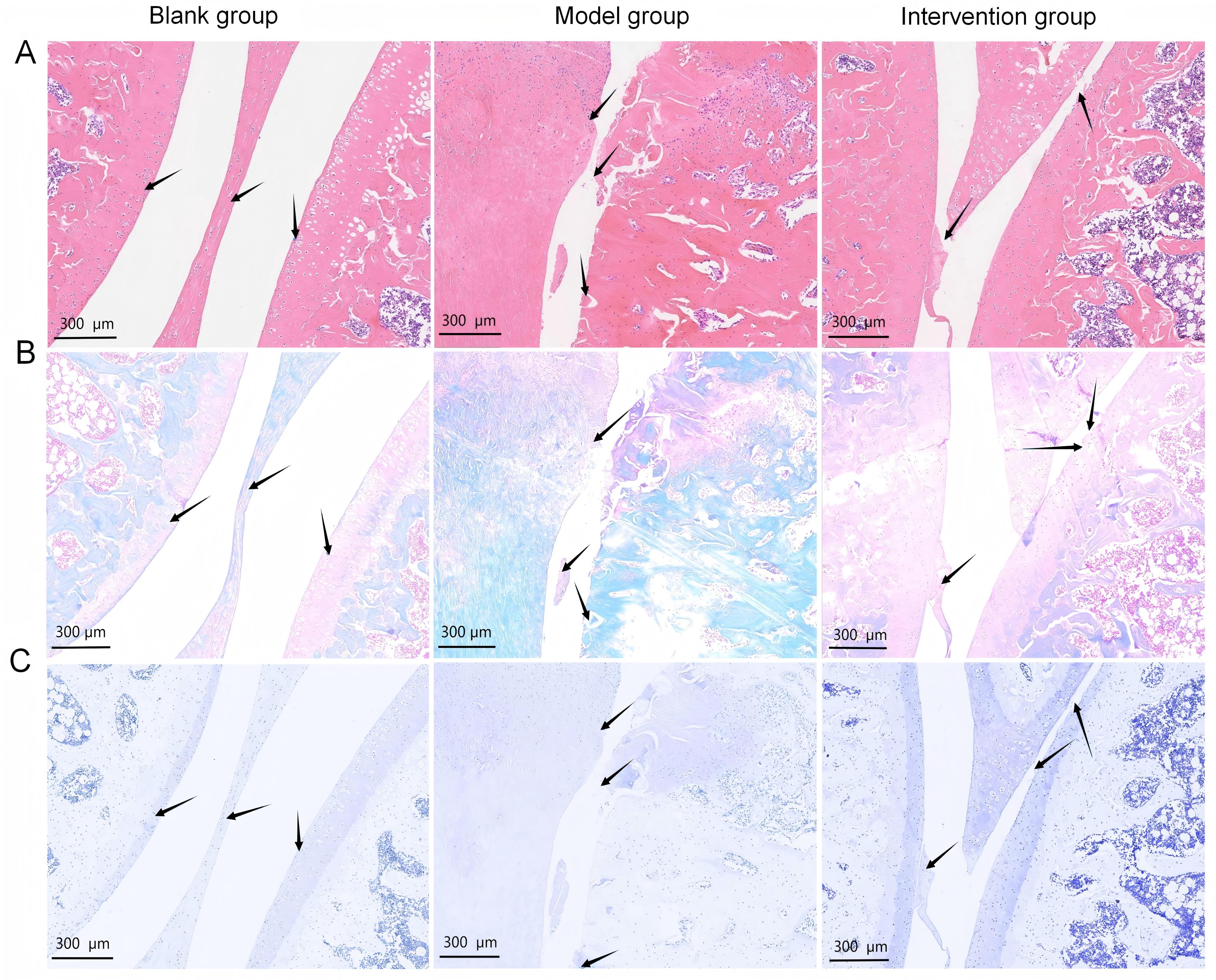
图1 碘乙酸钠注射造模及秦皮素药物干预后大鼠膝关节软骨组织病理学变化注:A、B、C分别为3组大鼠膝关节软骨组织的苏木精-伊红、番红O-固绿、甲苯胺蓝染色结果(比例尺为300 μm,黑色箭头指向膝关节软骨表面)。空白组指先向右膝关节腔注射50 μL生理盐水,再腔内连续7 d注射50 μL 生理盐水的大鼠;模型组指右膝关节腔内单次注射50 μL新鲜配制的(60 mg/mL)碘乙酸钠建立膝骨关节炎动物模型,再连续7 d注射50 μL 生理盐水的大鼠;干预组指右膝关节腔内单次注射50 μL新鲜配制的(60 mg/mL)碘乙酸钠后,再连续7 d每天注射5 mg/kg秦皮素(溶于50 μL 生理盐水)的大鼠。
Figure 1 Histopathological changes in rat knee joint cartilage after monosodium iodoacetate injection and fraxetin interventionNote: A, B, and C are the staining results of hematoxylin-eosin, safranin O–fast green, and toluidine blue in the knee cartilage tissue of three groups of rats (scale bar = 300 μm, the black arrow in the picture points to the surface of the knee cartilage). The blank group refers to rats that were first injected with 50 μL of normal saline into the right knee joint cavity, followed by intra-articular injections of 50 μL of normal saline for 7 consecutive days; the model group refers to rats that were injected with one time injection of 50 μL fresh prepared monosodium iodoacetate (60 mg/mL)in the right knee joint cavity to induce osteoarthritis, followed by injections of 50 μL of normal saline for 7 consecutive days; the intervention group refers to rats that were injected with one time injection of 50 μL fresh prepared monosodium iodoacetate (60 mg/mL)in the right knee joint cavity, followed by fraxetin injections (5 mg·kg-1·d-1, dissolved in 50 μL of normal saline) for 7 consecutive days.
分组 Group | 动物数量 n | Mankin评分 Mankin score | OARSI评分 OARSI sore | 骨体积分数 Tb.BV/TV | 骨表面积密度/(mm2·mm-3) Tb.BS/TV | 骨小梁数量/mm-1 Tb.N |
|---|---|---|---|---|---|---|
| 空白组 Blank group | 6 | 1.17±0.75 | 1.83±0.75 | 0.59±0.01 | 17.44±0.60 | 3.72±0.21 |
| 模型组 Model group | 6 | 10.67±1.86* | 8.00±1.41* | 0.25±0.02* | 13.28±0.86* | 2.47±0.19* |
| 干预组 Intervention group | 6 | 5.67±0.82# | 5.50±1.05# | 0.38±0.02# | 15.59±0.50# | 3.29±0.14# |
表1 不同组大鼠膝关节软骨的Mankin评分、OARSI评分、骨体积分数、骨表面积密度、骨小梁数量比较
Table 1 Comparison of Mankin score, OARSI score, Tb.BV/TV, Tb.BS/TV, and Tb.N of knee cartilage among different groups
分组 Group | 动物数量 n | Mankin评分 Mankin score | OARSI评分 OARSI sore | 骨体积分数 Tb.BV/TV | 骨表面积密度/(mm2·mm-3) Tb.BS/TV | 骨小梁数量/mm-1 Tb.N |
|---|---|---|---|---|---|---|
| 空白组 Blank group | 6 | 1.17±0.75 | 1.83±0.75 | 0.59±0.01 | 17.44±0.60 | 3.72±0.21 |
| 模型组 Model group | 6 | 10.67±1.86* | 8.00±1.41* | 0.25±0.02* | 13.28±0.86* | 2.47±0.19* |
| 干预组 Intervention group | 6 | 5.67±0.82# | 5.50±1.05# | 0.38±0.02# | 15.59±0.50# | 3.29±0.14# |
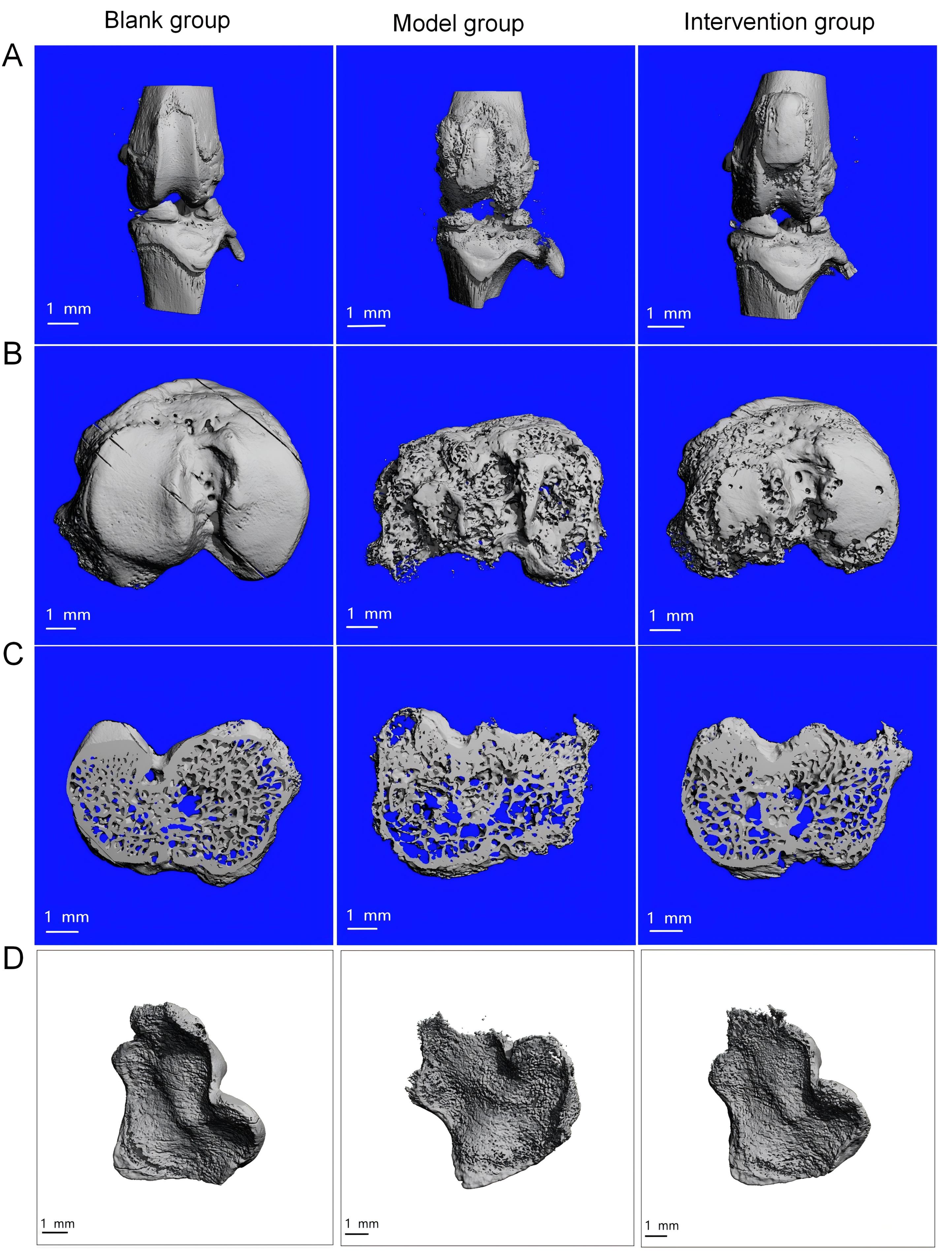
图2 碘乙酸钠注射造模及秦皮素药物干预后大鼠膝关节的micro-CT成像注:A、B、C、D分别从膝关节3D层面、胫骨平台、软骨下骨水平面以及横截面特定区域截面扫描3组大鼠膝关节,观察相关骨质情况,评价骨关节炎情况。空白组指先向右膝关节腔注射50 μL生理盐水,再腔内连续7 d注射50 μL生理盐水的大鼠;模型组指右膝关节腔内单次注射50 μL新鲜配制的60 mg/mL碘乙酸钠建立膝骨关节炎动物模型,再连续7 d注射50 μL生理盐水的大鼠;干预组指右膝关节腔内单次注射50 μL新鲜配制的60 mg/mL碘乙酸钠后,再连续7 d每天注射5 mg/kg秦皮素(溶于50 μL生理盐水)的大鼠。
Figure 2 Micro-CT imaging of the rat knee joint after monosodium iodoacetate injection and fraxetin treatmentNote: A, B, C, and D represent micro-CT images of the knee joints of three groups of rats, scanned at the levels of 3D reconstruction, the tibial platform, the subchondral bone, and specific cross-sectional regions, to assess bone structure and evaluate osteoarthritis changes. The blank group refers to rats that were first injected with 50 μL of normal saline into the right knee joint cavity, followed by intra-articular injections of 50 μL of normal saline for 7 consecutive days; the model group refers to rats that were injected with one time injection of 50 μL fresh prepared monosodium iodoacetate (60 mg/mL)in the right knee joint cavity to induce osteoarthritis, followed by injection of 50 μL of normal saline for 7 consecutive days; the intervention group refers to rats that were injected with one time injection of 50 μL fresh prepared monosodium iodoacetate (60 mg/mL)in the right knee joint cavity, followed by intra-articular injections of fraxetin (5 mg·kg-1·d-1, dissolved in 50 μL of normal saline) for 7 consecutive days.
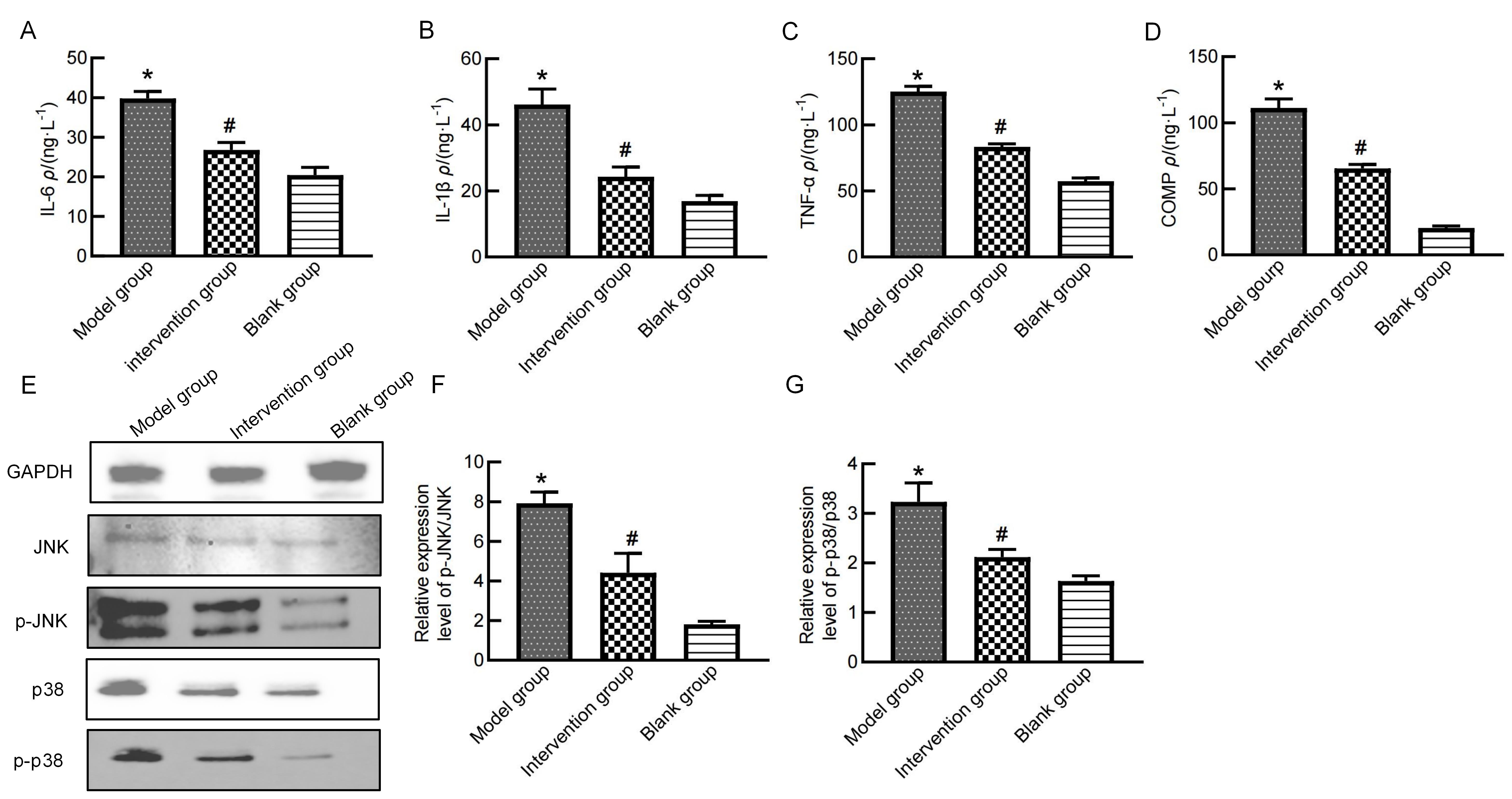
图3 秦皮素药物干预后大鼠血清和膝关节软骨组织中炎症相关蛋白的表达注:A~D分别为ELISA法检测各组大鼠血清IL-6、IL-1β、TNF-α、COMP含量变化。E为蛋白质印迹法检测各组大鼠膝关节软骨组织中p38 MAPK、JNK、p-p38 MAPK、p-JNK蛋白表达。F、G为p-JNK/JNK、p-p38 MAPK/p38 MAPK蛋白表达量比值(反映JNK、p38磷酸化程度,代表通路激活程度)。空白组指先向右膝关节腔注射50 μL生理盐水,再腔内连续7 d注射50 μL 生理盐水的大鼠;模型组指右膝关节腔内单次注射50 μL新鲜配制的60 mg/mL碘乙酸钠建立膝骨关节炎动物模型,再连续7 d注射50 μL生理盐水的大鼠;干预组指右膝关节腔内单次注射50 μL新鲜配制的60 mg/mL碘乙酸钠后,再连续7 d每天注射5 mg/kg秦皮素(溶于50 μL生理盐水)的大鼠。每组6只大鼠(n=6),与空白组相比,*P<0.05;与模型组相比,#P<0.05。
Figure 3 Expression of inflammation-related proteins in rat serum and knee cartilage after fraxetin treatmentNote: A-D, show the serum levels of IL-6, IL-1β, TNF-α, and COMP detected by ELISA. E shows the expression of p38 MAPK, JNK, p-p38 MAPK, and p-JNK proteins in knee cartilage tissues detected by Western blotting. F and G show the ratios of p-JNK/JNK and p-p38 MAPK/p38 MAPK, reflecting the phosphorylation levels of JNK and p38, which indicate the activation levels of the corresponding pathways. The blank group refers to rats that were first injected with 50 μL of normal saline into the right knee joint cavity, followed by intra-articular injections of 50 μL of normal saline for 7 consecutive days; the model group refers to rats that were injected with one time injection of 50 μL fresh prepared monosodium iodoacetate (60 mg/mL)in the right knee joint cavity to induce osteoarthritis, followed by intra-articular injections of 50 μL of normal saline for 7 consecutive days; the intervention group refers to rats that were injected with one time injection of 50 μL fresh prepared monosodium iodoacetate (60 mg/mL)in the right knee joint cavity, followed by intra-articular injections of fraxetin (5 mg·kg-1·d-1, dissolved in 50 μL of normal saline) for 7 consecutive days. There were 6 rats in each group (n=6). Compared with the blank group, *P<0.05; compared with the model group, #P<0.05.
| [1] | GIORGINO R, ALBANO D, FUSCO S, et al. Knee osteoarthritis: epidemiology, pathogenesis, and mesenchy- mal stem cells: what else is new? an update[J]. Int J Mol Sci, 2023, 24(7):6405. DOI:10.3390/ijms24076405 . |
| [2] | ZHAO J L, YANG W Y, LIANG G H, et al. The efficacy and safety of Jinwu Gutong capsule in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials[J]. J Ethnopharmacol, 2022, 293:115247. DOI:10.1016/j.jep.2022.115247 . |
| [3] | 王庆, 冯文昌, 庄迪, 等. 消瘀散贴膏联合针刀治疗膝骨关节炎的疗效及对炎症因子的影响[J]. 辽宁中医杂志, 2020, 47(10):155-159. DOI: 10.13192/j.issn.1000-1719.2020.10.048 . |
| WANG Q, FENG W C, ZHUANG D, et al. Clinical effect of Xiaoyusan plaster combined with acupotomy on knee osteoarthritis and its effect on inflammatory factors[J]. Liaoning J Tradit Chin Med, 2020, 47(10):155-159. DOI: 10.13192/j.issn.1000-1719.2020.10.048 . | |
| [4] | 崔若琳, 王庆, 杨玲, 等. 消瘀散新组方修复膝骨关节炎模型兔的软骨损伤及MMP-13表达[J]. 实验动物与比较医学, 2023, 43(1):30-38. DOI: 10.12300/j.issn.1674-5817.2022.099 . |
| CUI R L, WANG Q, YANG L, et al. Repair effects of Xiaoyusan new formula on cartilage injury and MMP-13 expression in knee osteoarthritis model rabbits[J]. Lab Anim Comp Med, 2023, 43(1):30-38. DOI: 10.12300/j.issn.1674-5817.2022.099 . | |
| [5] | 曹桂云, 宁波, 秦金淼, 等. 基于标准汤剂的秦皮(白蜡树)配方颗粒质量标准分析[J]. 中国实验方剂学杂志, 2023, 29(13):122-129. DOI: 10.13422/j.cnki.syfjx.20230148 . |
| CAO G Y, NING B, QIN J M, et al. Analysis on quality standard of fraxini cortex (Fraxinus chinensis) dispensing granules based on standard decoction[J]. Chin J Exp Tradit Med Formulae, 2023, 29(13):122-129. DOI: 10.13422/j.cnki.syfjx.20230148 . | |
| [6] | 杨凌, 庄迪, 金立伦. 转录组测序筛选大鼠滑膜炎差异表达基因及秦皮素治疗靶点的体外验证[J]. 实验动物与比较医学, 2023, 43(1):11-20. DOI: 10.12300/j.issn.1674-5817.2022.100 . |
| YANG L, ZHUANG D, JIN L L. Screening of differentially expressed genes in rat synovitis by transcriptome sequencing and in vitro verification of therapeutic target of fraxetin[J]. Lab Anim Comp Med, 2023, 43(1):11-20. DOI: 10.12300/j.issn.1674-5817.2022.100 . | |
| [7] | WANG Q, ZHUANG D, FENG W C, et al. Fraxetin inhibits interleukin-1β-induced apoptosis, inflammation, and matrix degradation in chondrocytes and protects rat cartilage in vivo [J]. Saudi Pharm J, 2020, 28(12):1499-1506. DOI:10.1016/j.jsps.2020.09.016 . |
| [8] | WU Q, YAO X B, SHAN N, et al. Platelet-rich plasma ameliorates cartilage degradation in rat models of osteoarthritis via the OPG/RANKL/RANK system[J]. Folia Histochem Cytobiol, 2024, 62(3):154-164. DOI:10.5603/fhc. 100179 . |
| [9] | 李培金, 韩倩, 王威, 等. 姜黄素治疗骨关节炎过程中潜在的铁死亡关键基因的分析与验证[J]. 世界科学技术-中医药现代化, 2024, 26(9):2455-2465. DOI:10.11842/wst.20230705004 . |
| LI P J, HAN Q, WANG W, et al. Analysis and verification of potential ferroptosis key genes in curcumin treatment of osteoarthritis[J]. World Sci Technol-Mod Tradit Chin Med Mater Med, 2024, 26(9):2455-2465. DOI:10.11842/wst. 20230705004 . | |
| [10] | XU Z X, KE T, ZHANG Y F, et al. Danshensu inhibits the IL-1β- induced inflammatory response in chondrocytes and osteoarthritis possibly via suppressing NF-κB signaling pathway[J]. Mol Med, 2021, 27(1):80. DOI:10.1186/s10020-021-00329-9 . |
| [11] | KOVÁCS B, VAJDA E, NAGY E E. Regulatory effects and interactions of the Wnt and OPG-RANKL-RANK signaling at the bone-cartilage interface in osteoarthritis[J]. Int J Mol Sci, 2019, 20(18):4653. DOI:10.3390/ijms20184653 . |
| [12] | ROSAS S, KWOK A, MOORE J, et al. Osteoarthritis as a systemic disease promoted prostate cancer in vivo and in vitro [J]. Int J Mol Sci, 2024, 25(11):6014. DOI:10.3390/ijms25116014 . |
| [13] | YAO Q, WU X H, TAO C, et al. Osteoarthritis: pathogenic signaling pathways and therapeutic targets[J]. Signal Transduct Target Ther, 2023, 8(1):56. DOI:10.1038/s41392-023-01330-w . |
| [14] | ULIVI V, GIANNONI P, GENTILI C, et al. p38/NF-kB-dependent expression of COX-2 during differentiation and inflammatory response of chondrocytes[J]. J Cell Biochem, 2008, 104(4):1393-1406. DOI:10.1002/jcb.21717 . |
| [15] | LU J S, ZHANG H B, PAN J Y, et al. Fargesin ameliorates osteoarthritis via macrophage reprogramming by downregulating MAPK and NF-κB pathways[J]. Arthritis Res Ther, 2021, 23(1):142. DOI:10.1186/s13075-021-02512-z . |
| [16] | DING D, YAN J B, FENG G N, et al. Dihydroartemisinin attenuates osteoclast formation and bone resorption via inhibiting the NF-κB, MAPK and NFATc1 signaling pathways and alleviates osteoarthritis[J]. Int J Mol Med, 2022, 49(1):4. DOI:10.3892/ijmm.2021.5059 . |
| [17] | LIAO J C, WEI Z X, ZHAO C, et al. Inhibition of osteoclastogenesis for periprosthetic osteolysis therapy through the suppression of p38 signaling by fraxetin[J]. Int J Mol Med, 2018, 42(3):1257-1264. DOI:10.3892/ijmm.2018.3698 . |
| [18] | 蔡宛儒, 余芳. 髋膝联合电针治疗早期膝骨关节炎的临床研究[J]. 湖北中医药大学学报, 2024, 26(5):88-90. DOI:10.3969/j.issn.1008-987x.2024.05.24 . |
| CAI W R, YU F. Clinical research on hip-knee coherent electro-acupuncture in treating early knee osteoarthritis[J]. J Hubei Univ Chin Med, 2024, 26(5):88-90. DOI:10.3969/j.issn.1008-987x.2024.05.24 . | |
| [19] | 任国飞, 李同林, 卞恒杰. 抗骨增生胶囊联合洛索洛芬钠治疗早期膝骨关节炎的临床研究[J]. 现代药物与临床, 2024, 39(10):2651-2655. DOI:10.7501/j.issn.1674-5515.2024.10.034 . |
| REN G F, LI T L, BIAN H J. Clinical study on Kanggu Zengsheng Capsules combined with loxoprofen sodium in treatment of early knee osteoarthritis[J]. Drugs Clin, 2024, 39(10):2651-2655. DOI:10.7501/j.issn.1674-5515.2024.10.034 . | |
| [20] | 韩杰, 柴源, 章晓云, 等. 中药复方治疗不同分期膝骨关节炎的研究进展[J]. 中医正骨, 2022, 34(11): 57-61. DOI: 10.3969/j.issn.1001-6015.2022.11.011 . |
| HAN J, CHAI Y, ZHANG X Y, et al. Advancement of research on the treatment of different stages of knee osteoarthritis with compounds[J]. J Tradit Chin Orthop Trauma, 2022, 34(11):57-61. DOI: 10.3969/j.issn.1001-6015.2022.11.011 . | |
| [21] | WU B, WANG R, LI S N, et al. Antifibrotic effects of Fraxetin on carbon tetrachloride-induced liver fibrosis by targeting NF-κB/IκBα, MAPKs and Bcl-2/Bax pathways[J]. Pharmacol Rep, 2019, 71(3):409-416. DOI:10.1016/j.pharep.2019.01.008 . |
| [22] | WITAICENIS A, SEITO L N, SILVEIRA CHAGAS A DA, et al. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives[J]. Phytomedicine, 2014, 21(3):240-246. DOI:10.1016/j.phymed.2013.09.001 . |
| [23] | DE SOUSA VALENTE J. The pharmacology of pain associated with the monoiodoacetate model of osteoarthritis[J]. Front Pharmacol, 2019, 10: 974. DOI: 10.3389/fphar.2019.00974 . |
| [24] | MA T W, ZHANG Z H, SONG X P, et al. Combined detection of COMP and CS846 biomarkers in experimental rat osteoarthritis: a potential approach for assessment and diagnosis of osteoarthritis[J]. J Orthop Surg Res, 2018, 13(1): 230. DOI: 10.1186/s13018-018-0938-3 . |
| [25] | CHEN J Y, ZHANG L, ZHANG H, et al. Triggering of p38 MAPK and JNK signaling is important for oleanolic acid-induced apoptosis via the mitochondrial death pathway in hypertrophic scar fibroblasts[J]. Phytother Res, 2014, 28(10): 1468-1478. DOI: 10.1002/ptr.5150 . |
| [26] | LI X H, ZHANG Z L, LIANG W N, et al. Tougu Xiaotong capsules may inhibit p38 MAPK pathway-mediated inflammation: In vivo and in vitro verification[J]. J Ethnopharmacol, 2020, 249: 112390. DOI: 10.1016/j.jep. 2019. 112390 . |
| [1] | 潘颐聪, 蒋汶洪, 胡明, 覃晓. 慢性肾脏病大鼠主动脉钙化模型的术式优化及效果评价[J]. 实验动物与比较医学, 2025, 45(3): 279-289. |
| [2] | 姜萌, 郝淑兰, 仝立国, 仲启明, 高振飞, 王永辉, 王晞星, 吉海杰. 长春瑞滨诱导大鼠足背静脉炎模型的动态评价[J]. 实验动物与比较医学, 2025, 45(3): 251-258. |
| [3] | 肖林林, 杨逸萱, 黎珊杉, 罗兰诗雨, 尹思威, 孙俊铭, 施维, 欧阳轶强, 李习艺. 利用脑立体定位技术将人源三突变APP基因导入海马区构建阿尔茨海默病大鼠模型[J]. 实验动物与比较医学, 2025, 45(3): 269-278. |
| [4] | 连辉, 姜艳玲, 刘佳, 张玉立, 谢伟, 薛晓鸥, 李健. 异常子宫出血大鼠模型的构建与评价[J]. 实验动物与比较医学, 2025, 45(2): 130-146. |
| [5] | 孙效容, 苏丹, 贵文娟, 陈玥. 手术诱导大鼠中重度膝骨关节炎模型的建立与评价[J]. 实验动物与比较医学, 2024, 44(6): 597-604. |
| [6] | 殷玉莲, 马丽娜, 屠思远, 陈玲, 叶媚娜, 陈红风. 非哺乳期乳腺炎大鼠模型的建立及评价[J]. 实验动物与比较医学, 2024, 44(6): 587-596. |
| [7] | 杨劲, 俞诗雅, 林楠, 方永超, 赵虎, 邱锦维, 林鸿铭, 陈惠燕, 王瑜, 吴伟航. 改良型十二指肠旷置术对2型糖尿病大鼠糖代谢的影响[J]. 实验动物与比较医学, 2024, 44(5): 523-530. |
| [8] | 戚龙菊, 陈世园, 廖泽华, 石袁虎, 孙郁雨, 王庆华. 经血干细胞移植联合运动训练促进大鼠脊髓损伤康复的转录组学分析[J]. 实验动物与比较医学, 2024, 44(5): 531-542. |
| [9] | 张乃群, 袁飘漂, 曹琳茸, 应娜, 杨涛涛. PNR检测在糖尿病肾脏疾病模型大鼠诊断及药效评价中的应用[J]. 实验动物与比较医学, 2024, 44(5): 543-549. |
| [10] | 郑艺清, 邓亚胜, 范燕萍, 梁天薇, 黄慧, 刘永辉, 倪召兵, 林江. 基于数据挖掘的盆腔炎性疾病动物模型应用分析[J]. 实验动物与比较医学, 2024, 44(4): 405-418. |
| [11] | 肖攀, 王红义, 陆璐, 张梅, 陈克明, 申栋帅, 牛廷献. 低氧敏感和低氧耐受型Wistar大鼠筛选及其G1代的低氧敏感性初探[J]. 实验动物与比较医学, 2024, 44(4): 374-383. |
| [12] | 朱晓雨, 袁韩涛, 李四波. 微RNA-887-3p能抑制大鼠椎间盘纤维环细胞中 MDM4表达和细胞增殖并促进细胞凋亡[J]. 实验动物与比较医学, 2024, 44(3): 270-278. |
| [13] | 张一粟, 刘欣茹, 武若杰, 刘睿, 欧阳红, 李晓红. 慢性不可预知性应激与完全弗氏佐剂、福尔马林诱导的妊娠期疼痛-抑郁共病小鼠模型的构建与评价[J]. 实验动物与比较医学, 2024, 44(3): 259-269. |
| [14] | 钟瑞华, 李国停, 杨文捷, 郭湘洁, 周洁芸, 胡颖怡, 倪其承, 杨野, 张敏, 朱焰. 同种异体子宫内膜异位症大鼠模型用于GnRH激动剂类药物的药效评价研究[J]. 实验动物与比较医学, 2024, 44(2): 127-138. |
| [15] | 胡锦华, 韩菁婕, 金旻, 胡滨, 娄月芬. 葛根素对大鼠和小鼠骨密度影响的Meta分析[J]. 实验动物与比较医学, 2024, 44(2): 149-161. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||