













实验动物与比较医学 ›› 2025, Vol. 45 ›› Issue (6): 726-737.DOI: 10.12300/j.issn.1674-5817.2025.119
收稿日期:2025-07-16
修回日期:2025-10-17
出版日期:2025-12-25
发布日期:2025-12-19
通讯作者:
沈义栋(1979—),男,博士,研究员,研究方向:衰老及衰老相关疾病的机理研究。E-mail:yidong.shen@sibcb.ac.cn。ORCID:000-0002-2841-7233作者简介:宋梦娇(1990—),女,本科,实验师,研究方向:秀丽隐杆线虫实验动物模型的构建及其在衰老生物学研究中的应用。E-mail:mengjiao.song@sibcb.ac.cn。ORCID: 0009-0003-1676-7556
基金资助:
SONG Mengjiao( ), SHEN Yidong(
), SHEN Yidong( )(
)( )
)
Received:2025-07-16
Revised:2025-10-17
Published:2025-12-25
Online:2025-12-19
Contact:
SHEN Yidong (ORCID: 0000-0002-2841-7233), E-mail: yidong.shen@sibcb.ac.cn摘要:
线粒体作为细胞的能量代谢中枢,其动态形态和氧化磷酸化功能异常与衰老及多种疾病直接相关。秀丽隐杆线虫(Caenorhabditis elegans,C. elegans,以下简称线虫)是被广泛使用的模式动物。本文系统总结了作者实验室以线虫为模型,多尺度分析了线粒体形态与功能的实验方法,主要包括:(1)形态定性分析,即采用单盲法人工分类(如点状、棒状、网状),该法虽然依赖主观经验,但操作简便,适用于初步表型的筛选;(2)形态定量分析,即依托Fiji/ImageJ平台,对特定组织(如表皮及体壁肌)中线粒体进行自动化参数提取,通过骨架化算法量化网络连通性(分支点数量、网络长度),结合二值化分析计算碎片化指数(如面积/周长比、碎片计数),实现客观表型比对;(3)高通量图像处理,即通过宏命令批量处理,整合形态学滤波、阈值分割及参数导出流程,显著提升大样本量的研究效率;(4)代谢功能监测,即应用Seahorse XF分析仪开展活体线虫呼吸代谢检测,通过序贯注入ATP合酶抑制剂N,N′-二环己基碳二亚胺(dicyclohexylcarbodiimide,DCCD)、解偶联剂羰基氰化物 4-(三氟甲氧基)苯腙[carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone,FCCP]及呼吸链抑制剂叠氮钠(sodium azide,NaN?),精准解析基础耗氧率(oxygen consumption rate,OCR)、ATP耦合呼吸效率及最大呼吸容量(呼吸潜力),进而揭示能量代谢规律。本文整合了形态-功能双维度方法,不仅为关于线虫的线粒体研究提供经验技术,其框架亦可拓展至哺乳动物细胞与类器官模型,以推动靶向线粒体的基础研究和药物开发。
中图分类号:
宋梦娇,沈义栋. 秀丽隐杆线虫的线粒体形态和功能研究方法及应用实例[J]. 实验动物与比较医学, 2025, 45(6): 726-737. DOI: 10.12300/j.issn.1674-5817.2025.119.
SONG Mengjiao,SHEN Yidong. Approaches and Application Examples for Studying Mitochondrial Morphology and Function in Caenorhabditis elegans[J]. Laboratory Animal and Comparative Medicine, 2025, 45(6): 726-737. DOI: 10.12300/j.issn.1674-5817.2025.119.
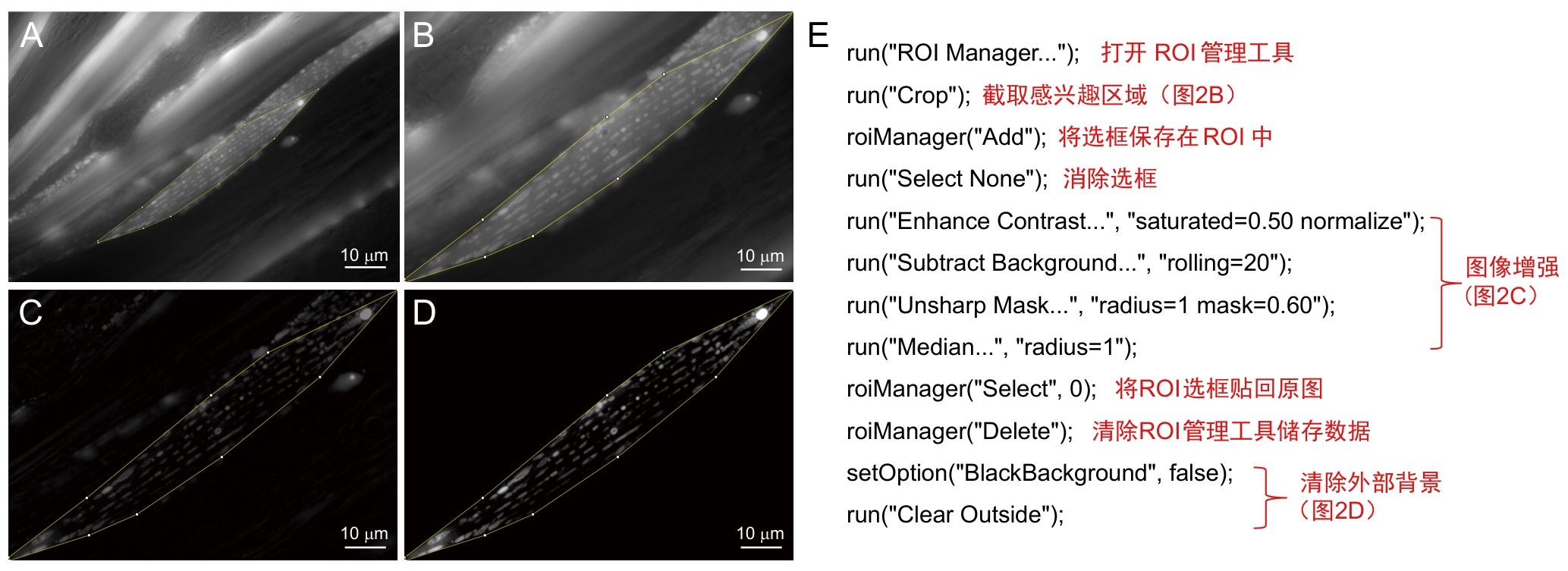
图2 前处理步骤及注释注:A~D,前处理模式图,从选取感兴趣区域(ROI)图像 (A) 中截取该区域 (B) 并进行图像增强处理 (C) 去掉外部背景 (D) 。E,A~D的批量处理宏及其注释。
Figure 2 Preprocessing procedures and annotationsNote: A-D, Preprocessing workflow, target region of interest (ROI) is selected from original image (A), cropped (B), subjected to image enhancement (C), and followed by external background removal (D). E, Batch-processing macro for steps A-D, with its annotated script.
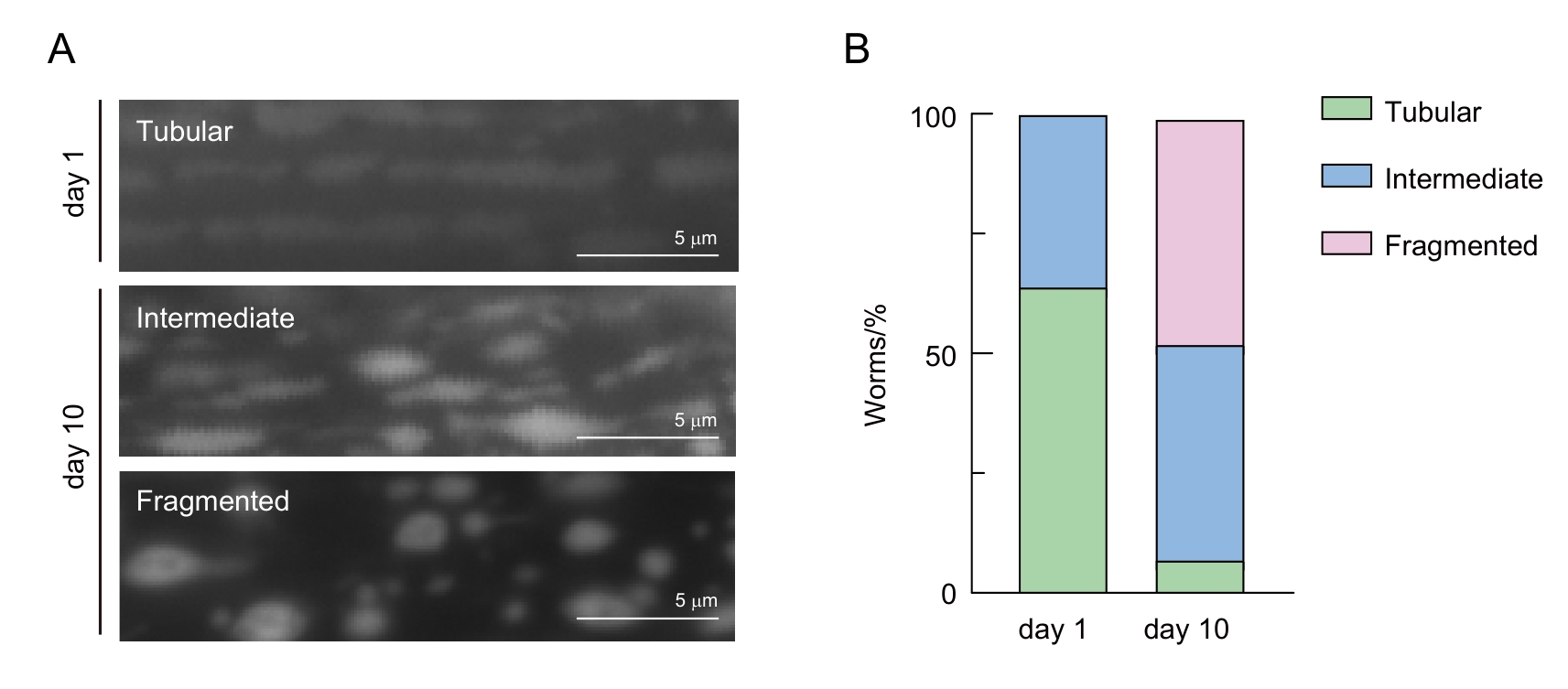
图3 线虫肌肉细胞线粒体形态分类注:A,年轻线虫(day 1)和年老线虫(day 10)不同线粒体形态的代表性照片。B,线粒体形态随年龄的定性分析结果,绿色、蓝色和粉色分别代表管状(tubular)、中间态(intermediate)和碎片化(fragmented)线粒体线虫占总数的百分比。
Figure 3 Classification of mitochondrial morphology in C. elegans muscle cellsNote: A, Representative images of different mitochondrial morphologies in young (day 1) and aged (day 10) C. elegans. B, Qualitative analysis of mitochondrial morphology changes with aging. Green, blue, and pink represent the percentages of C. elegans with tubular, intermediate, and fragmented mitochondria, respectively.
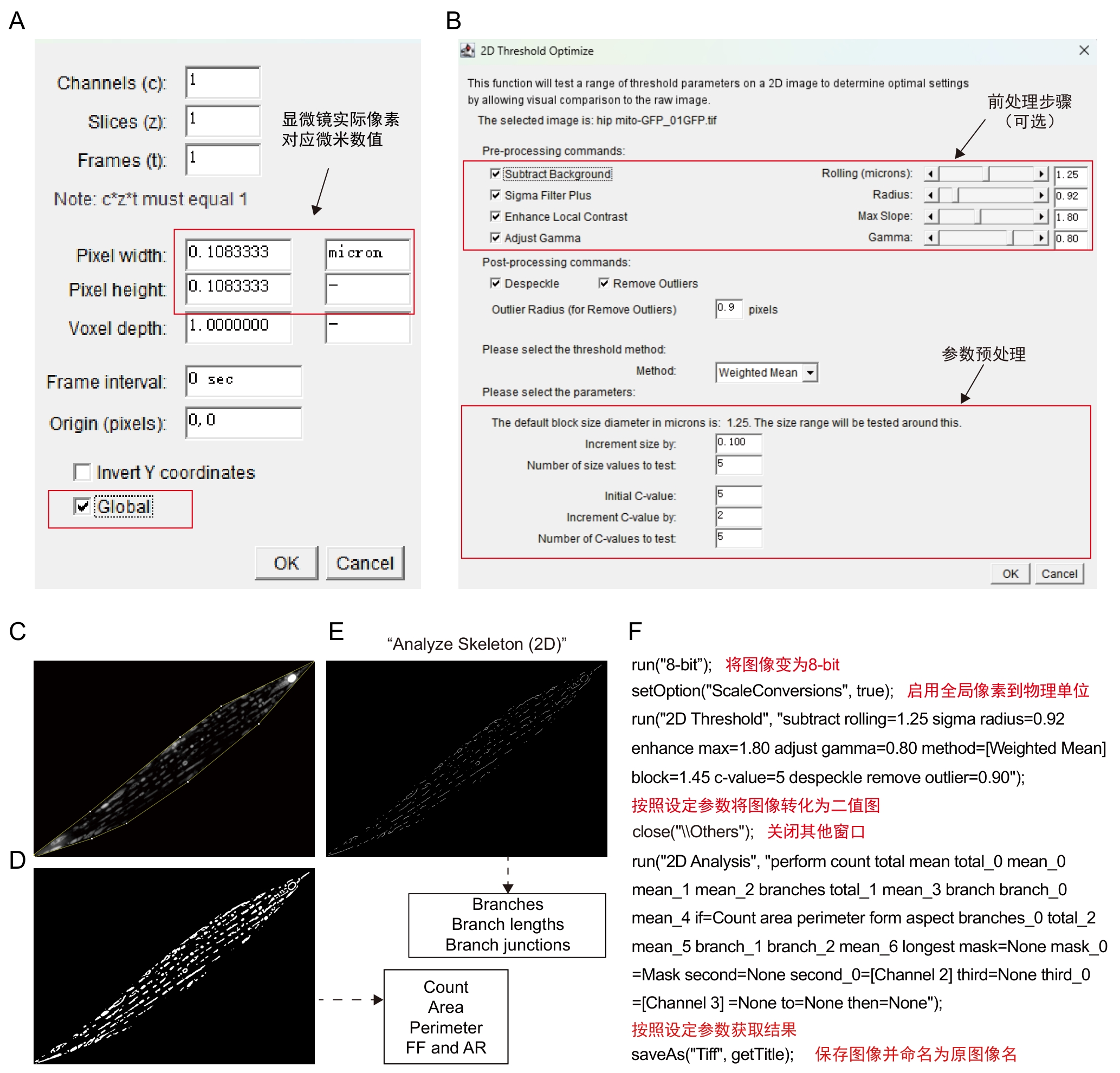
图4 Mitochondria Analyzer插件步骤及注释注:A~B,插件操作界面。C~E,软件处理模式图,将经过前处理的图像(C)以设定的block size和C-value参数获取二值化图像(D),并在此基础上通过Skeletonize(2D)获取骨架图像(E)。二值化图像(C)可进一步获取线粒体数量(Count)、面积(Area)、周长 (Perimeter)、外形因数(form factor,FF)、长宽比(aspect ratio,AR)等数值,骨架图像(E)可进一步获取骨架分支数(Branches)、分支长度(Branch lengths)、骨架交汇点数(Branch junctions)等数值。F,C~E的批量处理宏及其注释。
Figure 4 Procedures and annotations of Mitochondria Analyzer pluginNote: A-B, Plugin operation interface. C-E, Schematic of software processing workflow, the pre-processed image (C) is converted into a binarized image (D) using defined block sized and C-value parameters. The binarized image (D) is then skeletonized via the Skeletonize (2D) function to obtain the image (E) . From the binarized image (C), parameters such as mitochondrial count, area, perimeter, form factor and aspect ratio can be derived. From the skeleton image (E), data including branch number (Branches), branch lengths and branch junctions can be extracted. F, batch processing macro for steps C–E, accompanied by explanatory notes.
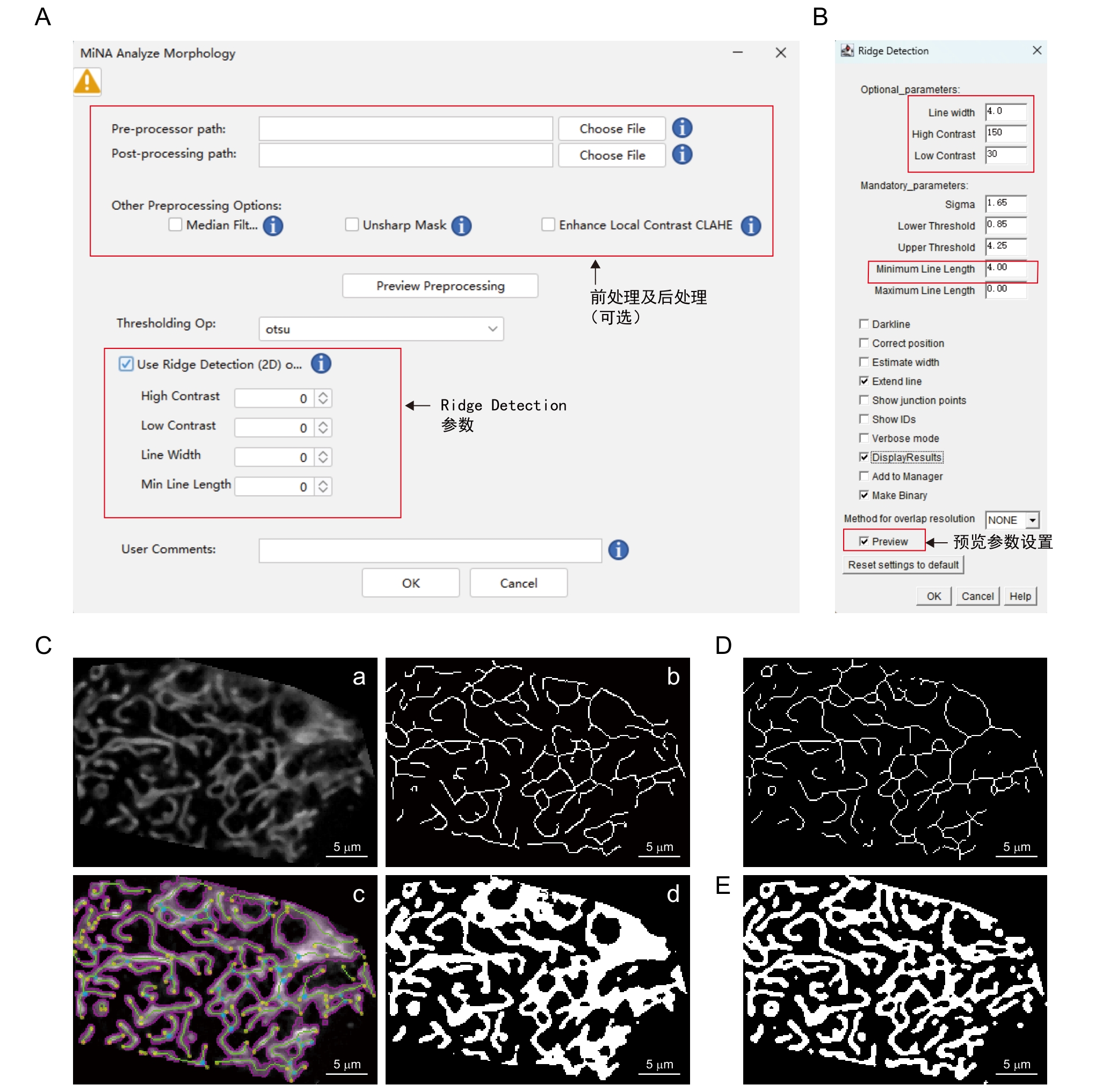
图5 MiNA - Mitochondrial Network Analysis插件步骤及注释注:A~B,插件操作界面。C,软件处理模式图,将经过前处理的图像(a)通过自动阈值(Auto threshold-otsu)二值化图像获取线粒体轮廓(b),以Ridge Detection界面设定的参数(B)获取骨架图像(c),最后分别通过二值化图像和骨架图像计算相关参数并生成轮廓图 (d)。D,Skeletonize骨架算法生成结果。E,Mitochondria Analyzer二值化算法生成结果。
Figure 5 Procedures and annotations of MiNA - Mitochondrial Network Analysis pluginNote: A-B, Plugin operation interface. C, Schematic diagram of the software processing workflow. The pre-processed image (a) undergoes binarization via the auto thresholding method (Auto threshold-Otsu) to extract mitochondrial contours (b). Using the parameters set for Ridge Detection in (B) to obtain the skeleton image (c). Relevant parameters are calculated from both the binarized image and skeleton image, and a contour map (d) is produced. D, Skeletonize algorithm(Skeletonization). E, Mitochondria Analyzer binarization algorithm.
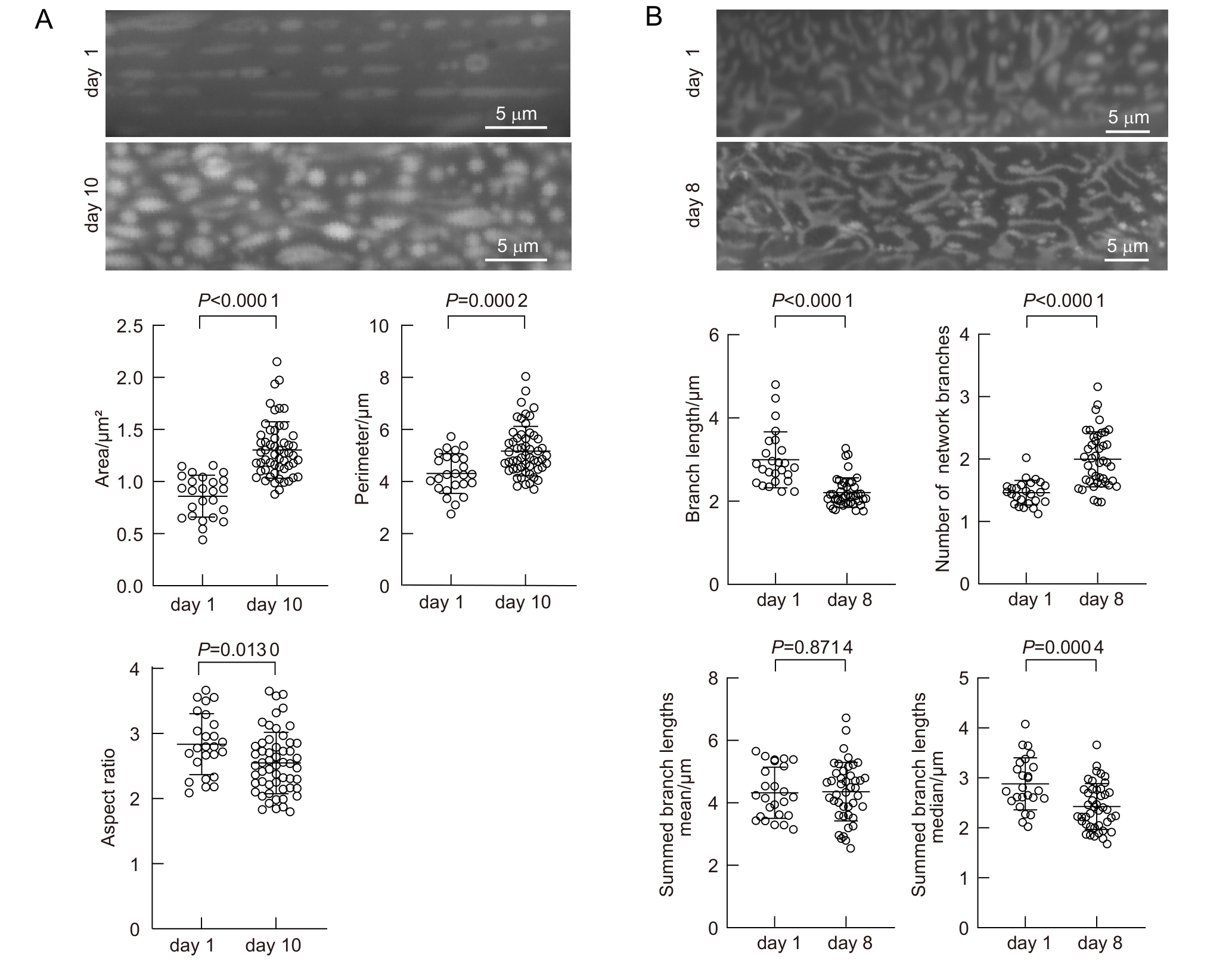
图6 老年线虫线粒体形态发生改变注:A,年轻线虫(day 1)和年老线虫(day 10)肌肉细胞线粒体平均线粒体面积(area)、周长(perimeter)和长宽比(aspect ratio)。B,年轻线虫(day 1)和年老线虫(day 8)表皮线粒体分支长度(branch length)、网络数量(number of network branches)、独立骨架长度平均值(summed branch lengths mean)和独立骨架长度中位数(summed branch lengths median)。A,n=25(day 1),n=57(day 10);B,n=25(day 1), n=43(day 8)。
Figure 6 Age-dependent mitochondrial morphology changes in C. elegansNote: A, Average mitochondrial area, perimeter, and aspect ratio in muscle cells of young adults (day 1) and aged adults (day 10). B, Branch length, number of network branches, summed branch lengths mean and summed branch lengths median in hypodermal mitochondria of young (day 1) and aged (day 8) C. elegans. A, n=25 (day 1), n=57 (day 10); B, n=25 (day 1), n=43 (day 8).
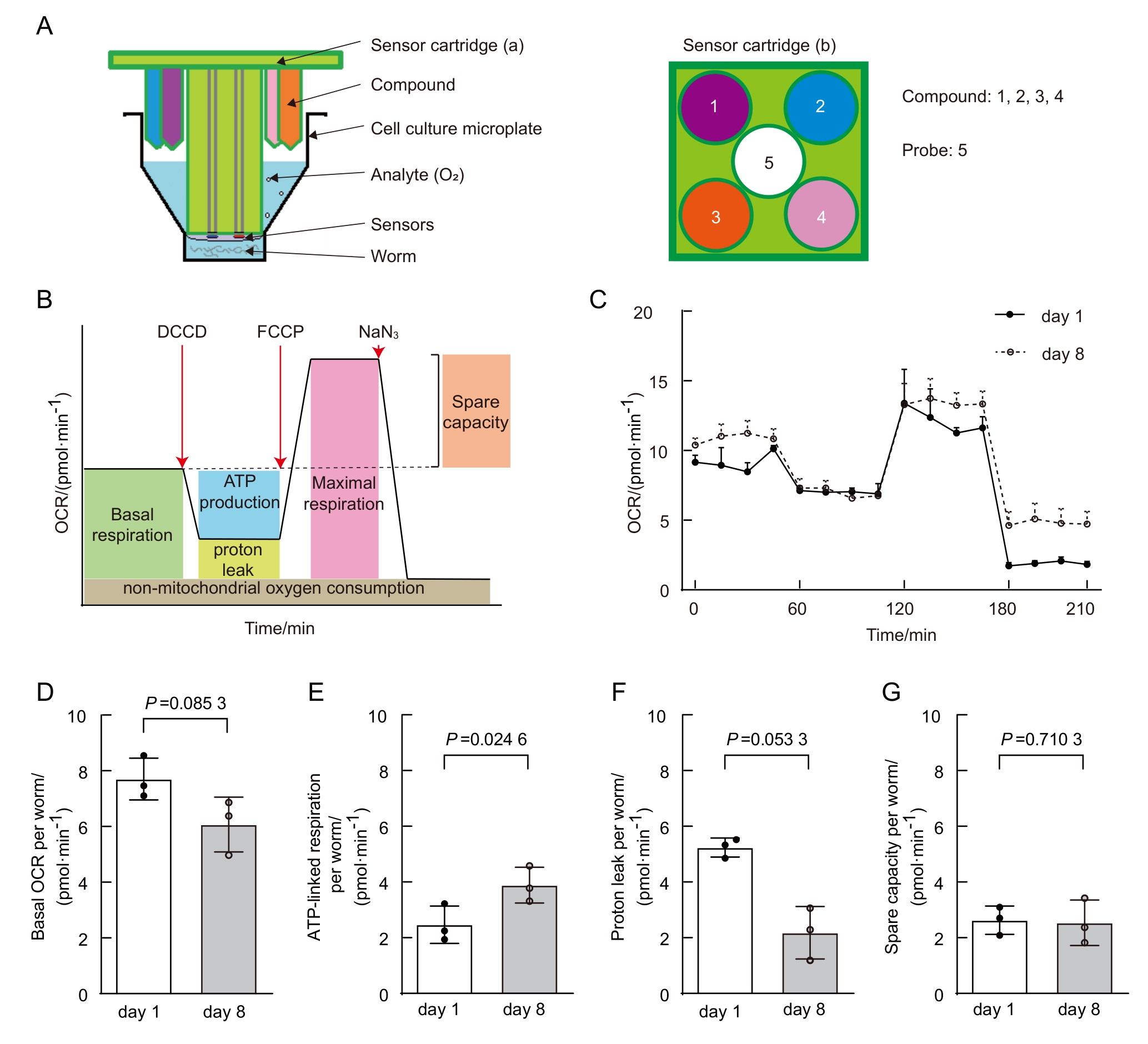
图8 年老线虫线粒体代谢发生改变(数据来自本实验室已发表文献[20])注:A,Seahorse分析仪板示意图。由传感器探针板(sensor cartridge)和细胞培养板(cell culture microplate)组合而成。a和b为传感器探针板的侧视图和俯视图,其中传感器位于中心,化合物的进样口位于每个孔的外围。添加同一个化合物选择相同的进样口(即相同颜色)。每个探头包含4个进样口,以及两个用于测量氧含量和溶液pH值的传感器。B,典型的OCR曲线阶段示意图。C,年轻和年老线虫的OCR比较。D~G,年轻与年老线虫的基础呼吸(D)、ATP相关呼吸 (E)、质子泄露 (F)和呼吸潜力 (G)比较。
Figure 8 Age-dependent mitochondrial function changes in C.elegans (data from published paper of our lab [20])Note: A, Schematic of Seahorse analyzer plates. The analyzer plates consist of a sensor cartridge and a cell culture microplate. a and b are side-view and top-view of the sensor cartridge, with the sensor at center and the compound injection ports at the periphery of each well. Same injection port (i.e., same color port) is selected for adding same compound. Each probe contains 4 injection ports and two sensors measuring oxygen content and solution pH. B, Typical OCR curve phases. C, Comparison of OCR between young and aged C. elegans. D-G, Comparison of basal respiration (D), ATP-linked respiration (E), proton leak (F), and spare respiratory capacity (G) between young and aged C. elegans.
| [1] | MUKHERJEE I, GHOSH M, MEINECKE M. MICOS and the mitochondrial inner membrane morphology–when things get out of shape[J]. FEBS Lett, 2021, 595(8):1159-1183. DOI:10.1002/1873-3468.14089 . |
| [2] | OKAMOTO K, SHAW J M. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes[J]. Annu Rev Genet, 2005, 39:503-536. DOI:10.1146/annurev.genet.38. 072902.093019 . |
| [3] | LÓPEZ-OTÍN C, BLASCO M A, PARTRIDGE L, et al. Hallmarks of aging: an expanding universe[J]. Cell, 2023, 186(2):243-278. DOI:10.1016/j.cell.2022.11.001 . |
| [4] | YAN C J, DUANMU X Y, ZENG L, et al. Mitochondrial DNA: distribution, mutations, and elimination[J]. Cells, 2019, 8(4):379. DOI:10.3390/cells8040379 . |
| [5] | KLIONSKY D J, ABDELMOHSEN K, ABE A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition)[J]. Autophagy, 2016, 12(1):1-222. DOI:10.1080/15548627.2015.1100356 . |
| [6] | MACK H I D, HEIMBUCHER T, MURPHY C T. The nematode Caenorhabditis elegans as a model for aging research[J]. Drug Discov Today Dis Models, 2018, 27:3-13. DOI:10.1016/j.ddmod.2018.11.001 . |
| [7] | JI T, ZHANG X L, XIN Z L, et al. Does perturbation in the mitochondrial protein folding pave the way for neurodegeneration diseases?[J]. Ageing Res Rev, 2020, 57:100997. DOI:10.1016/j.arr.2019.100997 . |
| [8] | SOHRABI S, MOR D E, KALETSKY R, et al. High-throughput behavioral screen in C. elegans reveals Parkinson's disease drug candidates[J]. Commun Biol, 2021, 4(1):203. DOI:10.1038/s42003-021-01731-z . |
| [9] | YE S W, SONG S D, LIU X J, et al. A small-molecule screen identifies novel aging modulators by targeting 5-HT/DA signaling pathway[J]. Aging Cell, 2025, 24(3): 1-13. DOI:10.1111/acel.14411 . |
| [10] | PANDA M, FAKITSA M, MARKAKI M, et al. Caenorhabditis elegans as an emerging high throughput chronotherapeutic drug screening platform for human neurodegenerative disorders[J]. Adv Drug Deliv Rev, 2025, 224:115655. DOI:10.1016/j.addr.2025.115655 . |
| [11] | YOO I, AHN I, LEE J, et al. Extracellular flux assay (Seahorse assay): Diverse applications in metabolic research across biological disciplines[J]. Mol Cells, 2024, 47(8):100095. DOI:10.1016/j.mocell.2024.100095 . |
| [12] | STIERNAGLE T. Maintenance of C. elegans [J]. WormBook, 2006:1-11. DOI:10.1895/wormbook.1.101.1 . |
| [13] | MALLICK A, RANAWADE A, VAN DEN BERG W, et al. Axin-mediated regulation of lifespan and muscle health in C. elegans requires AMPK-FOXO signaling[J]. iScience, 2020, 23(12):101843. DOI:10.1016/j.isci.2020.101843 . |
| [14] | REGMI S G, ROLLAND S G, CONRADT B. Age-dependent changes in mitochondrial morphology and volume are not predictors of lifespan[J]. Aging (Albany NY), 2014, 6(2):118-30. DOI: 10.18632/aging.100639 . |
| [15] | XIA Q, LI P L, CASAS-MARTINEZ J C, et al. Peroxiredoxin 2 regulates DAF-16/FOXO mediated mitochondrial remodelling in response to exercise that is disrupted in ageing[J]. Mol Metab, 2024, 88:102003. DOI: 10.1016/j.molmet.2024.102003 . |
| [16] | CHAUDHRY A, SHI R, LUCIANI D S. A pipeline for multidimensional confocal analysis of mitochondrial morphology, function, and dynamics in pancreatic β-cells[J]. Am J Physiol Endocrinol Metab, 2020, 318(2):E87-E101. DOI:10.1152/ajpendo.00457.2019 . |
| [17] | Mitochondria Analyzer[Z/OL]. [2025-07-16]. . |
| [18] | VALENTE A J, MADDALENA L A, ROBB E L, et al. A simple ImageJ macro tool for analyzing mitochondrial network morphology in mammalian cell culture[J]. Acta Histochem, 2017, 119(3):315-326. DOI:10.1016/j.acthis.2017.03.001 . |
| [19] | MiNA - Mitochondrial Network Analysis[Z/OL]. [2025-07-16]. . |
| [20] | NG L F, GRUBER J. Measurement of respiration rate in live Caenorhabditis elegans [J]. Bio Protoc, 2019, 9(10):1-12. DOI:10.21769/BioProtoc.3243 . |
| [21] | HAROON S, VERMULST M. Oxygen consumption measurements in Caenorhabditis elegans using the seahorse XF24[J]. Bio Protoc, 2019, 9(13):e3288. DOI:10.21769/BioProtoc.3288 . |
| [22] | SONG M J, DONG S M, ZHANG X F, et al. A moderate static magnetic field promotes C. elegans longevity through cytochrome P450s[J]. Sci Rep, 2022, 12:16108. DOI:10.1038/s41598-022-20647-0 . |
| [23] | DILLIN A, HSU A L, ARANTES-OLIVEIRA N, et al. Rates of behavior and aging specified by mitochondrial function during development[J]. Science, 2002, 298(5602):2398-2401. DOI: 10.1126/science/1077780 . |
| [24] | AMORIM J A, COPPOTELLI G, ROLO A P, et al. Mitochondrial and metabolic dysfunction in ageing and age-related diseases[J]. Nat Rev Endocrinol, 2022, 18(4):243-258. DOI:10.1038/s41574-021-00626-7 . |
| [25] | SUN N, YOULE R J, FINKEL T. The mitochondrial basis of aging[J]. Mol Cell, 2016, 61(5):654-666. DOI:10.1016/j.molcel.2016.01.028 . |
| [26] | BAR-ZIV R, BOLAS T, DILLIN A. Systemic effects of mitochondrial stress[J]. EMBO Rep, 2020, 21(6):e50094. DOI:10.15252/embr.202050094 . |
| [27] | REGMI S G, ROLLAND S G, CONRADT B. Age-dependent changes in mitochondrial morphology and volume are not predictors of lifespan[J]. Aging, 2014, 6(2):118-130. DOI:10.18632/aging.100639 . |
| [28] | SON H G, ALTINTAS O, KIM E J E, et al. Age-dependent changes and biomarkers of aging in Caenorhabditis elegans [J]. Aging Cell, 2019, 18(2):e12853. DOI:10.1111/acel.12853 . |
| [29] | MAGLIONI S, MELLO D F, SCHIAVI A, et al. Mitochondrial bioenergetic changes during development as an indicator of C. elegans health-span[J]. Aging, 2019, 11(16):6535-6554. DOI:10.18632/aging.102208 . |
| [30] | MITRA K, WUNDER C, ROYSAM B, et al. A hyperfused mitochondrial state achieved at G1–S regulates cyclin E buildup and entry into S phase[J]. Proc Natl Acad Sci U S A, 2009, 106(29):11960-11965. DOI:10.1073/pnas.0904875106 . |
| [31] | DAGDA R K, CHERRA S J, KULICH S M, et al. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission[J]. J Biol Chem, 2009, 284(20):13843-13855. DOI:10.1074/jbc.M808515200 . |
| [1] | 孙涵, 郭芃, 俞昕何, 张隽巧, 尧莹, 杨文. 秀丽隐杆线虫作为退行性疾病模型的分子通路研究进展[J]. 实验动物与比较医学, 2025, 45(6): 738-751. |
| [2] | 成慧, 方菲, 石嘉豪, 杨桦, 张梦杰, 杨平, 费俭. hil-1基因通过饮食限制通路调节秀丽隐杆线虫寿命[J]. 实验动物与比较医学, 2023, 43(3): 271-281. |
| [3] | 李晗, 张笑瑞, 张成芳. 间歇禁食法在改善奥氮平诱导小鼠代谢紊乱中的机制研究[J]. 实验动物与比较医学, 2023, 43(1): 3-10. |
| [4] | 李英娘, 戴玮, 盛健. 斑马鱼甲状腺肿大病例分析[J]. 实验动物与比较医学, 2019, 39(6): 462-466. |
| [5] | 沈艳, 徐汪洋, 朱后保. 氧化还原酶DHTKD1基因突变致病机制及小鼠模型研究进展[J]. 实验动物与比较医学, 2018, 38(6): 468-472. |
| [6] | 濮祥强, 王祥, 钱光辉, 马锦, 丁粤粤, 吕海涛. 小鼠免疫性冠状动脉炎内皮细胞线粒体的动态变化[J]. 实验动物与比较医学, 2018, 38(3): 169-175. |
| [7] | 常凯, 王裕, 庞文彪, 高继萍, 陈朝阳, 宋国华. 硒对氟致大鼠肾小管上皮细胞线粒体膜电位改变的拮抗作用[J]. 实验动物与比较医学, 2017, 37(3): 179-184. |
| [8] | 李媛, 张梅英. 自噬与帕金森疾病及相关性模型的研究进展[J]. 实验动物与比较医学, 2015, 35(4): 335-340. |
| [9] | 申幸娇, 岳秉飞, 马丽颖. 三个封闭群实验兔线粒体DNA D-loop区多态性分析[J]. 实验动物与比较医学, 2014, 34(1): 29-34. |
| [10] | 叶红梅, 钟春燕, 黄敏贤, 吕俊华. 荔枝核提取物对D-半乳糖诱发小鼠学习记忆损伤的保护机制[J]. 实验动物与比较医学, 2013, 33(4): 285-289. |
| [11] | 高骏, 倪丽菊, 孙凤萍, 王金祥, 胡建华, 高诚, 李凯, 肖君华, 周宇荀. 东方田鼠指名亚种的线粒体基因组序列分析及系统进化研究[J]. 实验动物与比较医学, 2013, 33(3): 167-173. |
| [12] | 张评浒, 陶元清, 江振洲, 王忠东, 范薇, 张陆勇. 喜马拉雅旱獭作为药物线粒体毒性替代模型的可行性分析[J]. 实验动物与比较医学, 2012, 32(5): 436-440. |
| [13] | 李洪涛1,吴清洪2,肖东2,袁进2,王万山2,张嘉宁2,顾为望2. 四种实验用小型猪mtDNA控制区5'端序列的比较研究[J]. 实验动物与比较医学, 2009, 29(4): 237-240. |
| [14] | 管敏强1,曹琼洁2,陈忠义2,阮冬芬2,楼哲丰2,金龙金2. 线粒体DNA序列分析封闭群小鼠遗传稳定性[J]. 实验动物与比较医学, 2009, 29(2): 113-116. |
| [15] | 谢建云1,2,冯洁1,柏熊3,胡建华1,高诚1. 四种群东方田鼠线粒体DNA[J]. 实验动物与比较医学, 2008, 28(5): 299-303. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||