
Laboratory Animal and Comparative Medicine ›› 2022, Vol. 42 ›› Issue (3): 187-193.DOI: 10.12300/j.issn.1674-5817.2021.138
• Animal Models of Human Diseases • Previous Articles Next Articles
Xiao LU1,2( ), Lin ZHANG2, Hui JI1, Shanxiang JIANG1(
), Lin ZHANG2, Hui JI1, Shanxiang JIANG1( )
)
Received:2021-08-17
Revised:2021-11-05
Online:2022-06-25
Published:2022-07-01
Contact:
Xiao LU, Shanxiang JIANG
About author:JIANG Shanxiang, E-mail: jiangshanxiang@163.com
CLC Number:
Xiao LU, Lin ZHANG, Hui JI, Shanxiang JIANG. Efficacy of DZ1462, a Novel Sodium-phosphate Transporter Inhibitor, on 5/6 Nephrectomy-induced Hyperphosphatemia Model Rats[J]. Laboratory Animal and Comparative Medicine, 2022, 42(3): 187-193.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2021.138
分组 Group | 动物数量 Animal n | 第1周 1st Week | 第3周 3rd Week | 第5周 5th Week | 第7周 7th Week | 第10周 10th Week | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Rd% | No. | Rd% | No. | Rd% | No. | Rd% | No. | Rd% | ||||||
| Ⅰ | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Ⅱ | 60 | 6 | 10 | 3 | 15 | 0 | 15 | 6 | 25 | 5 | 30 | ||||
| Ⅲ | 60 | 8 | 13 | 2 | 16 | 2 | 20 | 2 | 23 | 10 | 40 | ||||
| Ⅳ | 30 | 0 | 0 | 1 | 3 | 1 | 6 | 0 | 6 | 0 | 6 | ||||
Table 1 Mortality of different group rats within 10 weeks after modeling
分组 Group | 动物数量 Animal n | 第1周 1st Week | 第3周 3rd Week | 第5周 5th Week | 第7周 7th Week | 第10周 10th Week | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Rd% | No. | Rd% | No. | Rd% | No. | Rd% | No. | Rd% | ||||||
| Ⅰ | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Ⅱ | 60 | 6 | 10 | 3 | 15 | 0 | 15 | 6 | 25 | 5 | 30 | ||||
| Ⅲ | 60 | 8 | 13 | 2 | 16 | 2 | 20 | 2 | 23 | 10 | 40 | ||||
| Ⅳ | 30 | 0 | 0 | 1 | 3 | 1 | 6 | 0 | 6 | 0 | 6 | ||||
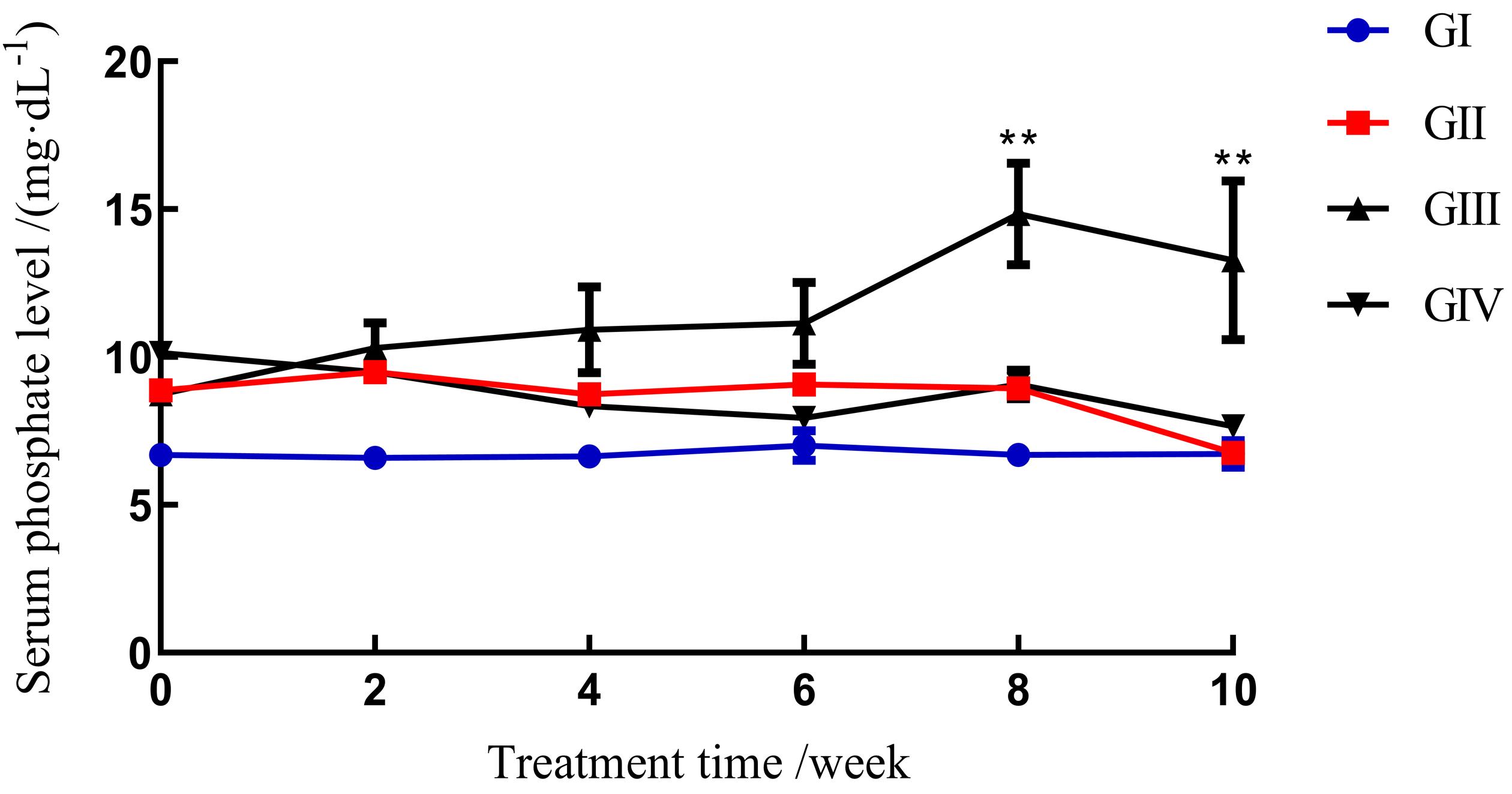
Figure1 Serum phosphorus levels in different groups of ratsNote: GⅠ, normal rats with a normal diet (groupⅠ);GⅡ, 5/6 nephrectomy rats with a normal diet (group Ⅱ);GⅢ, 5/6 nephrectomy rats with a high Pi diet (group Ⅲ);GⅣ, sham surgery rats with a high Pi diet (group Ⅳ). **Represent serum Pi has a highly significant difference between Group Ⅰ and Group Ⅲ at the 8th and 10th week (P < 0.01).
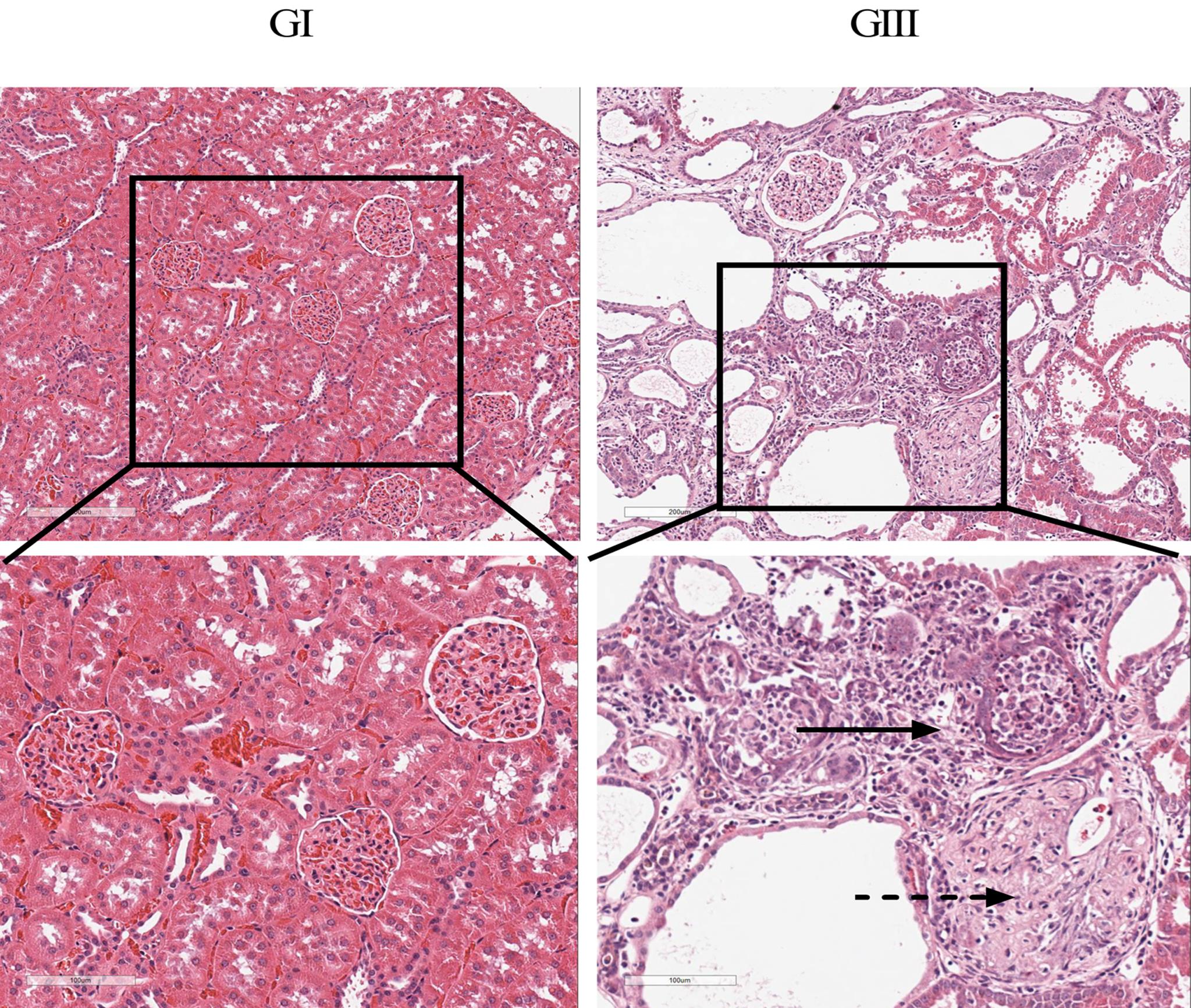
Figure 2 Pathological evaluation of kidney tissue in 5/6 nephrectomy model rats (HE staining)Note: GⅠ is normal rats with a normal diet (groupⅠ), GⅢ is 5/6 nephrectomy rats with a high Pi diet (groupⅢ). The magnification of the upper and the lower is 100 and 200, respectively. The solid arrow shows the interstitial fibrosis and inflammatory, and the dashed arrow shows glomerular sclerosis.
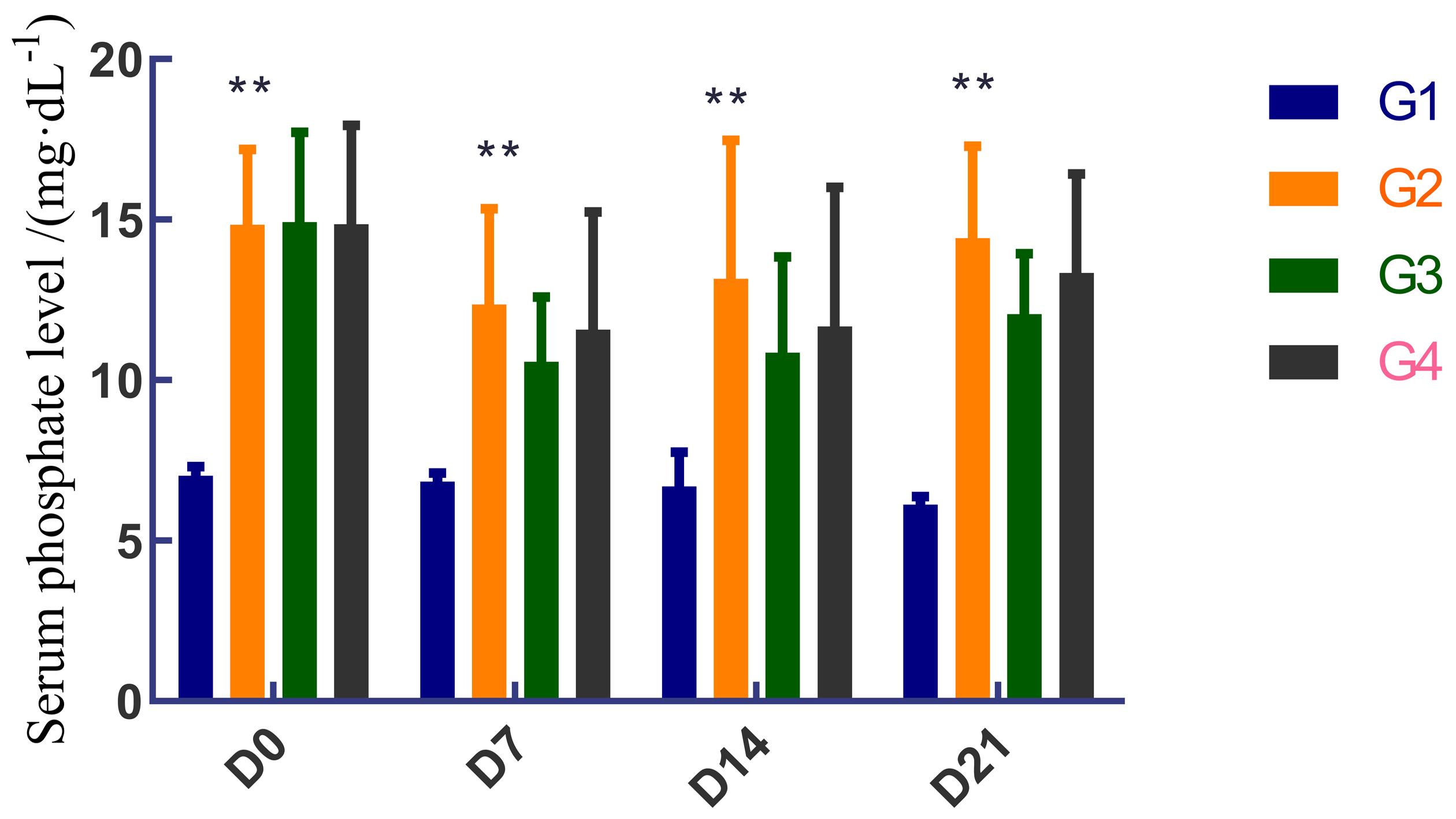
Figure 3 Comparison of serum phosphorus levels among different groups of rats after drug treatmentNote: G1 is normal rats with a normal diet; G2 is 5/6 nephrectomy and high diet model rats received a normal diet; G3 is 5/6 nephrectomy and high diet model rats received a high Pi diet and DZ1462 30 mg/kg; G4 is 5/6 nephrectomy and high diet model rats received a high Pi diet and Sevelamer 250 mg/kg. **Represent serum Pi in G2 is significantly higher than that in G1 on days 0, 7, 14, and 21 (P < 0.01).
| 1 | CHINYERE I R, MOUKABARY T, HUTCHINSON M D, et al. Progression of infarct-mediated arrhythmogenesis in a rodent model of heart failure[J]. Am J Physiol Heart Circ Physiol, 2021, 320(1): H108-H116. DOI:10.1152/ajpheart.00639. 2020 . |
| 2 | FOURNIER M L, CLÉMENT T, AUSSUDRE J, et al. Contusion rodent model of traumatic brain injury: controlled cortical impact[J]. Methods Mol Biol, 2021, 2193:49-65. DOI:10.1007/978-1-0716-0845-6_6 . |
| 3 | MARRA A. Animal models for drug development for MRSA[J]. Methods Mol Biol, 2020, 2069:253-266. DOI:10.1007/978-1-4939-9849-4_17 . |
| 4 | YOUNG F G. Growth and diabetes in normal animals treated with pituitary (anterior lobe) diabetogenic extract[J]. Biochem J, 1945, 39(5):515-536. DOI:10.1042/bj0390515 . |
| 5 | SARKAR D, AGRAWAL A, PAL D K. Clinical assessment of stabilisation of renal function after nephrectomy[J]. Urologia, 2021, 88(3):223-226. DOI:10.1177/0391560320987799 . |
| 6 | CHANTLER C, LIEBERMAN E, HOLLIDAY M A. A rat model for the study of growth failure in uremia[J]. Pediatr Res, 1974, 8(2):109-113. DOI:10.1203/00006450-197402000-00007 . |
| 7 | CZÉH B, SIMON M. Benefits of animal models to understand the pathophysiology of depressive disorders[J]. Prog Neuropsychopharmacol Biol Psychiatry, 2021, 106:110049. DOI:10.1016/j.pnpbp.2020.110049 . |
| 8 | PÉREZ-LÓPEZ L, BORONAT M, MELIÁN C, et al. Animal models and renal biomarkers of diabetic nephropathy[J]. Adv Exp Med Biol, 2021, 1307:521-551. DOI:10.1007/5584_2020_527 . |
| 9 | MCCARRON A, PARSONS D, DONNELLEY M. Animal and cell culture models for cystic fibrosis: which model is right for your application? [J]. Am J Pathol, 2021, 191(2):228-242. DOI:10.1016/j.ajpath.2020.10.017 . |
| 10 | ROBINSON N B, KRIEGER K, KHAN F M, et al. The Current state of animal models in research: a review[J]. Int J Surg, 2019, 72:9-13. DOI:10.1016/j.ijsu.2019.10.015 . |
| 11 | GUO W, WANG L Y, HAN X, et al. Effect of parathyroid hormone on intestinal mucosal sodium dependent phosphorus transporter[J]. Iran J Kidney Dis, 2021, 1(1):48-55. |
| 12 | ASKARI H, SEIFI B, KADKHODAEE M. Evaluation of renal-hepatic functional indices and blood pressure based on the progress of time in a rat model of chronic kidney disease[J]. Nephrourol Mon, 2016, 8(3): e37840. DOI:10.5812/numonthly. 37840 . |
| 13 | LEVEY A S, STEVENS L A, CORESH J. Conceptual model of CKD: applications and implications[J]. Am J Kidney Dis, 2009, 53(3 ): S4-S16. DOI:10.1053/j.ajkd.2008.07.048 . |
| 14 | HAN Y, LU J S, XU Y, et al. Rutin ameliorates renal fibrosis and proteinuria in 5/6-nephrectomized rats by anti-oxidation and inhibiting activation of TGFβ1-smad signaling[J]. Int J Clin Exp Pathol, 2015, 8(5):4725-4734. |
| 15 | GHOSH S S, MASSEY H D, KRIEG R, et al. Curcumin ameliorates renal failure in 5/6 nephrectomized rats: role of inflammation[J]. Am J Physiol Renal Physiol, 2009, 296(5): F1146-F1157. DOI:10.1152/ajprenal.90732.2008 . |
| 16 | JING W H, NUNES A C F, FARZANEH T, et al. Phosphate binder, ferric citrate, attenuates Anemia, renal dysfunction, oxidative stress, inflammation, and fibrosis in 5/6 nephrectomized CKD rats[J]. J Pharmacol Exp Ther, 2018, 367(1):129-137. DOI:10.1124/jpet.118.249961 . |
| 17 | 王婷玉. 虫草保肾颗粒对5/6肾切除大鼠钙磷代谢的影响[D]. 哈尔滨: 黑龙江省中医药科学院, 2019. |
| WANG T Y. Effect of Chongcao Baoshen Granules on calcium and phosphor metabolism with 5/6 nephrectomy rats[D]. Harbin: Heilongjiang College of Traditional Chinese Medicine, 2019. | |
| 18 | BAO Y W, YUAN Y, CHEN J H, et al. Kidney disease models: tools to identify mechanisms and potential therapeutic targets[J]. Zool Res, 2018, 39(2):72-86. DOI:10.24272/j.issn.2095-8137.2017.055 . |
| 19 | RANI L, GAUTAM N K. Drosophila renal organ as a model for identification of targets and screening of potential therapeutic agents for diabetic nephropathy[J]. Curr Drug Targets, 2018, 19(16):1980-1990. DOI:10.2174/1389450119666180808114533 . |
| 20 | HAMZAOUI M, DJERADA Z, BRUNEL V, et al. 5/6 nephrectomy induces different renal, cardiac and vascular consequences in 129/Sv and C57BL/6JRj mice[J]. Sci Rep, 2020, 10(1):1524. DOI:10.1038/s41598-020-58393-w . |
| 21 | 李鑫宇, 李丹, 赵学智. 一步法5/6肾切除大鼠加高磷饮食快速建立肾衰竭致血管钙化动物模型法[J]. 中国中西医结合肾病杂志, 2010, 11(10):862-864, 后插二. DOI:10.3969/j.issn.1009-587X.2010.10.006 . |
| LI X Y, LI D, ZHAO X Z. One-step approach of 5/6 nephrectomy plus high phosphorus diet to rapidly establish rat models of vessel calcification induced by renal failure[J]. Chinese Journal of Integrated Traditional and Western Nephrology, 2010, 11(10):862-864. DOI:10.3969/j.issn.1009-587X.2010.10.006 . | |
| 22 | RADLOFF J, LATIC N, PFEIFFENBERGER U, et al. A phosphate and calcium-enriched diet promotes progression of 5/6-nephrectomy-induced chronic kidney disease in C57BL/6 mice[J]. Sci Rep, 2021, 11(1):14868. DOI:10.1038/s41598-021-94264-8 . |
| 23 | TSAO C W, HSU Y J, CHANG T C, et al. A high phosphorus diet impairs testicular function and spermatogenesis in male mice with chronic kidney disease[J]. Nutrients, 2020, 12(9):2624. DOI:10.3390/nu12092624 . |
| 24 | LARSSON T E, KAMEOKA C, NAKAJO I, et al. NPT-Ⅱb inhibition does not improve hyperphosphatemia in CKD[J]. Kidney Int Rep, 2017, 3(1):73-80. DOI:10.1016/j.ekir.2017.08.003 . |
| 25 | MARUYAMA S, MARBURY T C, CONNAIRE J, et al. NaPi-Ⅱb inhibition for hyperphosphatemia in CKD hemodialysis patients[J]. Kidney Int Rep, 2020, 6(3):675-684. DOI:10.1016/j.ekir.2020.12.017 . |
| [1] | Liya ZHAO, Liju NI, Caiqin ZHANG, Jianping TANG, Yangzheng YAO, Yanyan NIE, Xiaoxue GU, Ying ZHAO. Establishing a Genetic Detection Protocol of Single Nucleotide Polymorphisms Panels in Inbred Rats Based on Multiplex PCR-LDR [J]. Laboratory Animal and Comparative Medicine, 2023, 43(5): 548-558. |
| [2] | Lingzhi YU, Jianyun XIE, Liping FENG, Xiaofeng WEI. Establishment of Fluorescence qPCR Method for Detection of Staphylococcus Aureus and Its Application in Feces Detection of Rats and Mice [J]. Laboratory Animal and Comparative Medicine, 2023, 43(5): 566-573. |
| [3] | Ziyin XIA, Yuanyuan CHAI, Yunxia XU, Qinwei YU, Xin HUANG, Luyong ZHANG, Zhenzhou JIANG. Quantification of Uric Acid of Rat Serum by Liquid Chromatography-ultraviolet Detection and Its Comparison Study [J]. Laboratory Animal and Comparative Medicine, 2023, 43(3): 314-322. |
| [4] | Ying TAN, Wenping LIAO, Qilong GAO, Yong LI, Xinhui SHI, Jingkun WANG. Physiological Indexes and Histopathology Analysis of Sodium Iodate-Induced Retinitis Pigmentosa in Rats [J]. Laboratory Animal and Comparative Medicine, 2023, 43(2): 124-135. |
| [5] | Jian GE, Jingfen SUN, Yongjie WU. Taurine Has no Protective Effect on Rat Corneal Endothelial Cells Injured by Benzalkonium Chloride [J]. Laboratory Animal and Comparative Medicine, 2023, 43(1): 39-43. |
| [6] | Qin XU, Yan NI, Wenhui SHI, Jianying LI, Jiangwei LIU, Hongqiong ZHAO, Xinming XU. Analysis on Ileum and Colon Microflora of SPF Male SD Rats based on High-throughput Sequencing [J]. Laboratory Animal and Comparative Medicine, 2023, 43(1): 53-60. |
| [7] | Bin WU, Xu WANG, Dongxu FU, Yujun ZHU, Jinlu HUANG, Shunxing ZHU. Effect of the Traditional Chinese Medicine Shuganjieyu Formula on Constipation Type Irritable Bowel Syndrome and Brain-gut Axis in Rats [J]. Laboratory Animal and Comparative Medicine, 2022, 42(6): 551-559. |
| [8] | Chen GAO, Chunling FAN, Yurong LI, Wenjuan PEI, Caiping GUAN. Changes in Expression of Monocarboxylate Transporters in the Rat Cerebral Cortex after Exercise-induced Fatigue Under Simulated High-altitude Hypoxia and its Significance [J]. Laboratory Animal and Comparative Medicine, 2022, 42(5): 384-392. |
| [9] | Bingxin XU, Kaijian FAN, Tingyu WANG, Huijin CHEN. Effect of Dexamethasone on Cartilage Degeneration in Rats with Collagen-induced Arthritis [J]. Laboratory Animal and Comparative Medicine, 2022, 42(5): 416-422. |
| [10] | Huiyan QIN, Huafeng CHEN, Hui YANG, Hailan LUO, Weizhong FU, Qingbo LI, Jiehong ZHANG. The Capacity of Silkworm Cocoon Water to Mitigate the Level of Oxidative Stress in Aged Rats [J]. Laboratory Animal and Comparative Medicine, 2022, 42(5): 393-400. |
| [11] | Xiaorui ZHANG, Jing CAO, Qianqian WU, Jijun LIU, Guoyuan CHEN, Baojin WU. Effects of Probucol Formulations on Mesenteric Lymphatic Trans-port Efficiency and Pharmacokinetics in Rats [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 275-283. |
| [12] | Sijia ZHAO, Xinyu HE, Quan JING, Lin MA, Chunlan GUO, Kuo WAN. Evaluation of Pain in Acute Pulpitis Hyperalgesia Model Rats [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 333-341. |
| [13] | Yiru WANG, Xiaoying JIANG, Ruoxi DONG, Yibin PAN, Xianghui HAN, Yongqing CAO. Modified Method for Inducing Acute Intestinal Fibrosis in Rats Using 2,4,6-Trinitrobenzene Sulfonic Acid [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 284-293. |
| [14] | Xiaorui ZHANG, Jing CAO, Qianqian WU, Kang KANG, Guoyuan CHEN, Baojin WU. A Preliminary Method for Continuous Drainage of Mesenteric Lymph Fluid in Rats [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 267-274. |
| [15] | Dingshan FENG, Yeyu HUANG, Xiaoxin ZHANG, Aiqin WU, Zhan WANG, Linliang SU. Effects of Storage Time on Electrolyte Content and pH Value in Rat Serum Samples [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 301-305. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||