
Laboratory Animal and Comparative Medicine ›› 2022, Vol. 42 ›› Issue (4): 333-341.DOI: 10.12300/j.issn.1674-5817.2021.171
• Animal Models of Human Diseases • Previous Articles Next Articles
Sijia ZHAO( ), Xinyu HE(
), Xinyu HE( ), Quan JING, Lin MA, Chunlan GUO, Kuo WAN(
), Quan JING, Lin MA, Chunlan GUO, Kuo WAN( )(
)( )
)
Received:2021-11-17
Revised:2022-03-29
Online:2022-08-25
Published:2022-09-01
Contact:
Kuo WAN
CLC Number:
Sijia ZHAO, Xinyu HE, Quan JING, Lin MA, Chunlan GUO, Kuo WAN. Evaluation of Pain in Acute Pulpitis Hyperalgesia Model Rats[J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 333-341.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2021.171
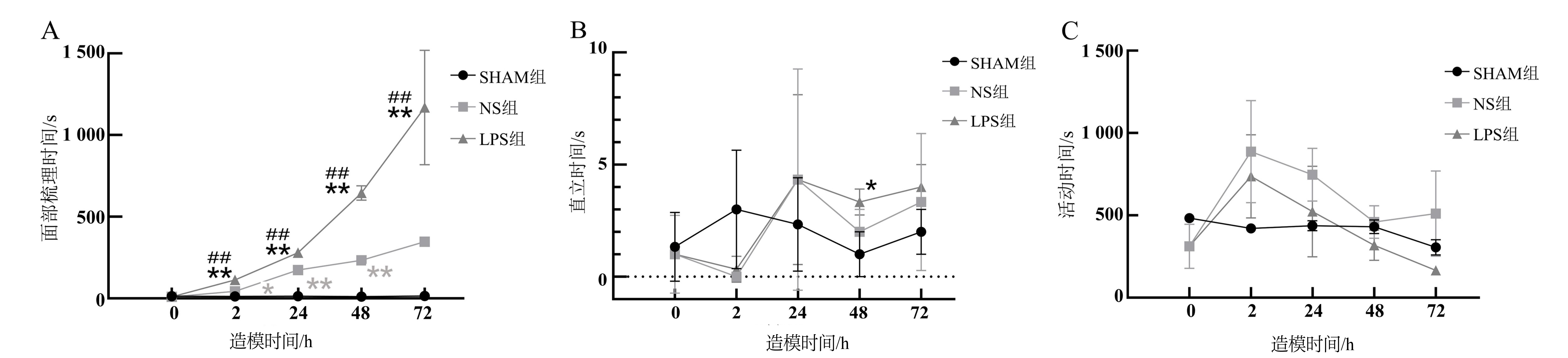
Figure 1 Evaluation of spontaneous pain in acute pulpitis model ratsNote: A, Facial grooming time; B, Upright time; C, Activity time. The rats in the SHAM group, a blank control group, only anesthetized with isoflurane; Under isoflurance anesthesia, the rats in the LPS group had their right upper molar teeth drilled and lipopolysaccharide was sealed temporarily with Caviton, while those in the NS group had their right upper molar teeth drilled and normal saline was sealed temporarily with Caviton. There were 15 rats in each group before modeling and 3 rats in each group at different time points after successful modeling. Compared with the SHAM group, *P<0.05, **P<0.01; Compared with the NS group, #P<0.05, P<0.01.
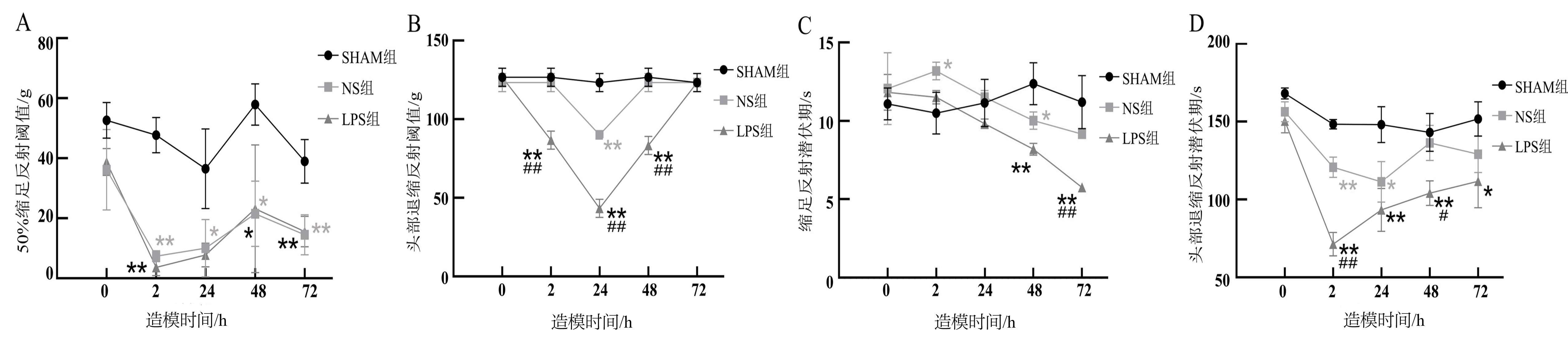
Figure 2 Mechanical and cold pain threshold of acute pulpitis model ratsNote: A, 50% Paw withdrawal threshold (50% PWT); B, Head withdrawal threshold (HWT); C, Paw withdrawal latency (PWL); D, Head withdrawal latency (HWL). The rats in the SHAM group, a blank control group, only anesthetized with isoflurane; Under isoflurance anesthesia, the rats in the LPS group had their right upper molar teeth drilled and lipopolysaccharide was sealed temporarily with Caviton, while those in the NS group had their right upper molar teeth drilled and normal saline was sealed temporarily with Caviton. There were 15 rats in each group before modeling and 3 rats in each group at different time points after successful modeling. Compared with the SHAM group, *P<0.05, **P<0.01; Compared with the NS group, #P<0.05, P<0.01.
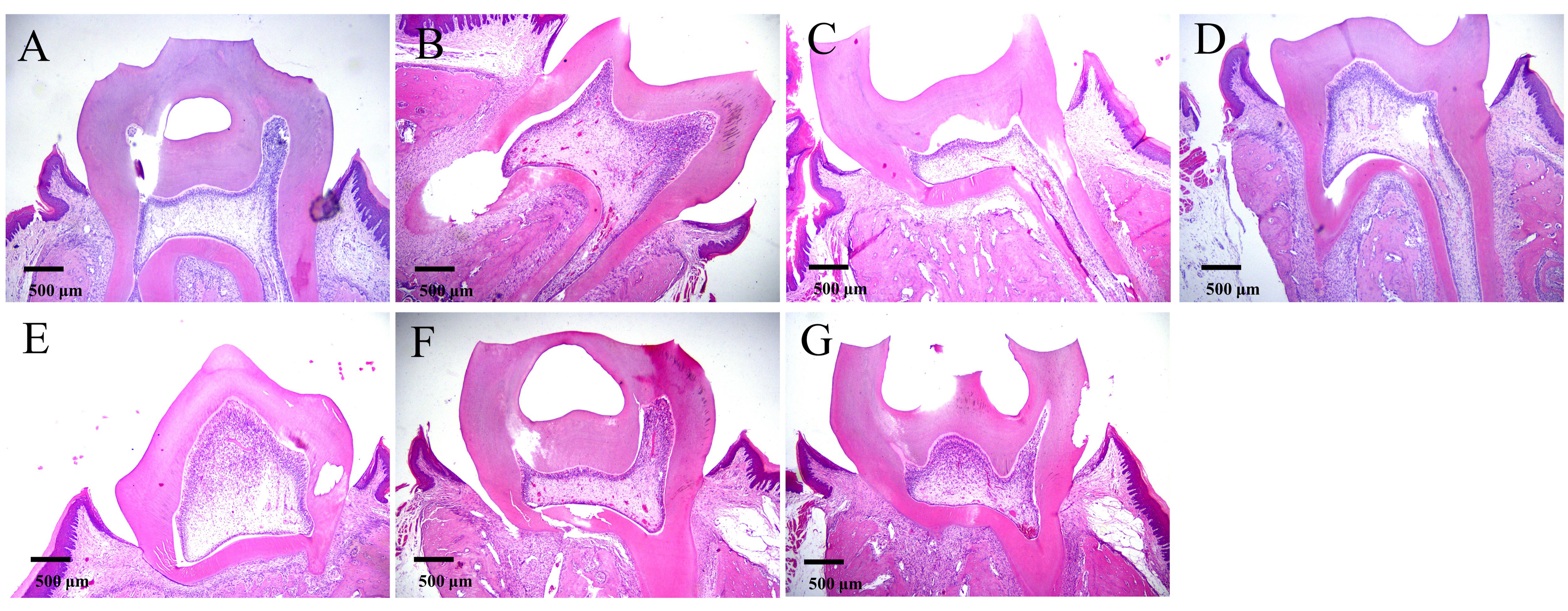
Figure 3 Histopathological changes of LPS and NS induced acute pulpitis in rats (HE,×40)Note: A-C, LPS groups at 24, 48, and 72 h after modeling, respectively; D, Normal control; E-G, NS groups at 24, 48, and 72 h after modeling, respectively. The rats in the SHAM group, a blank control group, only anesthetized with isoflurane; Under isoflurance anesthesia, the rats in the LPS group had their right upper molar teeth drilled and lipopolysaccharide was sealed temporarily with Caviton, while those in the NS group had their right upper molar teeth drilled and normal saline was sealed temporarily with Caviton. There were 15 rats in each group before modeling and 3 rats in each group at different time points after successful modeling.
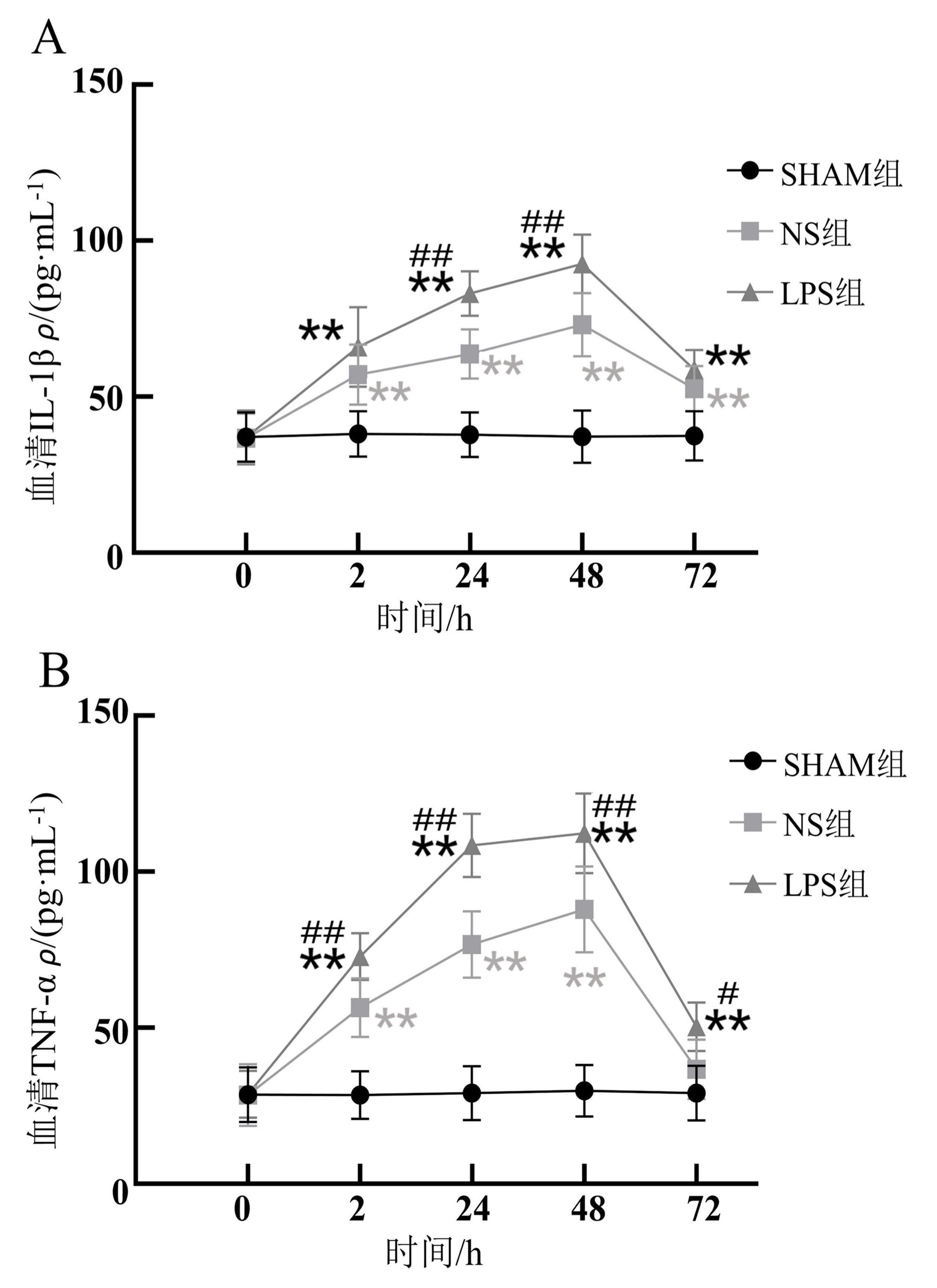
Figure 4 Changes in serum inflammatory factors in acute pulpitis model ratsNote: A, IL-1β levels in serum; B, TNF-α levels in serum. The rats in the SHAM group, a blank control group, only anesthetized with isoflurane; Under isoflurance anesthesia, the rats in the LPS group had their right upper molar teeth were drilled and lipopolysaccharide was sealed temporarily with Caviton, while those in the NS group had their right upper molar teeth were drilled and normal saline was sealed temporarily with Caviton. There were 15 rats in each group before modeling and 3 rats in each group at different time points after successful modeling. Compared with the SHAM group, *P<0.05, **P<0.01; Compared with the NS group, #P<0.05, P<0.01.
| 1 | JESPERSEN J J, HELLSTEIN J, WILLIAMSON A, et al. Evaluation of dental pulp sensibility tests in a clinical setting[J]. J Endod, 2014, 40(3):351-354. DOI:10.1016/j.joen.2013.11.009 . |
| 2 | BAI J, XU T, JI A P, et al. Impact of COVID-19 on oral emergency services[J]. Int Dent J, 2021, 71(1):27-31. DOI:10.1111/idj.12603 . |
| 3 | 林正梅,凌均启,凌征宇,等. 实验性牙髓炎疼痛动物模型的建立[J]. 广东牙病防治,1999, 7(4):249-251. |
| LIN Z M, LIN J Q, LING Z Y, et al. Establishmentof Animal Modelabout Dental Pain Caused by Experimental Pulpitis [J]. Journal of dental prevention and treatment, 1999, 7(4):249-251. | |
| 4 | LIN J J, DU Y, CAI W K, et al. Toll-like receptor 4 signaling in neurons of trigeminal ganglion contributes to nociception induced by acute pulpitis in rats[J]. Sci Rep, 2015, 5:12549. DOI:10.1038/srep12549 |
| 5 | LI M, TIAN J, XU Z, et al. Histology-based profile of inflammatory mediators in experimentally induced pulpitis in a rat model: screening for possible biomarkers[J]. Int Endod J, 2021, 54(8):1328-1341. DOI:10.1111/iej.13514 . |
| 6 | CHAPLAN S R, BACH F W, POGREL J W, et al. Quantitative assessment of tactile allodynia in the rat paw[J]. J Neurosci Methods, 1994, 53(1):55-63. DOI:10.1016/0165-0270(94)90144-9 . |
| 7 | OHARA K, SHIMIZU K, MATSUURA S, et al. Toll-like receptor 4 signaling in trigeminal ganglion neurons contributes tongue-referred pain associated with tooth pulp inflammation[J]. J Neuroinflammation, 2013, 10:139. DOI:10.1186/1742-2094-10-139 . |
| 8 | HARA K, HARANISHI Y, KATAOKA K, et al. Chlorogenic acid administered intrathecally alleviates mechanical and cold hyperalgesia in a rat neuropathic pain model[J]. Eur J Pharmacol, 2014, 723:459-464. DOI:10.1016/j.ejphar. 2013. 10.046 . |
| 9 | BYERS M R, NÄRHI M V. Dental injury models: experimental tools for understanding neuroinflammatory interactions and polymodal nociceptor functions[J]. Crit Rev Oral Biol Med, 1999, 10(1):4-39. DOI:10.1177/10454411990100010101 . |
| 10 | 高磊, 栗红师, 闫磊, 等. 大鼠实验性牙髓炎痛敏模型的建立及检验[J]. 牙体牙髓牙周病学杂志, 2015, 25(5):299-303, 328. DOI:10.15956/j.cnki.chin.j.conserv.dent.2015.05.008 . |
| GAO L, LI H S, YAN L, et al. Establishment and verification of rat experimental hyperalgesic pulpitis[J]. Chin J Conserv Dent, 2015, 25(5):299-303, 328. DOI:10.15956/j.cnki.chin.j.conserv.dent.2015.05.008 . | |
| 11 | NING T, SHAO J, ZHANG X, et al. Ageing affects the proliferation and mineralization of rat dental pulp stem cells under inflammatory conditions[J]. Int Endod J, 2020, 53(1):72-83. DOI:10.1111/iej.13205 . |
| 12 | 顾斌, 刘洪臣, 郭宏, 等. 大鼠急性牙髓炎动物模型的建立[J]. 中华老年口腔医学杂志, 2009, 7(5):304-307. DOI:10.3969/j.issn.1672-2973.2009.05.019 . |
| GU B, LIU H C, GUO H, et al. Establishment of central hyperalgesia animal model induced by acute pulpitis[J]. Chin J Geriatr Dent, 2009, 7(5):304-307. DOI:10.3969/j.issn.1672-2973.2009.05.019 . | |
| 13 | FAN W G, HUANG F, ZHU X, et al. Involvement of microglial activation in the brainstem in experimental dental injury and inflammation[J]. Arch Oral Biol, 2010, 55(9):706-711. DOI:10.1016/j.archoralbio.2010.06.007 . |
| 14 | NAKAMURA K, YAMASAKI M, NISHIGAKI N, et al. Effect of methotrexate-induced neutropenia on pulpal inflammation in rats[J]. J Endod, 2002, 28(4):287-290. DOI:10.1097/00004770-200204000-00007 . |
| 15 | ALGHAITHY R A, QUALTROUGH A J E. Pulp sensibility and vitality tests for diagnosing pulpal health in permanent teeth: a critical review[J]. Int Endod J, 2017, 50(2):135-142. DOI:10.1111/iej.12611 . |
| 16 | VIANA F. Nociceptors: thermal allodynia and thermal pain[J]. Handb Clin Neurol, 2018, 156:103-119. DOI:10.1016/B978-0-444-63912-7.00006-0 . |
| 17 | LE FUR-BONNABESSE A, BODÉRÉ C, HÉLOU C, et al. Dental pain induced by an ambient thermal differential: patho-physiological hypothesis[J]. J Pain Res, 2017, 10:2845-2851. DOI:10.2147/JPR.S142539 . |
| 18 | 张换鸽, 党延红, 胡柏平. 不同运动方式干预对攻击行为大鼠情绪行为的影响[J]. 中国比较医学杂志, 2020, 30(2):97-102. DOI:10.3969/j.issn.1671-7856.2020.02.015 . |
| ZHANG H G, DANG Y H, HU B P. Effects of different types of exercise on emotion and behavior in aggressive rats[J]. Chin J Comp Med, 2020, 30(2):97-102. DOI:10.3969/j.issn.1671-7856.2020.02.015 . | |
| 19 | WATASE T, SHIMIZU K, KOMIYA H, et al. Involvement of transient receptor potential vanilloid 1 channel expression in orofacial cutaneous hypersensitivity following tooth pulp inflammation[J]. J Oral Sci, 2018, 60(1):8-13. DOI:10.2334/josnusd.16-0854 . |
| 20 | WORSLEY M A, ALLEN C E, BILLINTON A, et al. Chronic tooth pulp inflammation induces persistent expression of phosphorylated ERK (pERK) and phosphorylated p38 (pp38) in trigeminal subnucleus caudalis[J]. Neuroscience, 2014, 269:318-330. DOI:10.1016/j.neuroscience.2014.03.056 . |
| 21 | COSTA Y M, DE SOUZA P R J, MARQUES V A S, et al. Intraoral somatosensory alterations impact pulp sensibility testing in patients with symptomatic irreversible pulpitis[J]. J Endod, 2020, 46(6):786-793. DOI:10.1016/j.joen.2020.03.012 . |
| 22 | RAHAL V, GALLINARI M O, BARBOSA J S, et al. Influence of skin cold sensation threshold in the occurrence of dental sensitivity during dental bleaching: a placebo controlled clinical trial[J]. J Appl Oral Sci, 2018, 26: e20170043. DOI:10.1590/1678-7757-2017-0043 . |
| 23 | BAAD-HANSEN L, PIGG M, IVANOVIC S E, et al. Intraoral somatosensory abnormalities in patients with atypical odontalgia: a controlled multicenter quantitative sensory testing study[J]. Pain, 2013, 154(8):1287-1294. DOI:10.1016/j.pain.2013.04.005 . |
| 24 | BERNAL L, SOTELO-HITSCHFELD P, KÖNIG C, et al. Odontoblast TRPC5 channels signal cold pain in teeth[J]. Sci Adv, 2021, 7(13): eabf5567. DOI:10.1126/sciadv.abf5567 . |
| [1] | Xiaorui ZHANG, Jing CAO, Qianqian WU, Jijun LIU, Guoyuan CHEN, Baojin WU. Effects of Probucol Formulations on Mesenteric Lymphatic Trans-port Efficiency and Pharmacokinetics in Rats [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 275-283. |
| [2] | Yiru WANG, Xiaoying JIANG, Ruoxi DONG, Yibin PAN, Xianghui HAN, Yongqing CAO. Modified Method for Inducing Acute Intestinal Fibrosis in Rats Using 2,4,6-Trinitrobenzene Sulfonic Acid [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 284-293. |
| [3] | Xiaorui ZHANG, Jing CAO, Qianqian WU, Kang KANG, Guoyuan CHEN, Baojin WU. A Preliminary Method for Continuous Drainage of Mesenteric Lymph Fluid in Rats [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 267-274. |
| [4] | Dingshan FENG, Yeyu HUANG, Xiaoxin ZHANG, Aiqin WU, Zhan WANG, Linliang SU. Effects of Storage Time on Electrolyte Content and pH Value in Rat Serum Samples [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 301-305. |
| [5] | Xinpeng LU, Rong LIU, Wenbo Huang, Jin ZHAO, Hongtao LI. A Comparative Study of Chronic Obstructive Pulmonary Disease Rat Models Established by Different Methods [J]. Laboratory Animal and Comparative Medicine, 2022, 42(3): 201-206. |
| [6] | ZHANG Chengcai, LIU Yinhang, CHEN Chao, JIANG Wenzheng. A Preliminary Study on Effects of Lipopolysaccharide on Lung Tissues of Naked Mole Rats [J]. Laboratory Animal and Comparative Medicine, 2020, 40(6): 523-527. |
| [7] | YUAN Xiao-hong, HE Feng, JIANG Ze-hui, ZHAO He, YE Chao, WU Shao-ming, YU Hai-chuan, LI Chun-gen. Establishment and Evaluation of Rat Model of Neurogenic Bladder after Spinal Cord Transection Injury [J]. Laboratory Animal and Comparative Medicine, 2016, 36(6): 423-427. |
| [8] | NIU Ting-xian, WAN Dong-jun, LU Lu, FENG Xiao-ming, LUO Xiao-hong, CAI Rang-cao. Establishment of Animal Model of Multiple Organ Dysfunction Syndrome on Highland Scene in Sheep [J]. Laboratory Animal and Comparative Medicine, 2012, 32(4): 285-289. |
| [9] | SHI Zhi-yong, LUO Xiao-hong, WAN Dong-jun, LU Lu, WANG Hong-yi, NIU Ting-xian. Effect of XUEBIJING on Multiple Organ Dysfunction Syndrome Induced by Lipopolysaccharide in Sheep [J]. Laboratory Animal and Comparative Medicine, 2012, 32(2): 120-124. |
| [10] | LI Xiang-cui, ZHANG Yi, LI Ji-ping, WANG Jia-dong. Establishment of Otitis Media with Effusion Induced by Lipopolysaccharides in Rat [J]. Laboratory Animal and Comparative Medicine, 2011, 31(6): 410-413. |
| [11] | NIU Ting-xian, LUO Xiao-hong, WAN Dong-jun, SHI Zhi-yong, WANG Hong-yi, LU Lu. Pathological Changes of Multi-organ in Acute Respiratory Distress Syndrome Induced by Lipopolysaccharide in Sheep [J]. Laboratory Animal and Comparative Medicine, 2011, 31(4): 248-251. |
| [12] | ZHENG Hai-ming,TAO Kai-zhong,ZHENG Ping. NF-κB Participates in LPS-induced Degranulation of Paneth Cells [J]. , 2010, 30(2): 109-112. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||