
Laboratory Animal and Comparative Medicine ›› 2022, Vol. 42 ›› Issue (4): 275-283.DOI: 10.12300/j.issn.1674-5817.2022.026
• Animal Experimental Techniques and Methods • Previous Articles Next Articles
Xiaorui ZHANG1( ), Jing CAO2, Qianqian WU1, Jijun LIU1, Guoyuan CHEN1, Baojin WU1(
), Jing CAO2, Qianqian WU1, Jijun LIU1, Guoyuan CHEN1, Baojin WU1( )(
)( )
)
Received:2022-03-04
Revised:2022-07-19
Online:2022-08-25
Published:2022-09-01
Contact:
Baojin WU
CLC Number:
Xiaorui ZHANG, Jing CAO, Qianqian WU, Jijun LIU, Guoyuan CHEN, Baojin WU. Effects of Probucol Formulations on Mesenteric Lymphatic Trans-port Efficiency and Pharmacokinetics in Rats[J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 275-283.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2022.026
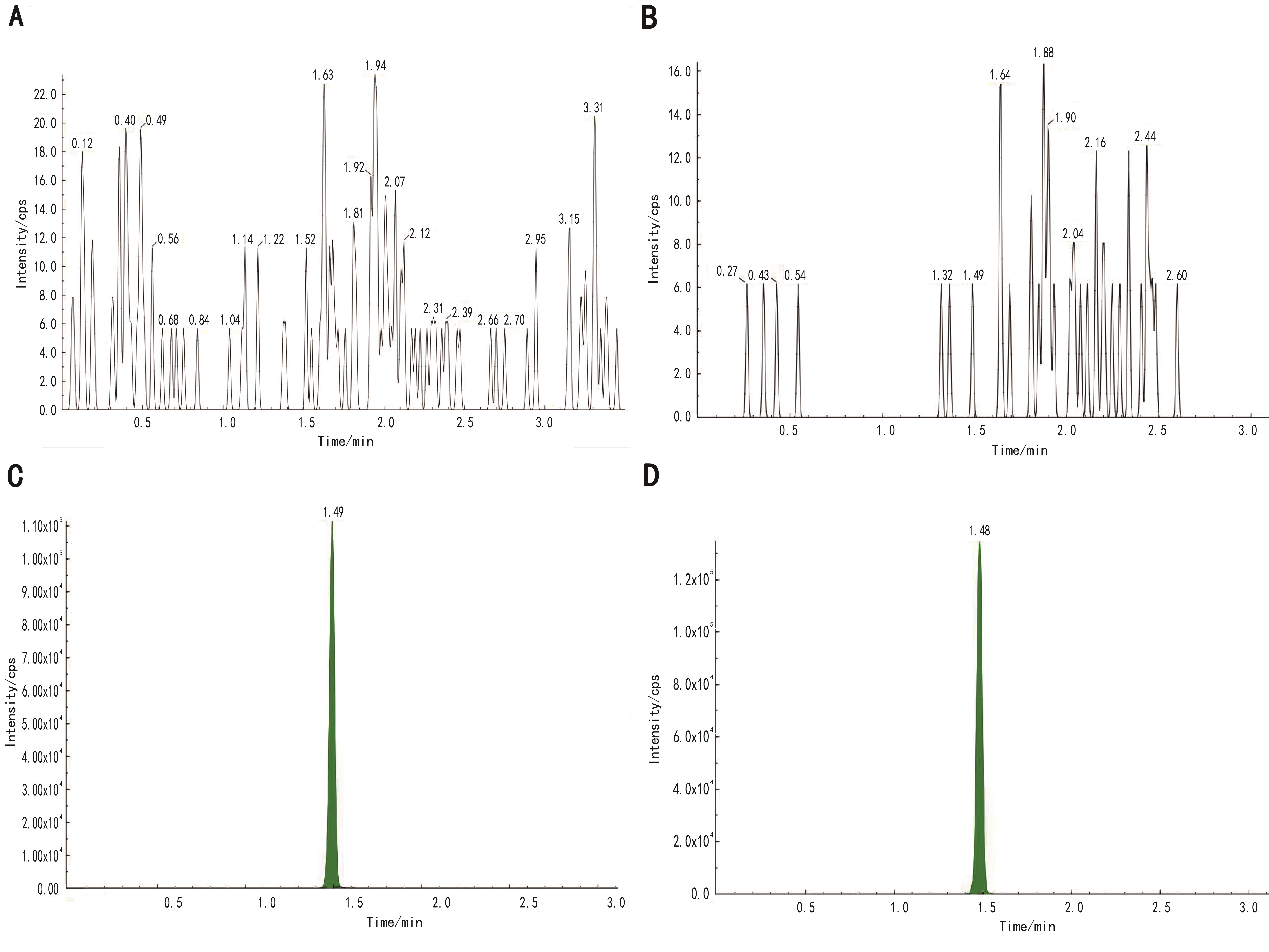
Figure 1 Chromatogram of blood and lymph with and without probucolNote: (A) Blank whole blood; (B) Blank lymph fluid; (C) Probucol (1 ng/mL) added to whole blood; (D) Probucol (1 ng/mL) added to lymph fluid.
采血时间 Blood collection time/h | 普罗布考Probucol ρ/(ng·mL-1) | |||
|---|---|---|---|---|
H-JD组 H-JD group | H-JCS组 H-JCS group | G-JD组 G-JD group | G-JCS组 G-JCS group | |
| 0 | - | - | - | - |
| 0.25 | - | - | - | - |
| 0.5 | 5 | - | - | - |
| 1 | 34±7 | 28±32 | 13±5 | - |
| 2 | 62±48 | 38±32 | 76±30 | 19±23 |
| 4 | 126±91 | 188±167 | 143±26 | 82±119 |
| 8 | 104±87 | 100±36 | 379±138 | 128±184 |
| 24 | 67±30 | 69±49 | 289±296 | 252±189 |
| 48 | 12±5 | 22±15 | 85±23 | 48±13 |
| 72 | 5±1 | 11±10 | 15±5 | 18±1 |
Table 1 Drug concentrations in the blood of rats in each group
采血时间 Blood collection time/h | 普罗布考Probucol ρ/(ng·mL-1) | |||
|---|---|---|---|---|
H-JD组 H-JD group | H-JCS组 H-JCS group | G-JD组 G-JD group | G-JCS组 G-JCS group | |
| 0 | - | - | - | - |
| 0.25 | - | - | - | - |
| 0.5 | 5 | - | - | - |
| 1 | 34±7 | 28±32 | 13±5 | - |
| 2 | 62±48 | 38±32 | 76±30 | 19±23 |
| 4 | 126±91 | 188±167 | 143±26 | 82±119 |
| 8 | 104±87 | 100±36 | 379±138 | 128±184 |
| 24 | 67±30 | 69±49 | 289±296 | 252±189 |
| 48 | 12±5 | 22±15 | 85±23 | 48±13 |
| 72 | 5±1 | 11±10 | 15±5 | 18±1 |
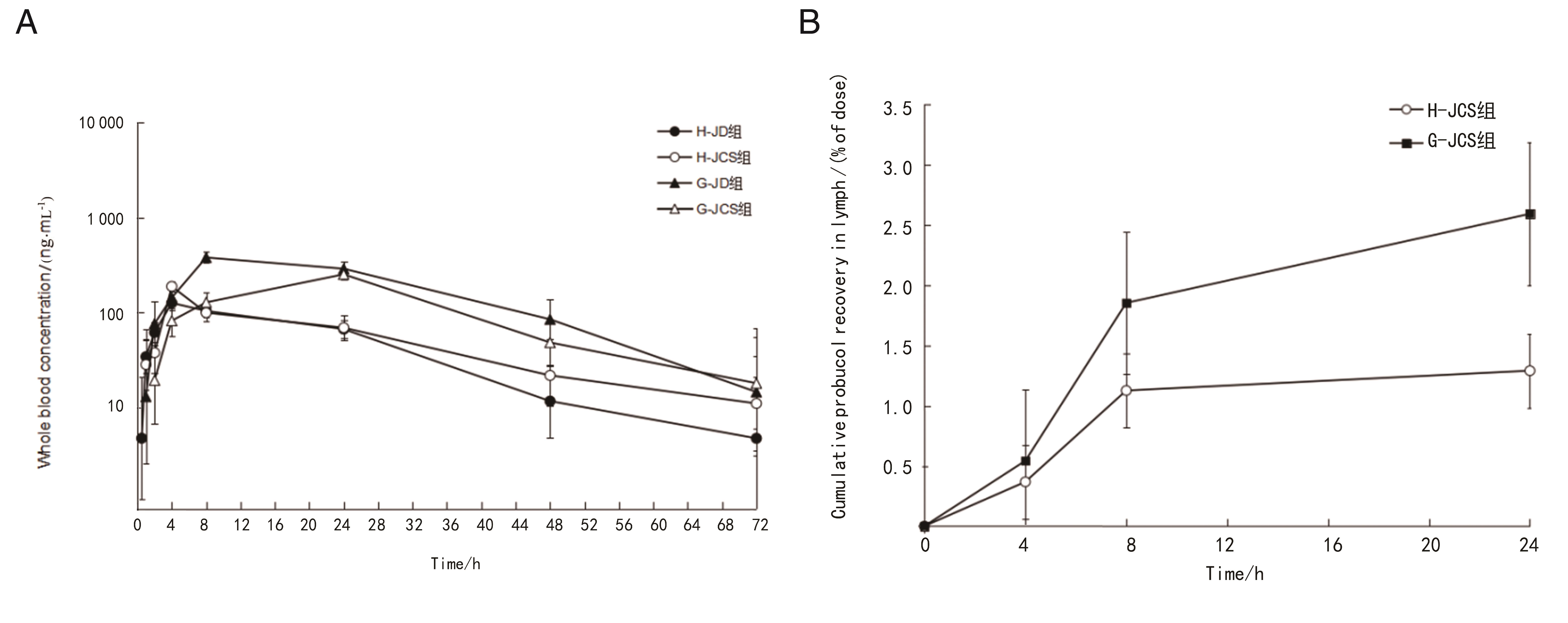
Figure 3 Drug concentration-time curve (A) and dose percentage curve of lymphatic fluid (B) in all groups of ratsNote: H-JD, suspension preparation-jugular vein single cannulation group; H-JCS, suspension preparation-jugular vein and intestinal lymphatic double cannulation group; G-JD, olive oil preparation-jugular vein single cannulation group; G-JCS, olive oil preparation-jugular vein and intestinal lymphatic double cannulation group.
参数 Parameters | H-JD组 H-JD group | H-JCS组 H-JCS group | G-JD组 G-JD group | G-JCS组 G-JCS group |
|---|---|---|---|---|
| 峰值时间Tmax/h | 11±12 | 5±2 | 13±9 | 19±9 |
| 峰值浓度Cmax/(ng·mL-1) | 148±60 | 207±137 | 453±204 | 309±177 |
| 半衰期T1/2/h | 12±1 | 17±3 | 13±3 | 19±11 |
| 药-时曲线下面积AUClast/(h·ng·mL-1) | 3 210±885 | 3 677±2 014 | 12 360±6 629 | 8 080±3 064 |
| 药-时曲线下面积AUCINF/(h·ng·mL-1) | 3 293±899 | 3 970±2 295 | 12 623±6 650 | 8 947±2 218 |
Table 2 Pharmacokinetic parameters of the whole blood of rats in each group
参数 Parameters | H-JD组 H-JD group | H-JCS组 H-JCS group | G-JD组 G-JD group | G-JCS组 G-JCS group |
|---|---|---|---|---|
| 峰值时间Tmax/h | 11±12 | 5±2 | 13±9 | 19±9 |
| 峰值浓度Cmax/(ng·mL-1) | 148±60 | 207±137 | 453±204 | 309±177 |
| 半衰期T1/2/h | 12±1 | 17±3 | 13±3 | 19±11 |
| 药-时曲线下面积AUClast/(h·ng·mL-1) | 3 210±885 | 3 677±2 014 | 12 360±6 629 | 8 080±3 064 |
| 药-时曲线下面积AUCINF/(h·ng·mL-1) | 3 293±899 | 3 970±2 295 | 12 623±6 650 | 8 947±2 218 |
动物 Rat | 药-时曲线下面积AUClast/(h·ng·mL-1) | Frel/% | 药-时曲线下面积AUClast/(h·ng·mL-1) | Frel/% | ||
|---|---|---|---|---|---|---|
H-JD组 H-JD group | G-JD组 G-JD group | H-JCS组 H-JCS group | G-JCS组 G-JCS group | |||
| 第1只No.1 | 2 750 | 19 800 | 720 | 3 360 | 8 680 | 258 |
| 第2只No.2 | 4 230 | 10 200 | 241 | 1 840 | 10 800 | 587 |
| 第3只No.3 | 2 650 | 7 080 | 267 | 5 830 | 4 760 | 82 |
| 平均数 | 3 210±885 | 12 360±6 629 | 409±269 | 3 677±2 014 | 8 080±3 064 | 309±256 |
Table 3 Relative bioavailability (Frel) of rats in each group
动物 Rat | 药-时曲线下面积AUClast/(h·ng·mL-1) | Frel/% | 药-时曲线下面积AUClast/(h·ng·mL-1) | Frel/% | ||
|---|---|---|---|---|---|---|
H-JD组 H-JD group | G-JD组 G-JD group | H-JCS组 H-JCS group | G-JCS组 G-JCS group | |||
| 第1只No.1 | 2 750 | 19 800 | 720 | 3 360 | 8 680 | 258 |
| 第2只No.2 | 4 230 | 10 200 | 241 | 1 840 | 10 800 | 587 |
| 第3只No.3 | 2 650 | 7 080 | 267 | 5 830 | 4 760 | 82 |
| 平均数 | 3 210±885 | 12 360±6 629 | 409±269 | 3 677±2 014 | 8 080±3 064 | 309±256 |
参数 Parameters | 采液时间/h Liquid collection time/h | H-JCS组 H-JCS group | G-JCS组 G-JCS group |
|---|---|---|---|
| 质量浓度ρ/(ng·mL-1) | 0~4 | 2 880±2 120 | 2 862±968 |
| 4~8 | 5 682±4 935 | 4 019±4 019 | |
| 8~24 | 417±189 | 1 345±1 695 | |
| 体积V/mL | 0~4 | 6.8±0.5 | 11.0±2.0 |
| 4~8 | 7.2±0.6 | 19.2±1.4 | |
| 8~24 | 20.8±2.4 | 30.4±3.0 | |
| 剂量百分比Dose percentage/% | 0~4 | 0.37±0.26 | 0.60±0.25 |
| 0~8 | 1.13±0.87 | 1.85±0.88 | |
| 0~24 | 1.29±0.50 | 2.59±0.43 |
Table 4 Dose percentage parameters of lymphatic fluid of rats in each group
参数 Parameters | 采液时间/h Liquid collection time/h | H-JCS组 H-JCS group | G-JCS组 G-JCS group |
|---|---|---|---|
| 质量浓度ρ/(ng·mL-1) | 0~4 | 2 880±2 120 | 2 862±968 |
| 4~8 | 5 682±4 935 | 4 019±4 019 | |
| 8~24 | 417±189 | 1 345±1 695 | |
| 体积V/mL | 0~4 | 6.8±0.5 | 11.0±2.0 |
| 4~8 | 7.2±0.6 | 19.2±1.4 | |
| 8~24 | 20.8±2.4 | 30.4±3.0 | |
| 剂量百分比Dose percentage/% | 0~4 | 0.37±0.26 | 0.60±0.25 |
| 0~8 | 1.13±0.87 | 1.85±0.88 | |
| 0~24 | 1.29±0.50 | 2.59±0.43 |
| 1 | HIRATA K I. New evidence of probucol on cardiovascular events[J]. J Atheroscler Thromb, 2021, 28(2):97-99. DOI:10.5551/jat.ED155 . |
| 2 | YAMASHITA S, MASUDA D, OHAMA T, et al. Rationale and design of the PROSPECTIVE trial: probucol trial for secondary prevention of atherosclerotic events in patients with prior coronary heart disease[J]. J Atheroscler Thromb, 2016, 23(6):746-756. DOI:10.5551/jat.32813 . |
| 3 | LIU J C, LI M H, LU H, et al. Effects of probucol on restenosis after percutaneous coronary intervention: a systematic review and meta-analysis[J]. PLoS One, 2015, 10(4): e0124021. DOI:10.1371/journal.pone.0124021 . |
| 4 | HIGASHI K, MORI A, SAKAMOTO K, et al. Probucol slows the progression of cataracts in streptozotocin-induced hyperglycemic rats[J]. Pharmacology, 2019, 103(3-4):212-219. DOI:10.1159/000496055 . |
| 5 | JUNG Y S, PARK J H, KIM H, et al. Probucol inhibits LPS-induced microglia activation and ameliorates brain ischemic injury in normal and hyperlipidemic mice[J]. Acta Pharmacol Sin, 2016, 37(8):1031-1044. DOI:10.1038/aps.2016.51 . |
| 6 | HAN L M, YANG Q S, SHEN T, et al. Lymphatic transport of orally administered probucol-loaded mPEG-DSPE micelles[J]. Drug Deliv, 2016, 23(6):1955-1961. DOI:10.3109/10717544.2015.1028600 . |
| 7 | QIAN Y W, CHEN G G, WANG J, et al. Preparation and evaluation of probucol-phospholipid complex with enhanced bioavailability and No food effect[J]. AAPS Pharmscitech, 2018, 19(8):3599-3608. DOI:10.1208/s12249-018-1157-2 . |
| 8 | GERSHKOVICH P, HOFFMAN A. Uptake of lipophilic drugs by plasma derived isolated chylomicrons: linear correlation with intestinal lymphatic bioavailability[J]. Eur J Pharm Sci, 2005, 26(5):394-404. DOI:10.1016/j.ejps.2005.07.011 . |
| 9 | YÁÑEZ J A, WANG S W J, KNEMEYER I W, et al. Intestinal lymphatic transport for drug delivery[J]. Adv Drug Deliv Rev, 2011, 63(10-11):923-942. DOI:10.1016/j.addr.2011.05.019 . |
| 10 | BEG S, ALAM M N, AHMAD F J, et al. Chylomicron mimicking nanocolloidal carriers of rosuvastatin calcium for lymphatic drug targeting and management of hyperlipidemia[J]. Colloids Surf B Biointerfaces, 2019, 177:541-549. DOI:10.1016/j.colsurfb.2019.02.039 . |
| 11 | HOKKANEN K, TIRRONEN A, YLÄ-HERTTUALA S. Intestinal lymphatic vessels and their role in chylomicron absorption and lipid homeostasis[J]. Curr Opin Lipidol, 2019, 30(5):370-376. DOI:10.1097/MOL.0000000000000626 . |
| 12 | KIM H, SEONG I, RO J, et al. Enhanced association of probucol with chylomicron by pharmaceutical excipients: an in vitro study[J]. Drug Dev Ind Pharm, 2015, 41(7):1073-1079. DOI:10.3109/03639045.2014.927479 . |
| 13 | 张笑瑞, 曹静, 吴倩倩, 等. 大鼠肠系膜淋巴液持续引流新方法的初步建立[J]. 实验动物与比较医学, 2022, 42(4):267-274. DOI:10.12300/j.issn.1674-5817.2022.024 . |
| ZHANG X R, CAO J, WU Q Q, et al. A preliminary method for continuous drainage of mesenteric lymph fluid in rat[J]. Laboratory Animal and Comparative Medicine, 2022, 42(4):267-274. DOI:10.12300/j.issn.1674-5817.2022.024 . | |
| 14 | ALEXANDER J S, GANTA V C, JORDAN P A, et al. Gastrointestinal lymphatics in health and disease[J]. Pathophysiology, 2010, 17(4):315-335. DOI:10.1016/j.pathophys. 2009.09.003 . |
| 15 | HYEONGMIN K, YEONGSEOK K, JAEHWI L E. Liposomal formulations for enhanced lymphatic drug delivery[J]. Asian J Pharm Sci, 2013(2):96-103. |
| 16 | MARWAHA R K, DABAS A. Bioavailability of nanoemulsion formulations vs conventional fat soluble preparations of cholecalciferol (D3) - An overview[J]. J Clin Orthop Trauma, 2019, 10(6):1094-1096. DOI:10.1016/j.jcot.2019.07.014 . |
| 17 | ZHANG Y M, ZHANG S K, CUI N Q. Intravenous infusion of mesenteric lymph from severe intraperitoneal infection rats causes lung injury in healthy rats[J]. World J Gastroenterol, 2014, 20(16):4771-4777. DOI:10.3748/wjg.v20.i16.4771 . |
| 18 | CHAMPAGNE D, PEARSON D, DEA D, et al. The cholesterol-lowering drug Probucol increases apolipoprotein E production in the hippocampus of aged rats: implications for Alzheimer's disease[J]. Neuroscience, 2003, 121(1):99-110. DOI:10.1016/S0306-4522(03)00361-0 . |
| 19 | BROCKS D R, DAVIES N M. Lymphatic drug absorption via the enterocytes: pharmacokinetic simulation, modeling, and considerations for optimal drug development[J]. J Pharm Pharm Sci, 2018, 21(1s):254s-270s. DOI:10.18433/jpps30217 . |
| 20 | LI J, YANG Y, ZHAO M H, et al. Improved oral bioavailability of probucol by dry media-milling[J]. Mater Sci Eng C, 2017, 78:780-786. DOI:10.1016/j.msec.2017.04.141 . |
| 21 | SCHÖNFELD P, WOJTCZAK L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective[J]. J Lipid Res, 2016, 57(6):943-954. DOI:10.1194/jlr.R067629 . |
| 22 | TANOUS D, BRÄSEN J H, CHOY K, et al. Probucol inhibits in-stent thrombosis and neointimal hyperplasia by promoting re-endothelialization[J]. Atherosclerosis, 2006, 189(2):342-349. DOI:10.1016/j.atherosclerosis.2006.01.025 . |
| 23 | KOSTIK V, MEMETI S, BAUER B. Fatty acid composition of edible oils and fats[J]. J Hyg Eng Des, 2013, 4: 112-116. |
| [1] | Chen GAO, Chunling FAN, Yurong LI, Wenjuan PEI, Caiping GUAN. Changes in Expression of Monocarboxylate Transporters in the Rat Cerebral Cortex after Exercise-induced Fatigue Under Simulated High-altitude Hypoxia and its Significance [J]. Laboratory Animal and Comparative Medicine, 2022, 42(5): 384-392. |
| [2] | Sijia ZHAO, Xinyu HE, Quan JING, Lin MA, Chunlan GUO, Kuo WAN. Evaluation of Pain in Acute Pulpitis Hyperalgesia Model Rats [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 333-341. |
| [3] | Yiru WANG, Xiaoying JIANG, Ruoxi DONG, Yibin PAN, Xianghui HAN, Yongqing CAO. Modified Method for Inducing Acute Intestinal Fibrosis in Rats Using 2,4,6-Trinitrobenzene Sulfonic Acid [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 284-293. |
| [4] | Xiaorui ZHANG, Jing CAO, Qianqian WU, Kang KANG, Guoyuan CHEN, Baojin WU. A Preliminary Method for Continuous Drainage of Mesenteric Lymph Fluid in Rats [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 267-274. |
| [5] | Dingshan FENG, Yeyu HUANG, Xiaoxin ZHANG, Aiqin WU, Zhan WANG, Linliang SU. Effects of Storage Time on Electrolyte Content and pH Value in Rat Serum Samples [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 301-305. |
| [6] | Xiao LU, Lin ZHANG, Hui JI, Shanxiang JIANG. Efficacy of DZ1462, a Novel Sodium-phosphate Transporter Inhibitor, on 5/6 Nephrectomy-induced Hyperphosphatemia Model Rats [J]. Laboratory Animal and Comparative Medicine, 2022, 42(3): 187-193. |
| [7] | Chen GAO, Wan WANG, Yurong LI, Wenjuan PEI. Expression and Significance of Monocarboxylate Transporters in Cortex of Rats After Exercise-induced Fatigue [J]. Laboratory Animal and Comparative Medicine, 2022, 42(1): 42-47. |
| [8] | WANG Xing-tong, CHEN Hong-yan, HAN Ling-xia. Advances in Research on Relationship between Transporter Associate with Antigen Processing Gene Polymorphism and Diseases [J]. Laboratory Animal and Comparative Medicine, 2017, 37(3): 252-256. |
| [9] | ZHANG Yun-li, WANG Lin, LIU Tie-min. PI3K/Akt and AMPK Signaling Pathway and Effect of Exercise on Rodent Skeletal Muscle GLUT4 Translocation and Expression [J]. Laboratory Animal and Comparative Medicine, 2017, 37(1): 76-82. |
| [10] | YUAN Xiao-hong, HE Feng, JIANG Ze-hui, ZHAO He, YE Chao, WU Shao-ming, YU Hai-chuan, LI Chun-gen. Establishment and Evaluation of Rat Model of Neurogenic Bladder after Spinal Cord Transection Injury [J]. Laboratory Animal and Comparative Medicine, 2016, 36(6): 423-427. |
| [11] | HUANG Hu, YANG Fan, WU Mao-bo, ZHANG Yu-jie, ZHONG Ling, HOU Yong-min. Expression, Activity and Pharmacokinetic Study of A Novel Long-Acting Recombinant Human Chorionic Gonadotropin [J]. Laboratory Animal and Comparative Medicine, 2014, 34(2): 113-119. |
| [12] | WANG Mei-shan, KONG Peng-cheng, ZHU Yan, ZHU Lian, JIANG Man-xi, CHEN Xue-jin, LI He-ping. A Modified Delivery of Mouse Cauda Epididymides at Room Temperature [J]. Laboratory Animal and Comparative Medicine, 2014, 34(2): 145-148. |
| [13] | YANG Yu-wei,CHEN Min-li,PAN Yong-ming,CHEN Liang,HE Huan,WANG De-jun. Effect of Long-term Transportation Stress on Autonomic Nervous Control of Heart in Two Strains of Wuzhishan Miniature Pigs [J]. , 2010, 30(5): 359-364. |
| [14] | PAN Yong-ming,CHEN Fang-ming,CHEN Liang,XU Jian-qin,PENG Ding-guo,ZHANG Li-zhong,CHEN Min-li,YING Hua-zhong,L(U) Jian-min. Effect of Long-distance Transportation on Cardiac Performance in Sub-health Beagle Dogs [J]. , 2007, 27(2): 107-111. |
| [15] | ZHANG Rong-Jun, LI Wei-Yi, CAO Guo-Xian, MU Zheng-Ming. Tissue Distribution and Pharmacokinetics of 125I labeled p-Iodophentermine as a Brain Perfusion Imaging Agent in Rat [J]. , 1995, 15(3): 143-145. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||