













实验动物与比较医学 ›› 2025, Vol. 45 ›› Issue (6): 762-772.DOI: 10.12300/j.issn.1674-5817.2025.111
收稿日期:2025-07-05
修回日期:2025-08-16
出版日期:2025-12-25
发布日期:2025-12-19
作者简介:基金资助:Received:2025-07-05
Revised:2025-08-16
Published:2025-12-25
Online:2025-12-19
Contact:
SHENG Lihong (ORCID: 0000-0001-8227-1856), E-mail: shenglihong@fudan.edu.cn摘要:
跳镰猛蚁(Harpegnathos saltator)是一类具有高度社会行为可塑性的真社会性昆虫,展现出独特的品级可逆性表型。与传统社会性蚂蚁不同,其工蚁在失去蚁后压制时,可通过一系列行为、神经及生理重编程,转变为有性工蚁(gamergate),且该过程可逆。因此,跳镰猛蚁成为研究社会等级建立与维持、行为调控和寿命可塑性的重要模型。得益于组学与成像技术的革新,跳镰猛蚁研究近年来取得了突破性进展。在社会等级转换过程中,个体在行为、神经系统活动、内分泌及基因表达水平等多个层面均呈现出高度动态和可塑的变化,揭示了环境信号如何整合为稳定的表型重编程。在寿命调控方面,跳镰猛蚁展现了与“繁殖-寿命对立”假说相矛盾的现象:繁殖个体寿命显著延长。相关研究揭示了端粒维持、表观遗传重塑、蛋白质稳态调控以及胰岛素/胰岛素样生长因子(insulin like growth factor,IGF)信号通路分叉等多重分子机制,为衰老与长寿研究提供了新视角。在社会化学通信与神经感知层面,跳镰猛蚁嗅觉系统进化显著,尤其是气味受体(odorant receptors,OR)基因家族的扩展,为其群体交互和等级维持提供了分子基础。神经肽与激素调控通路的研究也揭示了社会等级与行为状态之间的紧密联系。随着多种前沿工具的引入,如成簇规律间隔短回文重复序列(clustered regularly interspaced short palindromic repeats,CRISPR)/CRISPR相关蛋白核酸酶9(CRISPR-associated nuclease 9,Cas9)、基于绿色荧光蛋白(green fluorescent protein,GFP),钙调蛋白和M13肽的遗传编码钙指示器成像(genetically encoded calcium indicator imaging based on GFP, calmodulin, and M13 peptide,GCaMP imaging)、高通量测序的转座酶可及染色质检测(assay for transposase accessible chromatin with high-throughput sequencing,ATAC-seq)等,研究者能够在神经活动、基因调控与染色质可及性等多个层面进行更精确解析。这些工具的应用不仅推动了跳镰猛蚁社会行为和神经机制的深入研究,也为跨物种的比较提供了新手段。总体而言,跳镰猛蚁的研究框架涵盖社会行为、等级调控、寿命延长机制,以及基因表达与表观遗传重编程等多个方面。通过整合多组学与功能实验,研究者正逐步构建该物种社会可塑性与长寿机制的系统性图谱。这些成果不仅深化了人们对社会性昆虫行为和寿命调控的理解,也为人类抗衰老研究提供了潜在靶点和理论依据。
中图分类号:
盛李宏. 跳镰猛蚁:解码社会行为与衰老可塑性的模型昆虫[J]. 实验动物与比较医学, 2025, 45(6): 762-772. DOI: 10.12300/j.issn.1674-5817.2025.111.
SHENG Lihong. Harpegnathos saltator : A Model Insect for Decoding Plasticity of Social Behavior and Aging[J]. Laboratory Animal and Comparative Medicine, 2025, 45(6): 762-772. DOI: 10.12300/j.issn.1674-5817.2025.111.
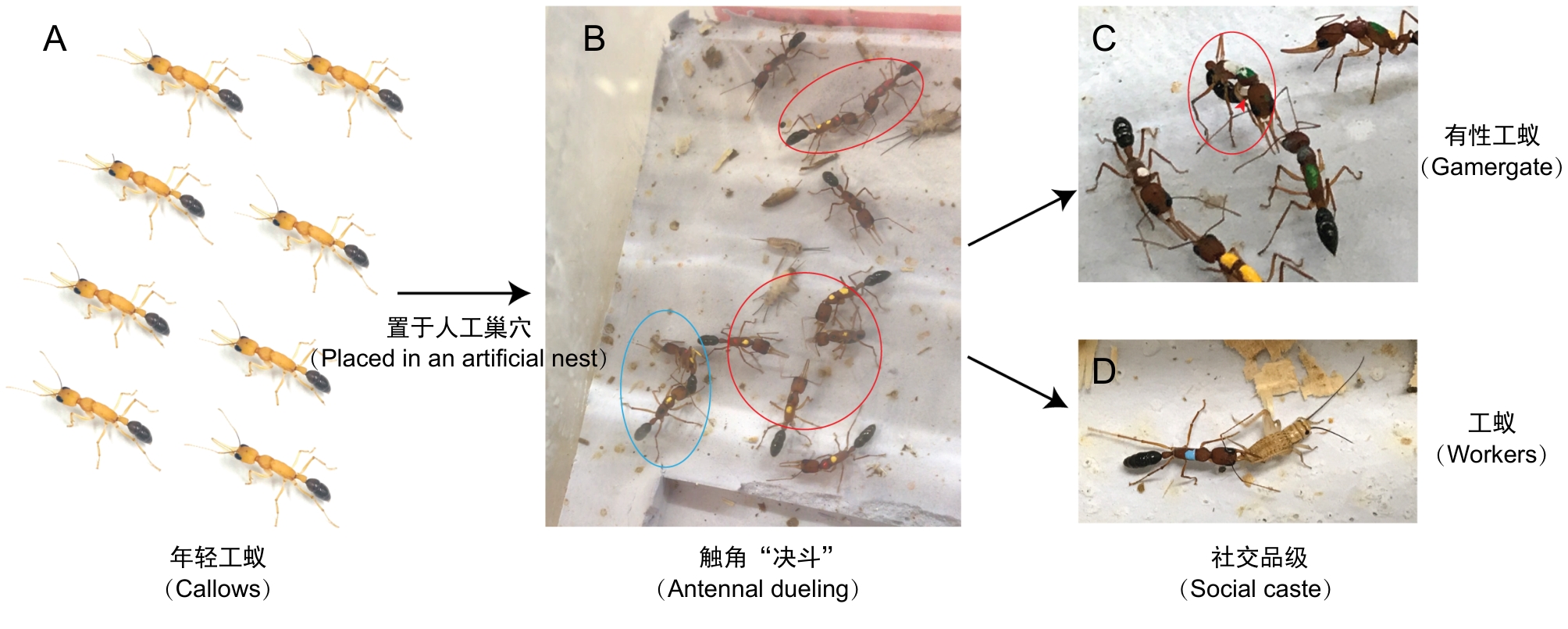
图2 实验室条件下跳镰猛蚁社会品级转变研究模型的建立注:A,年轻工蚁;B,品级转变,红色圈标记触角鞭打与决斗行为的个体,蓝色圈标记“警戒”行为,通常发生在当被单独饲养的有性工蚁被转移至宿主蚁群时;C,产卵的有性工蚁(红色圈),红色箭头标记白色的卵;D,觅食的工蚁。
Figure 2 Establishment of a research model for caste transition in Harpegnathos saltator under laboratory conditionsNote: A, Callows; B, Caste transition, individuals marked with red circles are exhibiting antennal dueling behavior, and those marked with blue circles are performing "policing" behavior, which typically occurs when an isolated gamergate is reintroduced into the host colony; C, Egg-laying gamergate (red circle), with the red arrow indicating white egg; D, Foraging workers.
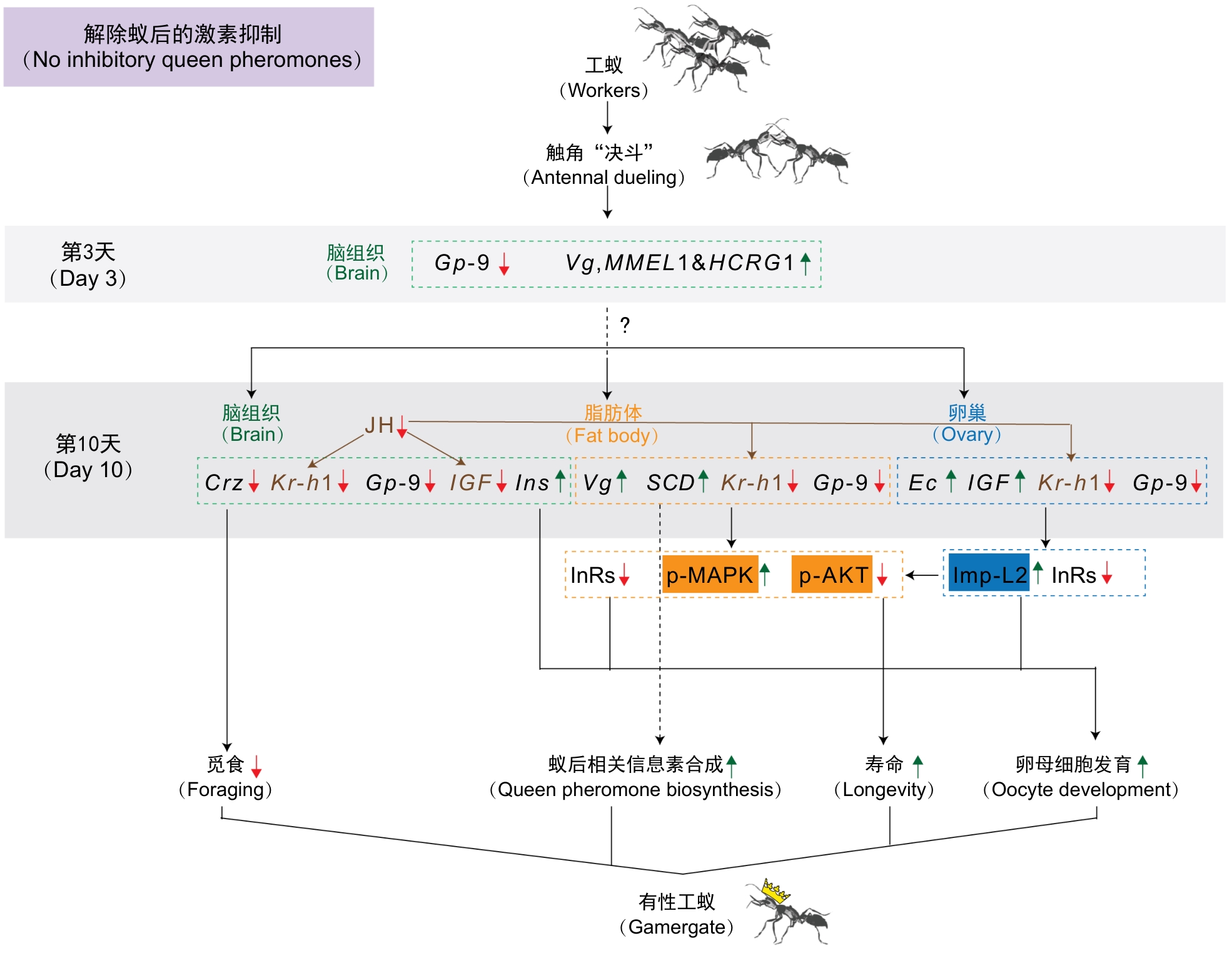
图3 跳镰猛蚁品级转变相关分子变化示意图(修改自参考文献[14])注:蚁后抑制性信息素的缺失解除对工蚁之间触角决斗行为的抑制,并引发多个组织中的一系列分子变化。假设模型:在预定将转变为有性工蚁的个体中,脑内基因表达的初始变化,如Gp-9的下调和Vg的上调,会引发一系列在脑内、脂肪体和卵巢中的基因表达级联变化,从而促进类似蚁后的表型(如蚁后信息素的生物合成和卵母细胞发育),并抑制工蚁特征(如觅食行为)和延长寿命。Ins是人类胰岛素的同源基因,IGF是人类IGF 1的同源基因。在跳镰猛蚁中,Ins先前被称为Ilp1,IGF先前被称为Ilp2[9,15],在蜜蜂和毕氏卵角蚁中Ins的同源基因被称为Ilp2[16-18]。图中红色箭头表示在决斗蚂蚁中下调的分子,绿色箭头表示上调的分子。实线表示该分子的已知功能,虚线表示假设性调控关系。
Figure 3 Schematic diagram of molecular changes related to caste transition in Harpegnathos saltator (modified from reference[14])Note: The absence of queen inhibitory pheromones relieves the suppression on antennal dueling behavior among workers and triggers a cascade of molecular changes across multiple tissues. Hypothetical model: initial gene expression changes in the destined gamergates brain, such as down-regulation of Gp-9 and up-regulation of vitellogenin, trigger a cascade of gene expression changes in the brain, fat body, and ovary that promote queen-like phenotypes, such as queen pheromone biosynthesis and oocyte development, and suppress worker-like phenotypes, such as foraging, while extending longevity. Ins is the homologous gene of human insulin, whereas IGF is the homologous gene of human IGF 1. Ins was previously known as Ilp1 and IGF as Ilp2 in H. saltator[9,15], while the homologous gene of Ins in honeybees and clonal raider ants was called Ilp2 [16-18]. Red arrows indicate downregulated molecules, green arrows indicate upregulated molecules in dueling ants. Solid lines represent confirmed molecule functions, while dashed lines represent hypothetical regulatory relationships.
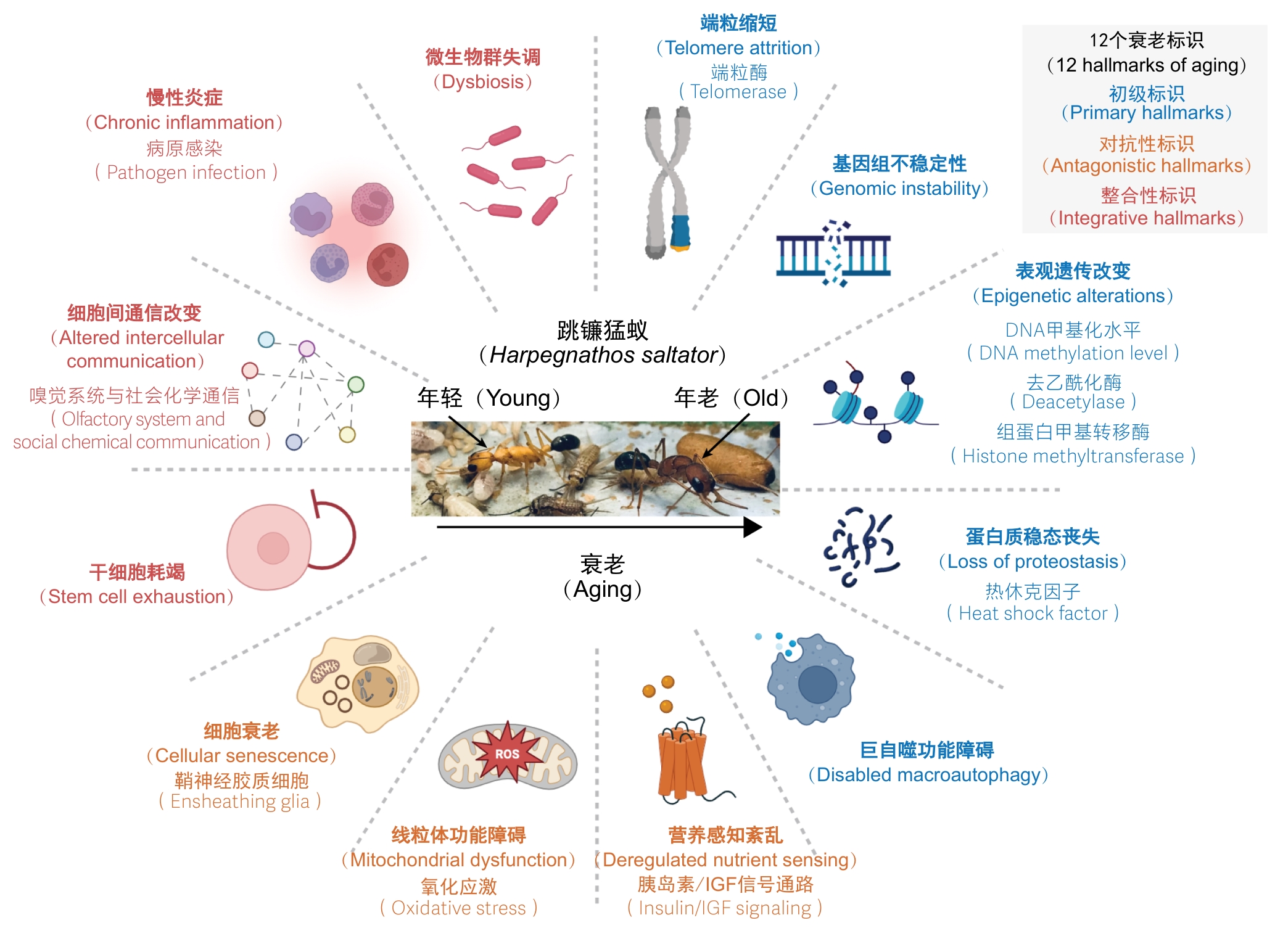
图4 基于衰老标识汇总跳镰猛蚁品级转变过程中衰老调控机制的研究进展
Figure 4 Research advances in aging regulatory mechanisms during caste transition in Harpegnathos saltator based on aging hallmarks
| [1] | PYENSON B, ALBIN-BROOKS C, BURHYTE C, et al. Worker-like behavioral and physiological phenotype in queens with removed wings in a ponerine ant[J]. J Exp Biol, 2022, 225(18): jeb243684. DOI:10.1242/jeb.243684 . |
| [2] | PENICK C A, PRAGER S S, LIEBIG J. Juvenile hormone induces queen development in late-stage larvae of the ant Harpegnathos saltator [J]. J Insect Physiol, 2012, 58(12):1643-1649. DOI:10.1016/j.jinsphys.2012.10.004 . |
| [3] | HAIGHT K L. Patterns of venom production and temporal polyethism in workers of Jerdon's jumping ant, Harpegnathos saltator [J]. J Insect Physiol, 2012, 58(12):1568-1574. DOI:10.1016/j.jinsphys.2012.09.011 . |
| [4] | LIEBIG J, PEETERS C, OLDHAM N J, et al. Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator?[J]. Proc Natl Acad Sci USA, 2000, 97(8):4124-4131. DOI: 10.1073/pnas.97.8.4124 . |
| [5] | GHANINIA M, HAIGHT K, BERGER S L, et al. Chemosensory sensitivity reflects reproductive status in the ant Harpegnathos saltator [J]. Sci Rep, 2017, 7(1):3732. DOI: 10.1038/s41598-017-03964-7 . |
| [6] | GELLERT H R, HALLEY D C, SIEB Z J, et al. Microstructures at the distal tip of ant chemosensory sensilla[J]. Sci Rep, 2022, 12(1):19328. DOI: 10.1038/s41598-022-21507-7 . |
| [7] | OPACHALOEMPHAN C, CARMONA-ALDANA F, YAN H. Caste transition and reversion in Harpegnathos saltator ant colonies[J]. Bio Protoc, 2023, 13(16): e4770. DOI: 10.21769/BioProtoc.4770 . |
| [8] | PENICK C A, GHANINIA M, HAIGHT K L, et al. Reversible plasticity in brain size, behaviour and physiology characterizes caste transitions in a socially flexible ant (Harpegnathos saltator)[J]. Proc Biol Sci, 2021, 288(1948):20210141. DOI: 10.1098/rspb.2021.0141 . |
| [9] | BONASIO R, ZHANG G J, YE C Y, et al. Genomic comparison of the ants Camponotus floridanus and Harpegnathos saltator [J]. Science, 2010, 329(5995):1068-1071. DOI: 10.1126/science.1192428 . |
| [10] | SHIELDS E J, SHENG L H, WEINER A K, et al. High-quality genome assemblies reveal long non-coding RNAs expressed in ant brains[J]. Cell Rep, 2018, 23(10):3078-3090. DOI: 10.1016/j.celrep.2018.05.014 . |
| [11] | LIU A K, WANG Y, DANG C W, et al. A genome-wide identification and analysis of the basic helix-loop-helix transcription factors in the ponerine ant, Harpegnathos saltator [J]. BMC Evol Biol, 2012, 12:165. DOI:10.1186/1471-2148-12-165 . |
| [12] | BONASIO R, LI Q Y, LIAN J M, et al. Genome-wide and caste-specific DNA methylomes of the ants Camponotus floridanus and Harpegnathos saltator [J]. Curr Biol, 2012, 22(19):1755-1764. DOI: 10.1016/j.cub.2012.07.042 . |
| [13] | SHIELDS E J, SORIDA M, SHENG L H, et al. Genome annotation with long RNA reads reveals new patterns of gene expression and improves single-cell analyses in an ant brain[J]. BMC Biol, 2021, 19(1):254. DOI: 10.1186/s12915-021-01188-w . |
| [14] | OPACHALOEMPHAN C, MANCINI G, KONSTANTINIDES N, et al. Early behavioral and molecular events leading to caste switching in the ant Harpegnathos [J]. Genes Dev, 2021, 35(5-6):410-424. DOI: 10.1101/gad.343699.120 . |
| [15] | GOSPOCIC J, SHIELDS E J, GLASTAD K M, et al. The neuropeptide corazonin controls social behavior and caste identity in ants[J]. Cell, 2017, 170(4):748-759.e12. DOI: 10.1016/j.cell.2017.07.014 . |
| [16] | WANG Y, AZEVEDO S V, HARTFELDER K, et al. Insulin-like peptides (AmILP1 and AmILP2) differentially affect female caste development in the honey bee (Apis mellifera L.)[J]. J Exp Biol, 2013, 216(Pt 23):4347-4357. DOI: 10.1242/jeb.085779 . |
| [17] | CORONA M, VELARDE R A, REMOLINA S, et al. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity[J]. Proc Natl Acad Sci USA, 2007, 104(17):7128-7133. DOI: 10.1073/pnas.0701909104 . |
| [18] | CHANDRA V, FETTER-PRUNEDA I, OXLEY P R, et al. Social regulation of insulin signaling and the evolution of eusociality in ants[J]. Science, 2018, 361(6400):398-402. DOI: 10.1126/science.aar5723 . |
| [19] | PENICK C A, BRENT C S, DOLEZAL K, et al. Neurohormonal changes associated with ritualized combat and the formation of a reproductive hierarchy in the ant Harpegnathos saltator [J]. J Exp Biol, 2014, 217(Pt 9):1496-1503. DOI: 10.1242/jeb.098301 . |
| [20] | WU Q, BROWN M R. Signaling and function of insulin-like peptides in insects[J]. Annu Rev Entomol, 2006, 51:1-24. DOI: 10.1146/annurev.ento.51.110104.151011 . |
| [21] | YAN H, OPACHALOEMPHAN C, CARMONA-ALDANA F, et al. Insulin signaling in the long-lived reproductive caste of ants[J]. Science, 2022, 377(6610):1092-1099. DOI: 10.1126/science.abm8767 . |
| [22] | LIBBRECHT R, OXLEY P R, KRONAUER D J C. Clonal raider ant brain transcriptomics identifies candidate molecular mechanisms for reproductive division of labor[J]. BMC Biol, 2018, 16(1):89. DOI: 10.1186/s12915-018-0558-8 . |
| [23] | PENICK C A, LIEBIG J, BRENT C S. Reproduction, dominance, and caste: endocrine profiles of queens and workers of the ant Harpegnathos saltator [J]. J Comp Physiol A Neuroethol Sens Neural Behav Physiol, 2011, 197(11):1063-1071. DOI: 10.1007/s00359-011-0667-0 . |
| [24] | BRENT C, PEETERS C, DIETEMANN V, et al. Hormonal correlates of reproductive status in the queenless ponerine ant, Streblognathus peetersi [J]. J Comp Physiol A Neuroethol Sens Neural Behav Physiol, 2006, 192(3):315-320. DOI: 10.1007/s00359-005-0065-6 . |
| [25] | BLOCH G, HEFETZ A, HARTFELDER K. Ecdysteroid titer, ovary status, and dominance in adult worker and queen bumble bees (Bombus terrestris)[J]. J Insect Physiol, 2000, 46(6):1033-1040. DOI: 10.1016/s0022-1910(99)00214-0 . |
| [26] | RACHINSKY A, STRAMBI C, STRAMBI A, et al. Caste and metamorphosis: hemolymph titers of juvenile hormone and ecdysteroids in last instar honeybee larvae[J]. Gen Comp Endocrinol, 1990, 79(1):31-38. DOI: 10.1016/0016-6480(90)90085-z . |
| [27] | GOSPOCIC J, GLASTAD K M, SHENG L H, et al. Kr-h1 maintains distinct caste-specific neurotranscriptomes in response to socially regulated hormones[J]. Cell, 2021, 184(23):5807-5823.e14. DOI: 10.1016/j.cell.2021.10.006 . |
| [28] | GLASTAD K M, ROESSLER J, GOSPOCIC J, et al. Long ant life span is maintained by a unique heat shock factor[J]. Genes Dev, 2023, 37(9-10):398-417. DOI: 10.1101/gad.350250.122 . |
| [29] | LÓPEZ-OTÍN C, BLASCO M A, PARTRIDGE L, et al. Hallmarks of aging: an expanding universe[J]. Cell, 2023, 186(2):243-278. DOI: 10.1016/j.cell.2022.11.001 . |
| [30] | SAHIN E, DEPINHO R A. Linking functional decline of telomeres, mitochondria and stem cells during ageing[J]. Nature, 2010, 464(7288):520-528. DOI: 10.1038/nature08982 . |
| [31] | SCHNEIDER S A, SCHRADER C, WAGNER A E, et al. Stress resistance and longevity are not directly linked to levels of enzymatic antioxidants in the ponerine ant Harpegnathos saltator [J]. PLoS One, 2011, 6(1): e14601. DOI: 10.1371/journal.pone.0014601 . |
| [32] | YAMAMOTO R, PALMER M, KOSKI H, et al. Aging modulated by the Drosophila insulin receptor through distinct structure-defined mechanisms[J]. Genetics, 2021, 217(2): iyaa037. DOI: 10.1093/genetics/iyaa037 . |
| [33] | LI W J, WANG C W, TAO L, et al. Insulin signaling regulates longevity through protein phosphorylation in Caenorhabditis elegans [J]. Nat Commun, 2021, 12(1):4568. DOI: 10.1038/s41467-021-24816-z . |
| [34] | MATHEW R, BHADRA M PAL, BHADRA U. Insulin/insulin-like growth factor-1 signalling (IIS) based regulation of lifespan across species[J]. Biogerontology, 2017, 18(1):35-53. DOI: 10.1007/s10522-016-9670-8 . |
| [35] | COHEN E, PAULSSON J F, BLINDER P, et al. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice[J]. Cell, 2009, 139(6):1157-1169. DOI: 10.1016/j.cell.2009.11.014 . |
| [36] | VIZUETA J, XIONG Z J, DING G, et al. Adaptive radiation and social evolution of the ants[J]. Cell, 2025, 188(18):4828-4848.e25. DOI: 10.1016/j.cell.2025.05.030 . |
| [37] | BONASIO R. Emerging topics in epigenetics: ants, brains, and noncoding RNAs[J]. Ann N Y Acad Sci, 2012, 1260:14-23. DOI: 10.1111/j.1749-6632.2011.06363.x . |
| [38] | SATO K, PELLEGRINO M, NAKAGAWA T, et al. Insect olfactory receptors are heteromeric ligand-gated ion channels[J]. Nature, 2008, 452(7190):1002-1006. DOI: 10.1038/nature06850 . |
| [39] | SIMOLA D F, GRAHAM R J, BRADY C M, et al. Epigenetic (re)programming of caste-specific behavior in the ant Camponotus floridanus [J]. Science, 2016, 351(6268): aac6633. DOI: 10.1126/science.aac6633 . |
| [40] | LINDQUIST S. The heat-shock response[J]. Annu Rev Biochem, 1986, 55:1151-1191. DOI: 10.1146/annurev.bi.55. 070186.005443 . |
| [41] | ÖSTLING P, BJÖRK J K, ROOS-MATTJUS P, et al. Heat shock factor 2 (HSF2) contributes to inducible expression of hsp genes through interplay with HSF1[J]. J Biol Chem, 2007, 282(10):7077-7086. DOI:10.1074/jbc.M607556200 . |
| [42] | SHENG L H, SHIELDS E J, GOSPOCIC J, et al. Social reprogramming in ants induces longevity-associated glia remodeling[J]. Sci Adv, 2020, 6(34): eaba9869. DOI:10.1126/sciadv.aba9869 . |
| [43] | SHENG L H, SHIELDS E J, GOSPOCIC J, et al. Ensheathing glia promote increased lifespan and healthy brain aging[J]. Aging Cell, 2023, 22(5): e13803. DOI:10.1111/acel.13803 . |
| [44] | FRAKES A E, METCALF M G, TRONNES S U, et al. Four glial cells regulate ER stress resistance and longevity via neuropeptide signaling in C. elegans [J]. Science, 2020, 367(6476):436-440. DOI: 10.1126/science.aaz6896 . |
| [45] | KOMIYAMA T, LUO L Q. Development of wiring specificity in the olfactory system[J]. Curr Opin Neurobiol, 2006, 16(1):67-73. DOI: 10.1016/j.conb.2005.12.002 . |
| [46] | YAN H, JAFARI S, PASK G, et al. Evolution, developmental expression and function of odorant receptors in insects[J]. J Exp Biol, 2020, 223(Pt Suppl 1): jeb208215. DOI: 10.1242/jeb.208215 . |
| [47] | ROBERTSON H M. Molecular evolution of the major arthropod chemoreceptor gene families[J]. Annu Rev Entomol, 2019, 64:227-242. DOI: 10.1146/annurev-ento-020117-043322 . |
| [48] | RENGARAJAN S, HALLEM E A. Olfactory circuits and behaviors of nematodes[J]. Curr Opin Neurobiol, 2016, 41:136-148. DOI: 10.1016/j.conb.2016.09.002 . |
| [49] | IMAI T, SAKANO H. Odorant receptor-mediated signaling in the mouse[J]. Curr Opin Neurobiol, 2008, 18(3):251-260. DOI: 10.1016/j.conb.2008.07.009 . |
| [50] | BENTON R. On the ORigin of smell: odorant receptors in insects[J]. Cell Mol Life Sci, 2006, 63(14):1579-1585. DOI: 10.1007/s00018-006-6130-7 . |
| [51] | BUTTERWICK J A, DEL MÁRMOL J, KIM K H, et al. Cryo-EM structure of the insect olfactory receptor Orco[J]. Nature, 2018, 560(7719):447-452. DOI: 10.1038/s41586-018-0420-8 . |
| [52] | WICHER D, SCHÄFER R, BAUERNFEIND R, et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels[J]. Nature, 2008, 452(7190):1007-1011. DOI: 10.1038/nature06861 . |
| [53] | BRAND P, ROBERTSON H M, LIN W, et al. The origin of the odorant receptor gene family in insects[J]. eLife, 2018, 7: e38340. DOI: 10.7554/eLife.38340 . |
| [54] | MIER P, FONTAINE J F, STOLDT M, et al. Annotation and analysis of 3902 odorant receptor protein sequences from 21 insect species provide insights into the evolution of odorant receptor gene families in solitary and social insects[J]. Genes (Basel), 2022, 13(5):919. DOI: 10.3390/genes13050919 . |
| [55] | ENGSONTIA P, SANGKET U, ROBERTSON H M, et al. Diversification of the ant odorant receptor gene family and positive selection on candidate cuticular hydrocarbon receptors[J]. BMC Res Notes, 2015, 8:380. DOI: 10.1186/s13104-015-1371-x . |
| [56] | ZHOU X F, SLONE J D, ROKAS A, et al. Phylogenetic and transcriptomic analysis of chemosensory receptors in a pair of divergent ant species reveals sex-specific signatures of odor coding[J]. PLoS Genet, 2012, 8(8): e1002930. DOI: 10.1371/journal.pgen.1002930 . |
| [57] | YAN H, OPACHALOEMPHAN C, MANCINI G, et al. An engineered orco mutation produces aberrant social behavior and defective neural development in ants[J]. Cell, 2017, 170(4):736-747.e9. DOI: 10.1016/j.cell.2017.06.051 . |
| [58] | TRIBLE W, OLIVOS-CISNEROS L, MCKENZIE S K, et al. Orco mutagenesis causes loss of antennal lobe glomeruli and impaired social behavior in ants[J]. Cell, 2017, 170(4):727-735.e10. DOI: 10.1016/j.cell.2017.07.001 . |
| [59] | SIERIEBRIENNIKOV B, SIEBER K R, KOLUMBA O, et al. Orco-dependent survival of odorant receptor neurons in ants[J]. Sci Adv, 2024, 10(23): eadk9000. DOI: 10.1126/sciadv.adk9000 . |
| [60] | PASK G M, SLONE J D, MILLAR J G, et al. Specialized odorant receptors in social insects that detect cuticular hydrocarbon cues and candidate pheromones[J]. Nat Commun, 2017, 8(1):297. DOI: 10.1038/s41467-017-00099-1 . |
| [61] | SLONE J D, PASK G M, FERGUSON S T, et al. Functional characterization of odorant receptors in the ponerine ant, Harpegnathos saltator [J]. Proc Natl Acad Sci USA, 2017, 114(32):8586-8591. DOI: 10.1073/pnas.1704647114 . |
| [62] | LI Q Y, WANG M Y, ZHANG P, et al. A single-cell transcriptomic atlas tracking the neural basis of division of labour in an ant superorganism[J]. Nat Ecol Evol, 2022, 6(8):1191-1204. DOI: 10.1038/s41559-022-01784-1 . |
| [63] | JU L Y, GLASTAD K M, SHENG L H, et al. Hormonal gatekeeping via the blood-brain barrier governs caste-specific behavior in ants[J]. Cell, 2023, 186(20):4289-4309.e23. DOI: 10.1016/j.cell.2023.08.002 . |
| [64] | MCKENZIE S K, KRONAUER D J C. The genomic architecture and molecular evolution of ant odorant receptors[J]. Genome Res, 2018, 28(11):1757-1765. DOI: 10.1101/gr.237123.118 . |
| [65] | GAO Q H, XIONG Z J, LARSEN R S, et al. High-quality chromosome-level genome assembly and full-length transcriptome analysis of the Pharaoh ant Monomorium pharaonis [J]. Gigascience, 2020, 9(12): giaa143. DOI: 10.1093/gigascience/giaa143 . |
| [66] | WURM Y, WANG J, RIBA-GROGNUZ O, et al. The genome of the fire ant Solenopsis invicta [J]. Proc Natl Acad Sci USA, 2011, 108(14):5679-5684. DOI: 10.1073/pnas.1009690108 . |
| [67] | WANG M Y, LIU Y, WEN T G, et al. Chromatin accessibility and transcriptome landscapes of Monomorium pharaonis brain[J]. Sci Data, 2020, 7(1):217. DOI: 10.1038/s41597-020-0556-x . |
| [68] | JONES B M, WAUGH A H, CATTO M A, et al. The fire ant social chromosome exerts a major influence on genome regulation[J]. Mol Biol Evol, 2025, 42(6): msaf112. DOI: 10.1093/molbev/msaf112 . |
| [69] | GLASTAD K M, JU L Y, BERGER S L. Tramtrack acts during late pupal development to direct ant caste identity[J]. PLoS Genet, 2021, 17(9): e1009801. DOI: 10.1371/journal.pgen. 1009801 . |
| [70] | GLASTAD K M, GRAHAM R J, JU L Y, et al. Epigenetic regulator CoREST controls social behavior in ants[J]. Mol Cell, 2020, 77(2):338-351.e6. DOI: 10.1016/j.molcel.2019.10.012 . |
| [71] | SIEBER K, SAAR M, OPACHALOEMPHAN C, et al. Embryo injections for CRISPR-mediated mutagenesis in the ant Harpegnathos saltator [J]. J Vis Exp, 2021(168):10.3791/61930. DOI: 10.3791/61930 . |
| [72] | IVASYK I, OLIVOS-CISNEROS L, VALDÉS-RODRÍGUEZ S, et al. DNMT1 mutant ants develop normally but have disrupted oogenesis[J]. Nat Commun, 2023, 14(1):2201. DOI: 10.1038/s41467-023-37945-4 . |
| [73] | SIERIEBRIENNIKOV B, REINBERG D, DESPLAN C. A molecular toolkit for superorganisms[J]. Trends Genet, 2021, 37(9):846-859. DOI: 10.1016/j.tig.2021.05.005 . |
| [74] | HART T, FRANK D D, LOPES L E, et al. Sparse and stereotyped encoding implicates a core Glomerulus for ant alarm behavior[J]. Cell, 2023, 186(14):3079-3094.e17. DOI: 10.1016/j.cell.2023.05.025 . |
| [75] | ZHOU K C, HARFOUCHE M, COOKE C L, et al. Parallelized computational 3D video microscopy of freely moving organisms at multiple gigapixels per second[J]. Nat Photonics, 2023, 17(5):442-450. DOI: 10.1038/s41566-023-01171-7 . |
| [76] | KHILA A, ABOUHEIF E. In situ hybridization on ant ovaries and embryos[J]. Cold Spring Harb Protoc, 2009, 2009(7): pdb.prot5250. DOI: 10.1101/pdb.prot5250 . |
| [1] | 宋梦娇, 沈义栋. 秀丽隐杆线虫的线粒体形态和功能研究方法及应用实例[J]. 实验动物与比较医学, 2025, 45(6): 726-737. |
| [2] | 王超, 李顺, 任晓楠, 杨华, 陈丽香, 徐春华, 周晓辉. 衰老抑制C57BL/6J小鼠肺部CD8+ T细胞对甲型H1N1流感病毒的记忆性免疫应答[J]. 实验动物与比较医学, 2025, 45(5): 515-523. |
| [3] | 肖文娴, 吕龙宝. 非人灵长类实验动物用于人类卵巢衰老研究进展[J]. 实验动物与比较医学, 2025, 45(1): 47-54. |
| [4] | 刘荣乐, 程灏, 尚付生, 常书福, 徐平. SHJH hr 小鼠的心脏衰老表型研究[J]. 实验动物与比较医学, 2025, 45(1): 13-20. |
| [5] | 李睿琪, 段涵, 甘罗, 郑媛, 杨文. 玻璃海鞘作为模式生物的优势及其应用[J]. 实验动物与比较医学, 2024, 44(2): 162-179. |
| [6] | 成慧, 方菲, 石嘉豪, 杨桦, 张梦杰, 杨平, 费俭. hil-1基因通过饮食限制通路调节秀丽隐杆线虫寿命[J]. 实验动物与比较医学, 2023, 43(3): 271-281. |
| [7] | 尹丹阳, 胡怡, 史仍飞. 动物衰老模型的研究进展[J]. 实验动物与比较医学, 2023, 43(2): 156-162. |
| [8] | 唐慧青, 常书福, 于志锋, 张雷, 谈小倩, 瞿伟, 李亮, 钱珍, 顾坚忠, 徐平. SHJH hr 小鼠部分生物学特性及衰老表型的测定与分析[J]. 实验动物与比较医学, 2023, 43(1): 44-52. |
| [9] | 潘永明, 刘瑞敏, 吴蔚, 王辉, 贾临超, 徐孝平, 朱科燕, 陈民利. 心率变异性分析评估不同因素致亚健康大鼠自主神经功能的影响[J]. 实验动物与比较医学, 2012, 32(6): 467-472. |
| [10] | 贾临超, 冷晓霞, 陈民利, 潘永明, 寿旗扬, 周卫民, 陶涛, 朱科燕. 抗衰老片对肾虚小鼠空间学习记忆和脑自由基代谢的影响[J]. 实验动物与比较医学, 2011, 31(6): 432-435. |
| [11] | 谢日青,冷晓霞,陈民利,朱科燕,林琳,陶涛,余佳,潘永明. 束缚应激致亚健康状态大鼠氧自由基变化及中药干预作用[J]. 实验动物与比较医学, 2010, 30(2): 121-123. |
| [12] | 沈志祥1, 乔伟伟2, 刘翠鲜1. 营养摄入限制对老年小鼠血甘油三酯及骨密度的影响[J]. 实验动物与比较医学, 2006, 26(1): 28-30. |
| [13] | 庄明1,王健2. SD大鼠股外侧肌形态结构及其酶活性的年龄性改变[J]. 实验动物与比较医学, 2002, 22(1): 53-55. |
| [14] | 陈冠敏, 林升清, 黄宗锈, 郑丽红, 何聆, 刘少娟. 去氢表雄酮抗衰老作用初探[J]. 实验动物与比较医学, 2000, 20(3): 151-153. |
| [15] | 杨斐, 丁宁, 杨昊, 桂立英. 老年雄性大鼠血清总超氧化物歧化酶活性、丙二醛含量和血浆雌二醇、睾酮含量的测定[J]. 实验动物与比较医学, 2000, 20(1): 49-51. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

