多囊卵巢综合征(polycystic ovary syndrome,PCOS)以高雄激素血症、卵巢多囊样改变、排卵异常、高抗米勒管激素(anti-Müllerian hormone,AMH)水平为主要诊断标准,同时伴有肥胖、胰岛素抵抗等代谢功能障碍;其作为一种生殖内分泌疾病,是导致无排卵性不孕最常见的原因[1],影响全球范围内大约10%~13%的女性[2]。PCOS是一种慢性疾病,目前尚无法治愈,一经诊断,常伴随终身。临床上通常会根据患者的临床表现及需求,在管理生活方式的基础上给予相应的对症治疗,从而改善PCOS患者出现的代谢、内分泌及不孕症状;然而PCOS的不良影响并不止这些,目前针对患者全身代谢和内分泌功能异常的治疗仍面临许多挑战[3]。
PCOS动物模型的建立方法多样,包括外源高雄激素造模、芳香化酶抑制剂造模、产前雄激素或AMH造模、胰岛素造模、肠道菌群造模等,但至今还没有一种模型能够完全复制模拟PCOS患者的临床特点。本研究组选择芳香化酶抑制剂造模方法,使用芳香化酶抑制剂来曲唑阻断雄激素向雌激素转化的过程。研究表明,在青春期前开始持续接受来曲唑治疗的小鼠可以呈现出许多PCOS的生殖和代谢特点,包括肥胖、雄激素和黄体生成素升高、卵巢多囊样表现和动情周期紊乱[4];而来曲唑结合高脂饮食,会在此基础上诱导出更明显的代谢异常,包括肥胖、胰岛素抵抗和血脂异常等[5]。另外,相较于其他方式,通过缓释片给药的造模方法简便,仅需要一次皮下包埋,且缓释稳定。因此,在青春期前接受来曲唑缓释片皮下包埋结合高脂饮食处理,可以模拟多数PCOS患者的内分泌和代谢表型。
肝脏是机体的重要代谢器官,与PCOS之间存在许多潜在的关联。以代谢功能障碍相关脂肪性肝病为例,多项研究已证实其与PCOS有很高的共患率和相关性,互为发病的风险因素[6-7]。肝脏分泌的多种因子在PCOS患者血液中水平异常,如性激素结合蛋白(sex hormone-binding globulin,SHBG)、FutuinA和成纤维细胞生长因子21(fibroblast growth factor-21,FGF21)等[8-10]。但PCOS模型中肝脏组织和功能的变化仍不清楚。本研究拟通过来曲唑缓释片皮下包埋和高脂饮食相结合的方法构建PCOS小鼠模型,然后对模型小鼠的肝脏进行转录组测序及差异基因分析,并进一步作功能富集分析,为探讨PCOS发病过程中肝脏的作用机制和有效筛选PCOS作用靶点提供模型和数据参考。
1 材料与方法
1.1 实验动物
16只SPF级雌性C57BL/6J小鼠,3周龄,体重为(11.9±1.1)g,购自上海灵畅生物科技有限公司[SCXK(沪)2023-0003],质量合格证号为20180003027971。所有实验小鼠均饲养于上海交通大学医学院实验动物科学部[SYXK(沪)2023-0041]的屏障环境实验动物设施中,每笼饲养4只小鼠,提供充足的无菌水和饲料,保持环境内温度20~22 ℃,相对湿度50%~60%,光照明暗12 h交替。本实验经上海交通大学医学院实验动物饲养管理与使用委员会批准(批号JUMC-2023-066-B),实验过程中严格遵守我国动物实验伦理和使用相关准则。
1.2 主要试剂与仪器
来曲唑缓释片(批号SX-999-2mg)及对照安慰剂缓释片(批号SC-111-2mg)通过美国IRA公司定制,均为直径约3 mm的片剂,缓释周期为40 d,缓释剂量为50 μg/d。高脂饲料D12492(批号 22090102)购自美国 Research Diets公司。舒泰(批号98CKA)购自法国Virbac公司。右美托咪定(批号190625BP)购自江苏恒瑞医药股份有限公司。异氟烷(批号 20211201)购自江苏恒丰强生物技术有限公司。通用型组织固定液(批号G1101)、F4/80免疫组织化学染色所用一抗(批号GB113373)和辣根过氧化物酶标记山羊抗兔二抗(批号GB23303)购自武汉赛维尔生物科技有限公司。TRIzol试剂(批号15596)购自美国 Thermo Fisher Scientific公司。RNA反转录试剂盒(批号 RR036A)与定量PCR试剂盒TB Green Premix Ex Taq(批号 RR420A)购自日本 TaKaRa公司。小鼠体重测量用电子天平(型号Secura612-1CN)和肝脏重量称量用电子天平(型号BSA224S-CW)均购自德国Sartorius公司。Matrx VWR小动物麻醉机购自美国MIDMARK公司。
1.3 PCOS模型小鼠构建
参考相关文献[11-12]所描述的方法,将16只3周龄C57BL/6J雌鼠随机分为两组:安慰剂对照组8只,来曲唑造模组8只。用试剂盒中的灭菌稀释液溶解舒泰粉末,取1 mL舒泰溶液(50 mg),加入等体积的右美托咪定溶液(0.1 mg),使用生理盐水稀释。所有小鼠在屏障环境内适应3 d后,按50 mg/kg舒泰联合0.1 mg/kg右美托咪定的剂量腹腔注射进行麻醉。使用剃毛机脱去小鼠后颈部毛发,用镊子提起皮肤,用眼科剪在颈后剪开约2~3 mm长的竖直小口,使用镊子钝性分离皮肤,将缓释片塞进皮下,缓释片会自动贴合小鼠皮肤组织,随后用镊子夹紧并缝合手术创口。术后,将小鼠放置在新的饲养笼里,每笼4只,并用高压灭菌后的纸巾覆盖保暖。术后8~24 h观察小鼠麻醉苏醒情况,并在24 h内更换两组小鼠饲料为新鲜高脂饲料,持续饲喂5周。
1.4 小鼠日增重与卵巢、肝脏的组织学检测
缓释片包埋后的第7、14、21和28天,测量所有动物体重,计算体重变化率[(被测当天体重-初始体重)/初始体重×100%]。从造模当天起,每周最后一天检测小鼠24 h进食量:于当天上午9点,给小鼠换用新鲜颗粒状的高脂饲料,称取饲料重量并记录;次日上午9点,再次称取每笼的高脂饲料重量并记录,计算得到24 h全笼摄食量(即饲料重量差值)及每笼中每只小鼠的平均日摄食量(饲料重量差值/每笼小鼠数目)。每周监测1 d,连续监测5周。
第36天称量小鼠体重后,使用小动物麻醉机和异氟烷麻醉小鼠。通过颈椎脱臼法安乐死小鼠后迅速剪开胸腔,收集所有小鼠的卵巢和肝脏,称量肝脏重量。将每只小鼠的肝脏左叶组织,用剪刀纵剖为左右两部分。将右侧卵巢和一半肝脏左叶迅速投入通用型组织固定液中,用于制备石蜡切片,通过HE染色观察组织病理学变化,通过Masson染色观察纤维化情况。另一半肝左叶迅速投入通用型组织固定液,用于制备冰冻切片,进行油红O染色,观察脂质沉积情况。肝脏剩余部分先在液氮中速冻,之后冻存于-80 ℃冰箱。
1.5 肝脏F4/80免疫组织化学染色
利用巨噬细胞表面标志物F4/80(即小鼠表皮生长因子样含模块黏蛋白样激素受体)进行肝脏免疫组织化学检测。将组织切片脱蜡脱水,并依次进行抗原修复、内源性过氧化氢酶阻断、山羊血清封闭;滴加F4/80抗体工作液,4 ℃过夜孵育;清洗后室温孵育二抗,然后滴加DAB显色液并在光学显微镜下观察;特异性棕色出现后,洗去DAB染色液,使用苏木精对比染色细胞核;分化、脱水、透明、封片固定后,在显微镜下观察。
1.6 肝脏转录组学分析
随机选取6个肝脏组织样本(安慰剂对照组和来曲唑造模组各3个),使用TRIzol提取样本总RNA。对RNA进行富集、片段化、逆转录、二链合成,末端修复并加接头后对cDNA进行PCR扩增、纯化,获得测序文库后上机进行双端测序。筛选差异表达基因的主要指标为差异倍数(fold change,FC)和P值,以|log2FC|≥1且P<0.05作为标准。对得到的差异基因进行GO功能注释、KEGG通路和GSEA富集分析。
1.7 实时荧光定量PCR验证肝组织中差异表达基因
选择2个上调基因和3个下调基因,运用定量PCR法对其转录水平进行验证。使用TRIzol试剂提取肝脏组织总RNA,利用反转录试剂盒反转录得到cDNA,质量浓度为5 ng/μL。每个反应体系10 μL,包括5 μL的2× TB Green、正向和反向引物各0.5 μL以及4 μL的cDNA。定量PCR反应条件:95 ℃预变性反应30 s,95 ℃变性反应5 s,60 ℃退火反应30 s,设置40次循环。以36B4基因为内参,采用2-△△Ct 方法计算目标基因3β-羟类固醇脱氢酶2(3 beta- and steroid delta-isomerase 2,HSD3B2)、3-羟基-3-甲基戊二酰辅酶A还原酶(3-hydroxy-3-methylglutaryl-coenzyme A reductase,HMGCR)、白细胞介素-4(interleukin-4,IL4)、C-C基序趋化因子配体2(C-C motif chemokine ligand 2,CCL2)和I型胶原α链(collagen,type I,alpha 1,COL1A1)的mRNA相对表达水平,基因引物序列见表1。
表 1 引物序列
Table 1
引物名称 Primer name | 引物序列 Sequence (5'→3') |
|---|---|
| 36B4-F | AGATTCGGGATATGCTGTTGGC |
| 36B4-R | TCGGGTCCTAGACCAGTGTTC |
| HSD3B2-F | GGTTTTTGGGGCAGAGGATCA |
| HSD3B2-R | GGTACTGGGTGTCAAGAATGTCT |
| HMGCR-F | CAAACATTGTCACCGCCATC |
| HMGCR-R | CCACCCACCGTTCCTATCTC |
| IL4-F | GGTCTCAACCCCCAGCTAGT |
| IL4-R | GCCGATGATCTCTCTCAAGTGAT |
| CCL2-F | TTAAAAACCTGGATCGGAACCAA |
| CCL2-R | GCATTAGCTTCAGATTTACGGGT |
| COL1A1-F | GCTCCTCTTAGGGGCCACT |
| COL1A1-R | CCACGTCTCACCATTGGGG |
1.8 统计学方法
应用GraphPad Prism 9.0软件进行统计分析与绘图,实验结果采用均数±标准误表示,采用独立样本t检验确定差异显著性,P<0.05表示差异有统计学意义。
2 结果
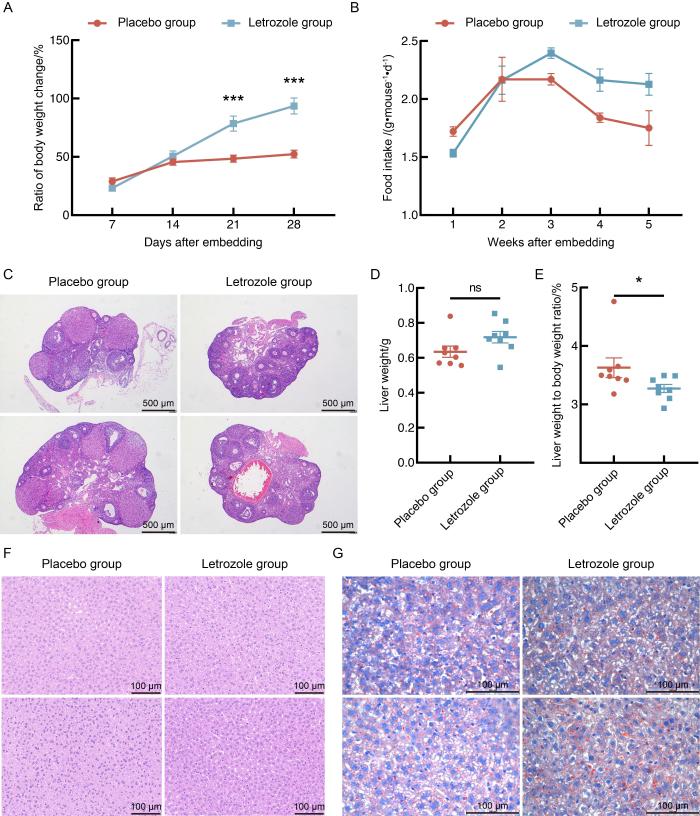
2.1 来曲唑和高脂饮食造模后的小鼠体重和肝脏状况
图 1
图 1
来曲唑和高脂饮食造模对小鼠体重、摄食量和肝脏的影响
Figure 1
Effects of letrozole and high-fat diet modeling on body weight, food intake and liver in mice
2.2 来曲唑和高脂饮食诱导的PCOS模型小鼠的肝脏转录组学分析
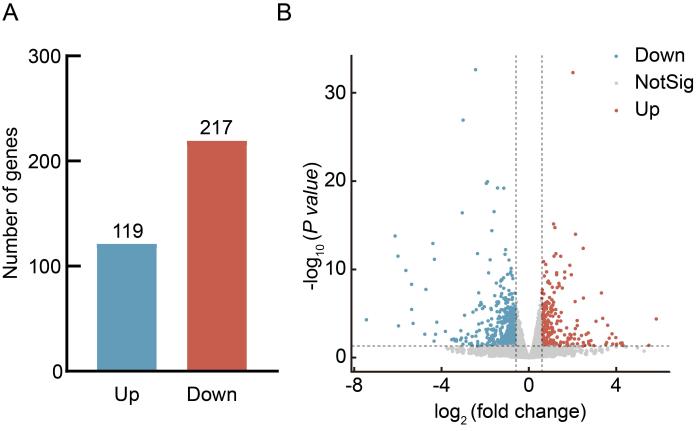
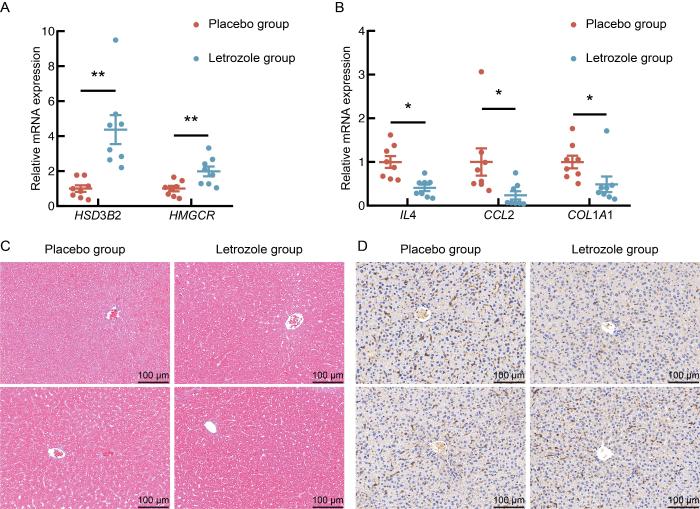
每组随机选取3个肝脏组织样本的转录组测序结果显示:与安慰剂对照组相比,来曲唑造模组小鼠的肝脏中共有336个差异表达基因(|log2FC|≥1且P<0.05),其中119个基因表达水平上调,217个基因表达水平下调(图2A和B)。
图 2
图 2
来曲唑和高脂饮食造模后小鼠肝脏组织中差异基因分析
Figure 2
Analysis of differentially expressed genes in liver of mice after letrozole and high-fat diet modeling
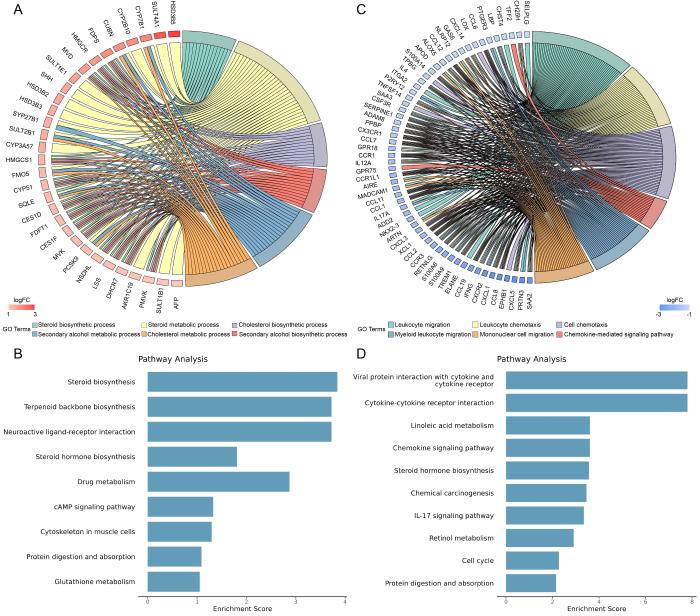
图 3
图 3
来曲唑和高脂饮食造模后小鼠差异表达基因的功能注释和富集分析
Figure 3
Functional annotation and enrichment analysis of differentially expressed genes in mice after letrozole and high-fat diet modeling
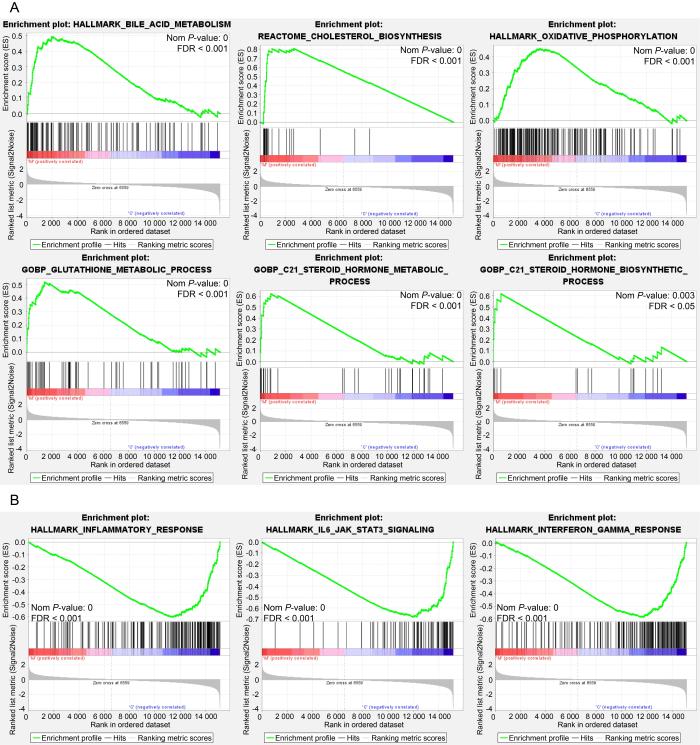
图 4
图 4
来曲唑和高脂饮食造模后差异表达基因的GSEA 富集分析
Figure 4
GSEA enrichment analysis of differentially expressed genes after letrozole and high-fat diet modeling
2.3 来曲唑和高脂饮食诱导的PCOS模型小鼠的差异基因验证
图 5
图 5
来曲唑和高脂饮食造模后小鼠差异基因的实时定量PCR验证
Figure 5
Real-time quantitative PCR verification of differentially expressed genes in mice after letrozole and high-fat diet modeling
3 讨论
肝脏是机体重要的代谢器官,与PCOS之间存在许多潜在的关联。本研究分析PCOS小鼠模型肝脏的转录组测序结果发现,来曲唑造模组小鼠肝脏表达上调的差异表达基因主要富集在类固醇合成、类固醇激素合成、胆固醇合成和代谢通路。PCOS患者常伴有血浆胆固醇水平异常[19],而PCOS大鼠模型的卵巢转录组中也发现了胆固醇、类固醇代谢相关基因表达失调[20],这些变化可能影响卵巢性激素的合成。胆固醇代谢异常在脂肪肝的发生中也起到重要作用[21]。本研究发现,模型组小鼠肝脏中胆固醇合成限速酶HMGCR的表达显著上调,这与既往研究结果一致,即HMGCR上调可促进饮食诱导的肥胖小鼠的脂肪组织炎症、胰岛素抵抗和肝脏脂肪变性[22],而这些病理变化在PCOS的发生发展中又发挥重要作用[23]。同时,本研究还发现,肝脏参与类固醇激素合成的HSD3B2表达也显著增强。因此,肝脏可能通过调控胆固醇和类固醇代谢,影响脂肪组织功能和糖脂代谢,进而参与PCOS的发病机制。
此外,对下调差异基因的分析显示,白细胞迁移、IL-17、IL6-JAK-STAT3等炎症相关的信号通路被显著富集[24-25],荧光定量PCR结果也显示来曲唑造模组小鼠肝脏炎症及纤维化相关基因IL4、CCL2、COL1A1的mRNA表达水平下调。这与既往文献中双氢睾酮诱导的PCOS大鼠模型中肝脏IL6表达无显著变化、TNFα和IL-1β表达水平升高,以及PCOS患者存在肥胖、脂肪组织炎症、胰岛炎症和全身慢性炎症的表现存在一定差异[26-29]。同时,本研究发现,在同样的高脂饮食喂养条件下,相较于安慰剂对照组,来曲唑造模组的小鼠体重增加更为显著,但肝重比却有所下降,且肝脏中与炎症反应相关的信号通路富集也呈现下调趋势。这些结果提示,来曲唑可能通过某种机制在一定程度上影响了肝脏的炎症反应和纤维化水平。然而,肝脏相关染色结果却未表现出明显的巨噬细胞浸润和纤维化程度变化,这表明来曲唑的作用程度和具体机制仍需进一步研究。
需要说明,本研究未设置普通饮食的来曲唑组和安慰剂组,在一定程度上限制了来曲唑单独作用和高脂饮食单独作用的肝脏影响评估。未来本研究组将在机制研究时完善分组,以更全面地揭示来曲唑和高脂饮食在PCOS发病中的独立和协同作用。另外,本研究缺少对胰岛素抵抗和血脂相关指标的检测,可能限制了对PCOS模型代谢状态的全面评估。未来将纳入这些指标,以更全面地揭示PCOS的代谢异常及其与肝脏功能的关系。
综上,本研究通过构建来曲唑结合高脂饮食诱导PCOS小鼠模型,成功模拟了PCOS的生殖系统组织学表型,并从组织形态学和转录组学水平对模型组和对照小鼠的肝脏结构和功能进行比较,且分析了肝脏在小鼠PCOS模型发病过程中的作用机制。本研究结果提示,肝脏可能是通过胆固醇和类固醇代谢改变参与PCOS的发生发展,为进一步探索PCOS的发病机制和治疗策略提供了新思路。
[引用本文]
许秋雨, 严国锋, 付丽, 等. 来曲唑缓释片皮下给药构建小鼠多囊卵巢综合征模型及其肝脏转录组学分析[J]. 实验动物与比较医学, 2025, 45(2): 119-129. DOI: 10.12300/j.issn.1674-5817.2024.186.
XU Q Y, YAN G F, FU L, et al. A mouse model of polycystic ovary syndrome established through subcutaneous administration of letrozole sustained-release pellets and hepatic transcriptome analysis[J]. Lab Anim Comp Med, 2025, 45(2): 119-129. DOI: 10.12300/j.issn.1674-5817.2024.186.
医学伦理声明
本研究涉及的所有动物实验均已通过上海交通大学医学院实验动物饲养管理与使用委员会审批通过 (批准编号 JUMC-2023-066-B)。所有实验过程均遵照中国实验动物相关法律法规条例要求进行。
Medical Ethics Statement
All animal experiments involved in this study have been approved by the Institutional Animal Care & Use Committee of Shanghai Jiao Tong University School of Medicine (Approval No. JUMC-2023-066-B). All experiments were performed in accordance with the requirements of laws and regulations on laboratory animals in China.
作者贡献声明
许秋雨负责研究方案策划、实验操作、数据整理和分析、论文撰写和修改;
严国锋、付丽、范文华、周晶和朱莲负责提供资源和动物实验操作;
仇淑雯负责实验结果的有效性验证;
张洁负责项目管理、监督指导、论文修订;
吴铃负责研究方案策划、获取资助、项目管理、监督指导、论文修订。
利益冲突声明
所有作者均声明本文不存在利益冲突。
参考文献
Consensus on infertility treatment related to polycystic ovary syndrome
[J].
Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome
[J].
Update on PCOS: consequences, challenges, and guiding treatment
[J].
A novel letrozole model recapitulates both the reproductive and metabolic phenotypes of polycystic ovary syndrome in female mice
[J].
Role of high-fat diet on letrozole-induced polycystic ovarian syndrome in rats
[J].
Increased prevalence of polycystic ovary syndrome in premenopausal women with nonalcoholic fatty liver disease
[J].
Nonalcoholic fatty liver disease in women with polycystic ovary syndrome
[J].
Hepatokine profile in adolescents with polycystic ovary syndrome: a case-control study
[J].
Serum Fetuin-a levels are increased and associated with insulin resistance in women with polycystic ovary syndrome
[J].
Serum β-Klotho concentrations are increased in women with polycystic ovary syndrome
[J].
Role of diacerein on steroidogenesis and folliculogenesis related genes in ovary of letrozole-induced PCOS mice
[J].
Roxadustat alleviates metabolic traits in letrozole-induced PCOS mice
[J].
The liver
[J].
The hepato-ovarian axis: genetic evidence for a causal association between non-alcoholic fatty liver disease and polycystic ovary syndrome
[J].
Naringenin regulates gut microbiota and SIRT1/PGC-1ɑ signaling pathway in rats with letrozole-induced polycystic ovary syndrome
[J].
The role of a high-fat, high-fructose diet on letrozole-induced polycystic ovarian syndrome in prepubertal mice
[J].
Effects of time-restricted feeding on letrozole-induced mouse model of polycystic ovary syndrome
[J].
Sodium oligomannate's amelioration of reproductive and metabolic phenotypes in a letrozole-induced PCOS-like mouse model depends on the gut microbiome
[J].
Lipids in polycystic ovary syndrome: role of hyperinsulinemia and effects of metformin
[J].
Polycystic ovarian syndrome is accompanied by repression of gene signatures associated with biosynthesis and metabolism of steroids, cholesterol and lipids
[J].
Hepatic cholesterol transport and its role in non-alcoholic fatty liver disease and atherosclerosis
[J].
Myeloid HMG-CoA reductase determines adipose tissue inflammation, insulin resistance, and hepatic steatosis in diet-induced obese mice
[J].
A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS)
[J].
Targeting the IL-6/JAK/STAT3 signalling axis in cancer
[J].
An interferon gamma response signature links myocardial aging and immunosenescence
[J].
Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis
[J].
Leucocytosis in women with polycystic ovary syndrome (PCOS) is incompletely explained by obesity and insulin resistance
[J].
Macrophages, cytokines and beta-cell death in Type 2 diabetes
[J].
Effect of DHT-induced hyperandrogenism on the pro-inflammatory cytokines in a rat model of polycystic ovary morphology
[J].