骨关节炎(osteoarthritis,OA)是一种复杂的退行性关节疾病,发病率高,导致骨关节疼痛、僵硬和功能受限,严重影响患者生活质量,并增加医疗负担,目前亟需有效的治疗药物[1]。中医药治疗骨关节炎拥有悠久的历史,且越来越多的证据表明中医药具有更好的治疗效果[2]。上海魏氏伤科外治敷贴中疗效突出的经验方消瘀散具有消肿止痛、活血化瘀等功效,其贴膏外敷联合针刀治疗膝OA效果显著,不良反应少[3];动物实验发现,消瘀散可修复OA兔关节软骨损伤,降低软骨组织中基质金属蛋白酶(matrix metallo-proteinase,MMP)13的表达[4]。然而,该方药物成分多,有时会出现皮肤瘙痒不适等反应,因此本课题组考虑在消瘀散精简方(大黄、紫荆皮、三七、乳香)基础上再加入秦皮。秦皮的有效成分之一秦皮素,有收涩止痢、止带、明目之功效,可用于治疗湿热泻痢、赤白带下、目赤肿痛和目生翳膜,亦有抗炎镇痛、清热解毒的作用[5]。本课题组在前期的体外细胞实验中通过转录组学分析发现,秦皮素能够调控OA大鼠膝关节滑膜组织中MMP12、乌头酸脱羧酶1(aconitate decarboxylase 1,Acod1)和ATP6V0D2蛋白(ATPase H+ transporting lysosomal 38kDa V0 subunit D isoform 2,Atp6v0d2)等基因的表达[6],且通过大鼠软骨细胞中的Toll样受体4(Toll-like receptor 4,TLR4)/髓样分化因子88(myeloid differentiation factor 88,MyD88)/核因子κB(nuclear factor kappa-B,NF-κB)通路抑制IL-1β诱导的细胞炎症[7]。秦皮素对OA动物模型的作用及其机制亟待深入挖掘。
OA的主要特征是关节软骨丢失,导致骨关节的结构、功能和代谢状态不断变化[8]。炎症和细胞凋亡贯穿于OA的整个进展过程[9]。促炎细胞因子如肿瘤坏死因子α(tumour necrosis factor-α,TNF-α)[10]、白细胞介素(interleukin,IL)-1β[10]和IL-6[11]被认为是诱导细胞凋亡和破坏软骨的关键细胞因子,在血清中的表达水平可作为OA的生物标志。此外,软骨寡聚基质蛋白(cartilage oligomeric matrix protein,COMP)也是OA的血清学指标之一[12]。同时,OA的发展还与Wnt/β- catenin、NF-κB等信号通路有关[13],其中NF-κB信号通路可诱导降解酶的分泌。例如,基质金属蛋白酶类(matrix metalloproteinases,MMPs)、整合素样金属蛋白酶与凝血酶4/5型抗体(a disintegrin and metalloproteinase with thrombospondin motifs 4/5,ADAMTS4/5)可导致关节软骨降解[14]。此外,磷酸化-丝裂原活化蛋白激酶p38(phosphorylated p38 mitogen-activated protein kinase,p-p38 MAPK)抗体[15]和磷酸化c-Jun氨基末端激酶(phosphorylated c-Jun N-terminal kinase,p-JNK)在OA大鼠中表达显著升高[16],提示阻断p-p38 MAPK信号通路可能抑制OA软骨细胞的凋亡和相关促炎细胞因子的表达。本课题组结合以上研究结果推测,秦皮素治疗OA的作用机制也可能与这些信号通路有关。因此,本研究通过碘乙酸钠(monosodium iodoacetate,MIA)诱导建立OA动物模型,在动物体内验证秦皮素治疗OA的作用机制,从而为魏氏伤科消瘀散的改良并用于治疗OA提供理论基础。
1 材料与方法
1.1 实验动物
8周龄SPF级雄性SD大鼠18只,体重290~330 g,由上海吉辉实验动物饲养有限公司[SCXK(沪)2022-0009]提供,质量合格证号为20220009010285。实验动物饲养于上海交通大学医学院附属新华医院实验动物屏障设施 [SYXK(沪)2023-0048],室温(22±2)℃,相对湿度40%~60%,12 h明/暗循环,且提供高压蒸汽和过滤除菌后的实验动物饮用水及钴60辐照灭菌饲料(由江苏省协同医药生物工程有限责任公司提供),饲养1周以适应环境。动物实验方案获得上海交通大学医学院附属新华医院医学伦理委员会审查批准 (XHEC-F-2024-077)。
1.2 主要试剂与仪器
秦皮素(F396636,化学名称为7,8-二羟基-6-甲氧基香豆素,纯度≥98%)与MIA(S104897,纯度≥99%)购自上海阿拉丁生化科技股份有限公司。蛋白酶抑制剂混合物(P1005,通用型,100×)、RIPA裂解液(P0013B)、苏木精-伊红(hematoxylin-eosin,HE)染色试剂盒(C0105S)、番红O-固绿软骨染色试剂盒(C0621S)、甲苯胺蓝染色液(C0637)和高灵敏TMB显色液(P0206)均购自上海碧云天生物技术有限公司。p38 MAPK单抗(A4771)、p-p38 MAPK多抗(AP1508)、JNK多抗(A25329)、p-JNK多抗(AP0105)和GAPDH多抗(AC054)及辣根过化物酶标记的山羊抗兔二抗(RM70003)购自武汉爱博泰克生物科技有限公司;测定TNF-α(ab236712)、IL-1β(ab221834)、IL-6(ab234570)、COMP(ab11056)的酶联免疫吸附试验(enzyme linked immunosorbent assay,ELISA)试剂盒购自英国Abcam公司。
CO2窒息安乐死设备为本院动物房自制;5430R超速离心机购自美国 LI-COR Biosciences公司;KA-1000台式离心机购自上海安亭科学仪器厂;SpectraMax® iD3微板阅读器、BCA试剂盒和ChemiDoc MP成像系统均为美国Bio-Rad公司产品;活体型micro-CT扫描系统(vivaCT80)为瑞士Scanco Medical AG公司产品。
1.3 实验动物模型的建立与药物干预
将18只SD大鼠分为空白组、模型组和干预组,每组6只。3组大鼠用剃刀备皮且经碘伏消毒后,腹腔注射2%戊巴比妥钠(0.3 mL/100 g),使动物深度麻醉。动物取仰卧位,屈曲右侧膝关节,充分暴露关节内侧,对右膝关节进行碘伏和95%乙醇溶液擦拭消毒后,于髌骨内侧进针入髌上囊,向模型组和干预组关节腔内单次注射50 μL新鲜配制的MIA(60 mg/mL)[7],同时向空白组单次注射50 μL生理盐水。MIA注射后的第3天开始,干预组右膝关节腔内注射50 μL秦皮素(5 mg·kg-1·d-1,溶于50 μL生理盐水),连续7 d。模型组和空白组大鼠的右膝关节腔内注射等量生理盐水,连续7 d。术后观察大鼠伤口有无渗血、红肿、化脓,并检测大鼠的生命体征,维持环境的清洁与干燥。
1.4 血液及膝关节软骨取材
药物干预结束后4周,采集各组大鼠的腹主动脉血5 mL。静置1 h后离心(4 000×g,15 min)取血清,保存于-80 ℃冰箱,待后续ELISA检测。采用CO2窒息法安乐死大鼠,收集膝关节软骨,用4%多聚甲醛溶液浸泡固定后,一部分置于液氮中暂时保存,另一部分置于-80 ℃冰箱长期保存。
1.5 膝关节软骨的组织病理学观察及评分
膝关节软骨在4%多聚甲醛溶液中浸泡固定24 h后,经脱钙液脱钙1周、石蜡包埋、切片,分别采用HE、番红O-固绿和甲苯胺蓝染色法进行骨组织病理学观察,根据试剂说明书进行操作。
采用Mankin评分标准[5]评估软骨退变程度,从软骨结构完整性、软骨细胞、软骨基质番红染色、软骨基质甲苯胺蓝染色和潮标线完整性共5个方面进行评分。总分范围为0~14分,分值判定标准:0~1分为正常,2~6 分为OA早期,7~10分为OA中期,11~14分为OA晚期。
采用国际骨关节炎研究学会(Osteoarthritis Research Society International,OARSI)半定量评分标准[16]评估OA严重程度。评分指标及其赋分:软骨结构(0~8分)、软骨基质情况(0~6分)、软骨细胞分布及数量(0~3分)、潮线完整性(0~1分)及骨赘形成(0~3分)。合计分值越大,OA炎症程度越严重。
1.6 膝关节的micro-CT成像及微观定量分析
将在4%多聚甲醛溶液中固定24 h后的完整膝关节,用活体型 micro-CT扫描系统(vivaCT80) 以5 μm的空间分辨率(55 kV,114 mA,500 ms的积分时间)扫描胫骨,采用NRecon软件重构图片,使用CTAn软件分析3D定量参数,分析膝关节软骨骨量变化[17]。测量软骨下骨小梁区域的相关参数:骨体积分数(trabecular bone volume/total volume,Tb.BV/TV)、骨表面积密度(trabecular bone surface density/total volume,Tb.BS/TV)、骨小梁数量(trabecular number,Tb.N)。
1.7 ELISA法检测血清炎症因子含量
按照TNF-α、IL-1β、IL-6、COMP测定用ELISA试剂盒说明,将标准品和各组大鼠血清样品(100 μL)分别加入96孔板中,室温孵育2 h。PBS清洗后加入100 μL一抗,室温孵育1 h。清洗后每孔加入100 μL二抗,室温孵育45 min。清洗后每孔加入100 μL显色剂,室温避光孵育30 min。加入50 μL终止液终止反应。用微板阅读器检测各样品中TNF-α、IL-1β、IL-6和COMP含量,检测波长为450 nm。
1.8 蛋白提取与免疫印迹法检测
从液氮中取出各组大鼠的膝关节软骨,放入EP管,加入含20 μL蛋白酶抑制剂苯甲基磺酰氟的180 μL RIPA裂解液及钢珠,置入预冷的组织研磨器中研磨,然后在4 ℃条件下离心(12 000×g)10 min后,吸出上清液。使用BCA试剂盒测定蛋白浓度。每组样本的上样量为30 μg,用10%十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(浓缩胶80 V,分离胶120 V 60 min)分离蛋白后,电转移到PVDF膜(300 mA,400 V,45 min),然后用5%牛血清白蛋白封闭溶液快速封闭。加入p38 MAPK、p-p38 MAPK、JNK、p-JNK和GAPDH(内参)一抗,4 ℃孵育过夜。PBS洗膜后,加入对应的二抗,37 ℃孵育1 h。使用增强化学发光法检测免疫反应条带,并使用ChemiDoc MP成像系统和Image J软件定量分析目的蛋白条带的相对表达量。
1.9 统计学分析
采用GraphPad Prism 9.0软件进行统计分析,结果数据以平均值±标准差表示。采用单因素方差分析和Tukey多重比较检验进行各组间差异分析。P<0.05表示差异有统计学意义。
2 结果
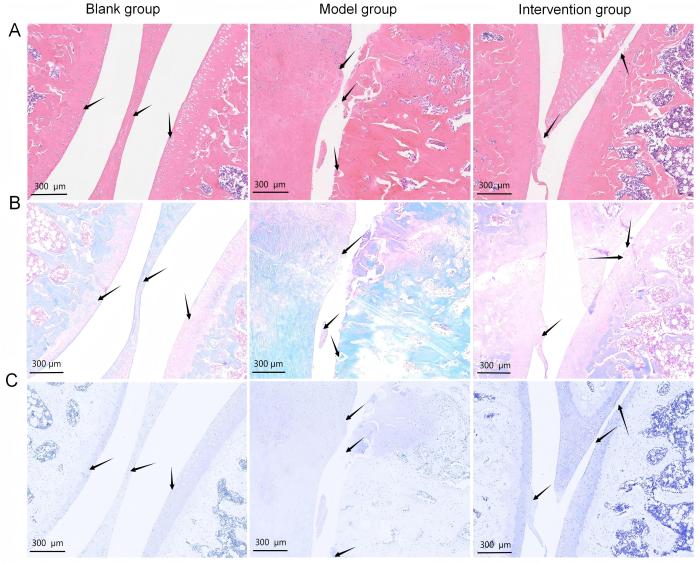
2.1 大鼠膝关节软骨组织学染色与评分
图1
图1
碘乙酸钠注射造模及秦皮素药物干预后大鼠膝关节软骨组织病理学变化
Figure 1
Histopathological changes in rat knee joint cartilage after monosodium iodoacetate injection and fraxetin intervention
表1 不同组大鼠膝关节软骨的Mankin评分、OARSI评分、骨体积分数、骨表面积密度、骨小梁数量比较
Table 1
分组 Group | 动物数量 n | Mankin评分 Mankin score | OARSI评分 OARSI sore | 骨体积分数 Tb.BV/TV | 骨表面积密度/(mm2·mm-3) Tb.BS/TV | 骨小梁数量/mm-1 Tb.N |
|---|---|---|---|---|---|---|
| 空白组 Blank group | 6 | 1.17±0.75 | 1.83±0.75 | 0.59±0.01 | 17.44±0.60 | 3.72±0.21 |
| 模型组 Model group | 6 | 10.67±1.86* | 8.00±1.41* | 0.25±0.02* | 13.28±0.86* | 2.47±0.19* |
| 干预组 Intervention group | 6 | 5.67±0.82# | 5.50±1.05# | 0.38±0.02# | 15.59±0.50# | 3.29±0.14# |
2.2 大鼠膝关节的micro-CT成像及骨小梁微观结构
图2
图2
碘乙酸钠注射造模及秦皮素药物干预后大鼠膝关节的micro-CT成像
Figure 2
Micro-CT imaging of the rat knee joint after monosodium iodoacetate injection and fraxetin treatment
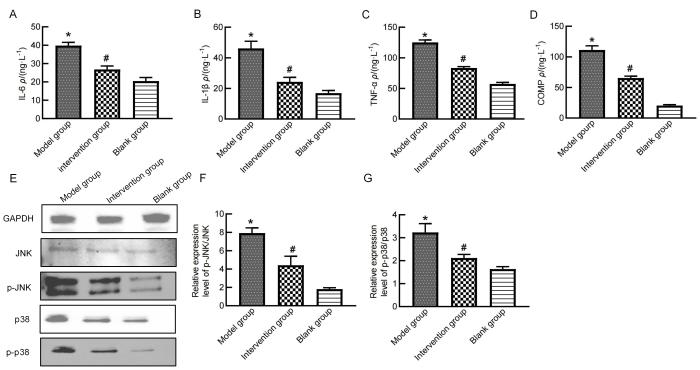
2.3 大鼠血清和膝关节软骨中炎症因子及其通路蛋白的表达
图3
图3
秦皮素药物干预后大鼠血清和膝关节软骨组织中炎症相关蛋白的表达
Figure 3
Expression of inflammation-related proteins in rat serum and knee cartilage after fraxetin treatment
3 讨论
OA是全球范围内导致残疾和慢性疼痛的主要原因之一[1]。随着世界人口老龄化和肥胖症发病率的升高,人们对治疗骨关节的需求日益增加[18-19],这促使科研工作者不断探索治疗OA的潜在药物。传统药用植物是治疗OA的潜在药物宝库,许多植物及其提取物在体内外都已被证明治疗OA有效,但其作用机制不明确,且缺乏有力的实验证据支持[20]。已有研究表明,秦皮素通过丝裂原活化蛋白激酶(mitogen-activated protein kinases,MAPKs)通路产生抗肝纤维化作用[21],在肠道炎症性疾病中发挥抗炎作用[22],还可通过抑制p38信号通路在体内外抑制破骨细胞分化和形成[17]。本课题组前期研究了秦皮素对OA细胞体外模型的作用,本研究进一步探索其在OA大鼠模型中对软骨破坏和炎症的治疗作用及机制。
OA大鼠的造模方法有很多,其中MIA注射已成为在大鼠和小鼠中模拟OA关节破坏的标准造模法,且该方法简便易行[23]。本研究中造模结果显示,模型组大鼠出现软骨细胞死亡、新血管形成、软骨下骨塌陷坏死以及炎症反应,其形态学变化和软骨破坏程度与临床OA患者的膝关节病理变化高度相似。模型组的膝关节软骨组织学染色及评分都显示软骨破坏严重,在CT三维重建的基础上,模型组大鼠的胫骨平台骨质缺失严重。以上结果提示,本研究成功建立了OA大鼠模型。
OA的关键特点之一是软骨下骨骨质丢失[8]。本研究发现,模型组大鼠经秦皮素药物干预后,软骨破坏及骨质缺失程度得到了明显缓解,Mankin 和OARSI评分相对于模型组显著降低,提示秦皮素对大鼠膝关节软骨有保护作用;进一步分析发现,秦皮素干预组的骨体积分数、骨表面积密度以及骨小梁数目相比模型组均明显增加(P<0.05),提示秦皮素治疗可以减轻膝关节软骨下骨骨质丢失,但具体机制还有待进一步探索。
本研究结果表明,秦皮素是治疗OA的潜在药物,且其作用机制可能与p38 MAPK通路有关。本课题组下一步将纳入西药对照,例如非甾体抗炎药、氨基葡萄糖、透明质酸等,必要时结合中药、西药联合疗法分组,对中药、西药、中西药联合治疗OA的临床实践开展深入研究。
[引用本文]
刘智伟, 杨然, 连浩, 等. 秦皮素对碘乙酸钠诱导骨关节炎模型大鼠的软骨保护与抗炎作用[J]. 实验动物与比较医学, 2025, 45(3): 259-268.DOI: 10.12300/j.issn.1674-5817.2024.165
LIU Z W, YANG R, LIAN H, et al. Cartilage protection and antiinflammatory effects of fraxetin on monosodium iodoacetateinduced rat model of osteoarthritis[J]. Lab Anim and Comp Med,2025, 45(3): 259-268. DOI: 10.12300/j.issn.1674-5817.2024.165.
医学伦理声明
该研究涉及的动物实验方案获得上海交通大学医学院附属新华医院医学伦理委员会审查批准(批号:XHEC-F-2024-077)。所有实验过程均遵照中国实验动物相关法律法规条例要求进行。
Medical Ethics Statement
All animal experimental protocols involved in this study were reviewed and approved by the Medical Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Approval No. XHEC-F-2024-077), and all experimental procedures were carried out in compliance with relevant Chinese laws, regulations and rules on laboratory animals.
作者贡献声明
刘智伟负责动物实验、结果数据的统计分析及Prism作图、初稿写作及修改;
杨然参与动物实验及其结果分析;
连浩负责实验方案策划;
张玉负责文献检索,参与动物实验;
金立伦负责实验方案的有效性验证,以及项目资金管理和实验监督指导。
利益冲突声明
所有作者均声明本文不存在利益冲突。
参考文献
Knee osteoarthritis: epidemiology, pathogenesis, and mesenchy- mal stem cells: what else is new? an update
[J].
The efficacy and safety of Jinwu Gutong capsule in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials
[J].
消瘀散贴膏联合针刀治疗膝骨关节炎的疗效及对炎症因子的影响
[J].
Clinical effect of Xiaoyusan plaster combined with acupotomy on knee osteoarthritis and its effect on inflammatory factors
[J].
消瘀散新组方修复膝骨关节炎模型兔的软骨损伤及MMP-13表达
[J].
Repair effects of Xiaoyusan new formula on cartilage injury and MMP-13 expression in knee osteoarthritis model rabbits
[J].
基于标准汤剂的秦皮(白蜡树)配方颗粒质量标准分析
[J].
Analysis on quality standard of fraxini cortex (Fraxinus chinensis) dispensing granules based on standard decoction
[J].
转录组测序筛选大鼠滑膜炎差异表达基因及秦皮素治疗靶点的体外验证
[J].
Screening of differentially expressed genes in rat synovitis by transcriptome sequencing and in vitro verification of therapeutic target of fraxetin
[J].
Fraxetin inhibits interleukin-1β-induced apoptosis, inflammation, and matrix degradation in chondrocytes and protects rat cartilage in vivo
[J].
Platelet-rich plasma ameliorates cartilage degradation in rat models of osteoarthritis via the OPG/RANKL/RANK system
[J].
姜黄素治疗骨关节炎过程中潜在的铁死亡关键基因的分析与验证
[J].
Analysis and verification of potential ferroptosis key genes in curcumin treatment of osteoarthritis
[J].
Danshensu inhibits the IL-1β- induced inflammatory response in chondrocytes and osteoarthritis possibly via suppressing NF-κB signaling pathway
[J].
Regulatory effects and interactions of the Wnt and OPG-RANKL-RANK signaling at the bone-cartilage interface in osteoarthritis
[J].
Osteoarthritis as a systemic disease promoted prostate cancer in vivo and in vitro
[J].
Osteoarthritis: pathogenic signaling pathways and therapeutic targets
[J].
p38/NF-kB-dependent expression of COX-2 during differentiation and inflammatory response of chondrocytes
[J].
Fargesin ameliorates osteoarthritis via macrophage reprogramming by downregulating MAPK and NF-κB pathways
[J].
Dihydroartemisinin attenuates osteoclast formation and bone resorption via inhibiting the NF-κB, MAPK and NFATc1 signaling pathways and alleviates osteoarthritis
[J].
Inhibition of osteoclastogenesis for periprosthetic osteolysis therapy through the suppression of p38 signaling by fraxetin
[J].
髋膝联合电针治疗早期膝骨关节炎的临床研究
[J].
Clinical research on hip-knee coherent electro-acupuncture in treating early knee osteoarthritis
[J].
抗骨增生胶囊联合洛索洛芬钠治疗早期膝骨关节炎的临床研究
[J].
Clinical study on Kanggu Zengsheng Capsules combined with loxoprofen sodium in treatment of early knee osteoarthritis
[J].
中药复方治疗不同分期膝骨关节炎的研究进展
[J].
Advancement of research on the treatment of different stages of knee osteoarthritis with compounds
[J].
Antifibrotic effects of Fraxetin on carbon tetrachloride-induced liver fibrosis by targeting NF-κB/IκBα, MAPKs and Bcl-2/Bax pathways
[J].
Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives
[J].
The pharmacology of pain associated with the monoiodoacetate model of osteoarthritis
[J].
Combined detection of COMP and CS846 biomarkers in experimental rat osteoarthritis: a potential approach for assessment and diagnosis of osteoarthritis
[J].
Triggering of p38 MAPK and JNK signaling is important for oleanolic acid-induced apoptosis via the mitochondrial death pathway in hypertrophic scar fibroblasts
[J].
Tougu Xiaotong capsules may inhibit p38 MAPK pathway-mediated inflammation: In vivo and in vitro verification
[J].