
Laboratory Animal and Comparative Medicine ›› 2023, Vol. 43 ›› Issue (4): 371-380.DOI: 10.12300/j.issn.1674-5817.2023.036
• Excellent Papers at the East China Conference • Previous Articles Next Articles
Jin LU1( ), Jian WANG1, Lian ZHU1, Guofeng YAN1, Zhengwen MA1, Yao LI1, Jianjun DAI2, Yinqiu ZHU1, Jing ZHOU1(
), Jian WANG1, Lian ZHU1, Guofeng YAN1, Zhengwen MA1, Yao LI1, Jianjun DAI2, Yinqiu ZHU1, Jing ZHOU1( )(
)( )
)
Received:2023-03-21
Revised:2023-06-06
Online:2023-08-25
Published:2023-08-25
Contact:
Jing ZHOU
CLC Number:
Jin LU,Jian WANG,Lian ZHU,et al. Establishment of Preeclampsia Model in Goat and Evaluation on Maternal Biological Characteristics[J]. Laboratory Animal and Comparative Medicine, 2023, 43(4): 371-380. DOI: 10.12300/j.issn.1674-5817.2023.036.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2023.036
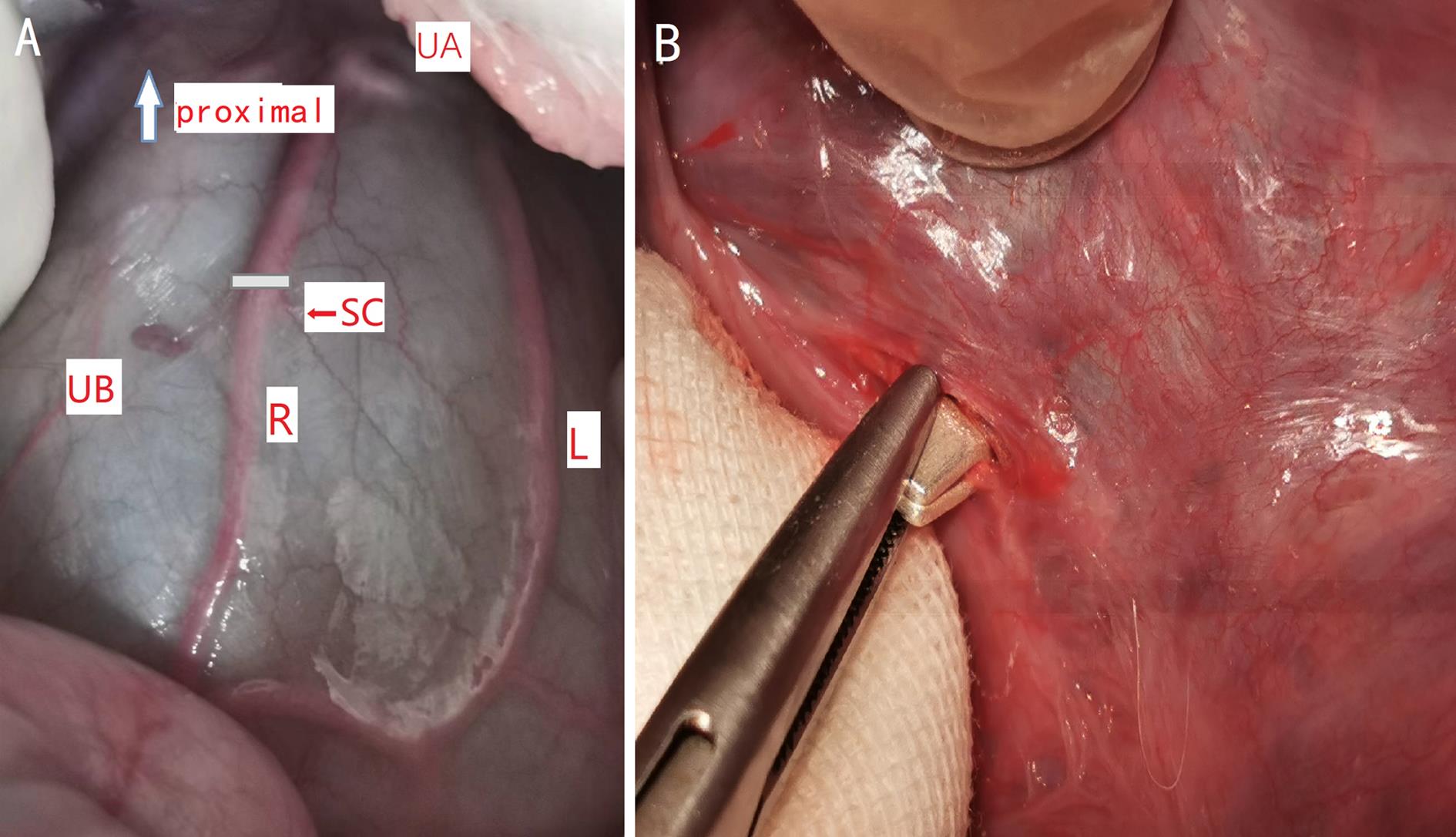
Figure 1 Anterior exposure of uterine arteries and the position of the silver clamp placement on uterine body arteryNote:A, Anterior exposure of uterine arteries (UA, uterine artery;UB, uterine body;R, right uterine body artery;L, left uterine body artery;SC, placement of silver clamp); B, Uterine body artery been partially closed by silver clamp.
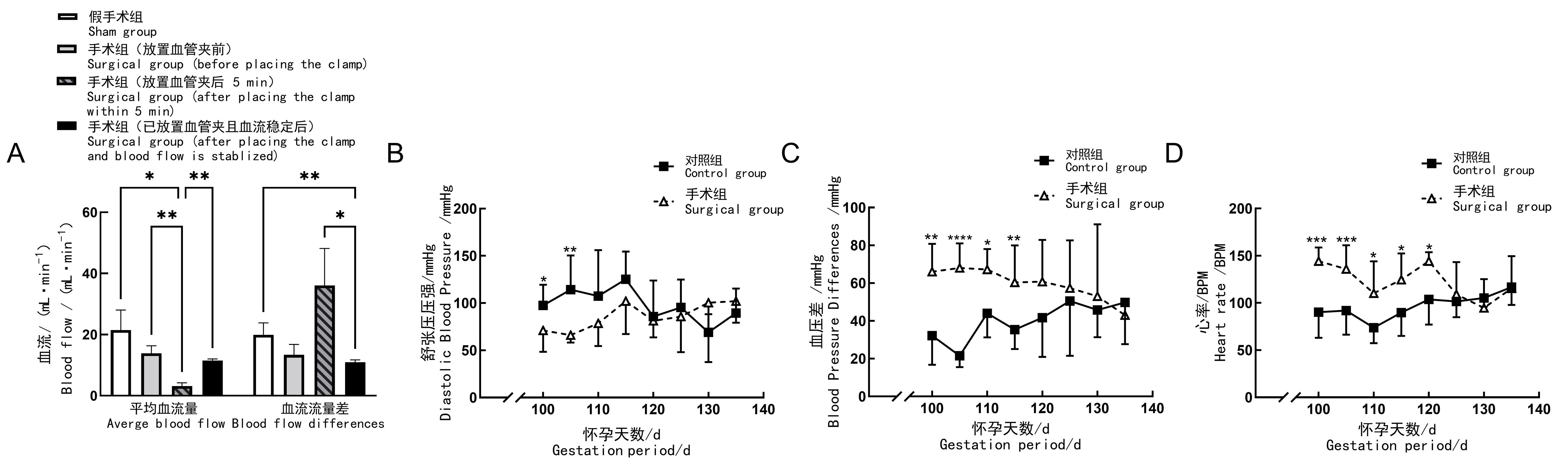
Figure 2 Blood flow, blood pressure and heart rate of pregnant goats after preeclampsia modellingNote:A, Blood flow changes of uterine body artery; B, Changes of hind limb diastolic blood pressure;C, Changes of hind limb pulse pressure; D, Change of heart rate (BPM, beat per min). In control group (n=11), no surgical operation was done on animals; in sham group (n=4), sham surgery was performed on the 100th day of gestation, to be specific, only their uterine body arteries were exposed and freed; in surgical group (n=8), preeclampsia modeling was performed by placing silver vascular clamps on one of the uterine body arteries of the animals on the 100th day of gestation to close their internal diameters partially. For comparison between groups, *P<0.05, **P<0.01, ***P<0.001, ****P<0.000 1.
项目 Item | 手术组(n=8) Surgical group (n=8) | 对照组(n=11) Control group (n=11) | |
|---|---|---|---|
术后5 d Postoperation 5 d | 产前 Before parturition | ||
白细胞计数/(103·μL-1) White blood cell count/(103·μL-1) | 38.26±2.81 | 34.00±2.99 | 40.62±1.70 |
淋巴细胞百分比/% Lymphocyte/% | 58.68±2.28 | 58.42±4.22 | 60.94±2.05 |
中间细胞百分比/% Intermediate cell/% | 7.20±1.07 | 7.60±1.09 | 6.61±0.73 |
粒细胞百分比/% Granulocyte/% | 34.12±1.77 | 33.98±3.18 | 32.45±1.63 |
淋巴细胞计数/(103·μL-1) Lymphocyte count/(103·μL-1) | 22.50±1.86 | 20.13±2.85 | 24.81±1.44 |
粒细胞计数/(103·μL-1) Granulocyte count/(103·μL-1) | 12.90±0.81 | 11.30±0.64 | 13.06±0.68 |
红细胞计数/(106·μL-1) Red blood cell count/(106·μL-1) | 1.14±0.14 | 0.65±0.04* | 1.58±0.17 |
血红蛋白计数/(g·L-1) haemoglobin count/(g·L-1) | 101.40±4.24 | 87.75±4.71* | 117.27±5.32 |
血细胞压积/% Packed cell volume/% | 4.52±0.62 | 2.50±0.16* | 6.49±0.76 |
平均红细胞压积/fL Mean corpuscular volume/fL | 40.18±0.31 | 39.53±0.30* | 41.16±0.30 |
平均红细胞血红蛋白量/pg Mean corpuscular hemoglobin/pg | 94.00±10.30 | 135.18±6.02**** | 77.99±4.33 |
平均红细胞血红蛋白浓度/(g·L-1) Mean corpusular hemoglobin concerntration/(g·L-1) | 2 397±286 | 3 526±154**** | 1 924±118 |
红细胞分别宽度变异系数/% coefficient variation of red blood cell volume distribution width/% | 17.26±0.61 | 16.33±0.69 | 17.41±0.43 |
中间细胞计数/(103·μL-1) Intermediate cell count/(103·μL-1) | 2.58±0.44 | 2.40±0.22 | 2.99±0.34 |
Table 1 Comparison of blood routine between groups in preeclampsia goat model
项目 Item | 手术组(n=8) Surgical group (n=8) | 对照组(n=11) Control group (n=11) | |
|---|---|---|---|
术后5 d Postoperation 5 d | 产前 Before parturition | ||
白细胞计数/(103·μL-1) White blood cell count/(103·μL-1) | 38.26±2.81 | 34.00±2.99 | 40.62±1.70 |
淋巴细胞百分比/% Lymphocyte/% | 58.68±2.28 | 58.42±4.22 | 60.94±2.05 |
中间细胞百分比/% Intermediate cell/% | 7.20±1.07 | 7.60±1.09 | 6.61±0.73 |
粒细胞百分比/% Granulocyte/% | 34.12±1.77 | 33.98±3.18 | 32.45±1.63 |
淋巴细胞计数/(103·μL-1) Lymphocyte count/(103·μL-1) | 22.50±1.86 | 20.13±2.85 | 24.81±1.44 |
粒细胞计数/(103·μL-1) Granulocyte count/(103·μL-1) | 12.90±0.81 | 11.30±0.64 | 13.06±0.68 |
红细胞计数/(106·μL-1) Red blood cell count/(106·μL-1) | 1.14±0.14 | 0.65±0.04* | 1.58±0.17 |
血红蛋白计数/(g·L-1) haemoglobin count/(g·L-1) | 101.40±4.24 | 87.75±4.71* | 117.27±5.32 |
血细胞压积/% Packed cell volume/% | 4.52±0.62 | 2.50±0.16* | 6.49±0.76 |
平均红细胞压积/fL Mean corpuscular volume/fL | 40.18±0.31 | 39.53±0.30* | 41.16±0.30 |
平均红细胞血红蛋白量/pg Mean corpuscular hemoglobin/pg | 94.00±10.30 | 135.18±6.02**** | 77.99±4.33 |
平均红细胞血红蛋白浓度/(g·L-1) Mean corpusular hemoglobin concerntration/(g·L-1) | 2 397±286 | 3 526±154**** | 1 924±118 |
红细胞分别宽度变异系数/% coefficient variation of red blood cell volume distribution width/% | 17.26±0.61 | 16.33±0.69 | 17.41±0.43 |
中间细胞计数/(103·μL-1) Intermediate cell count/(103·μL-1) | 2.58±0.44 | 2.40±0.22 | 2.99±0.34 |
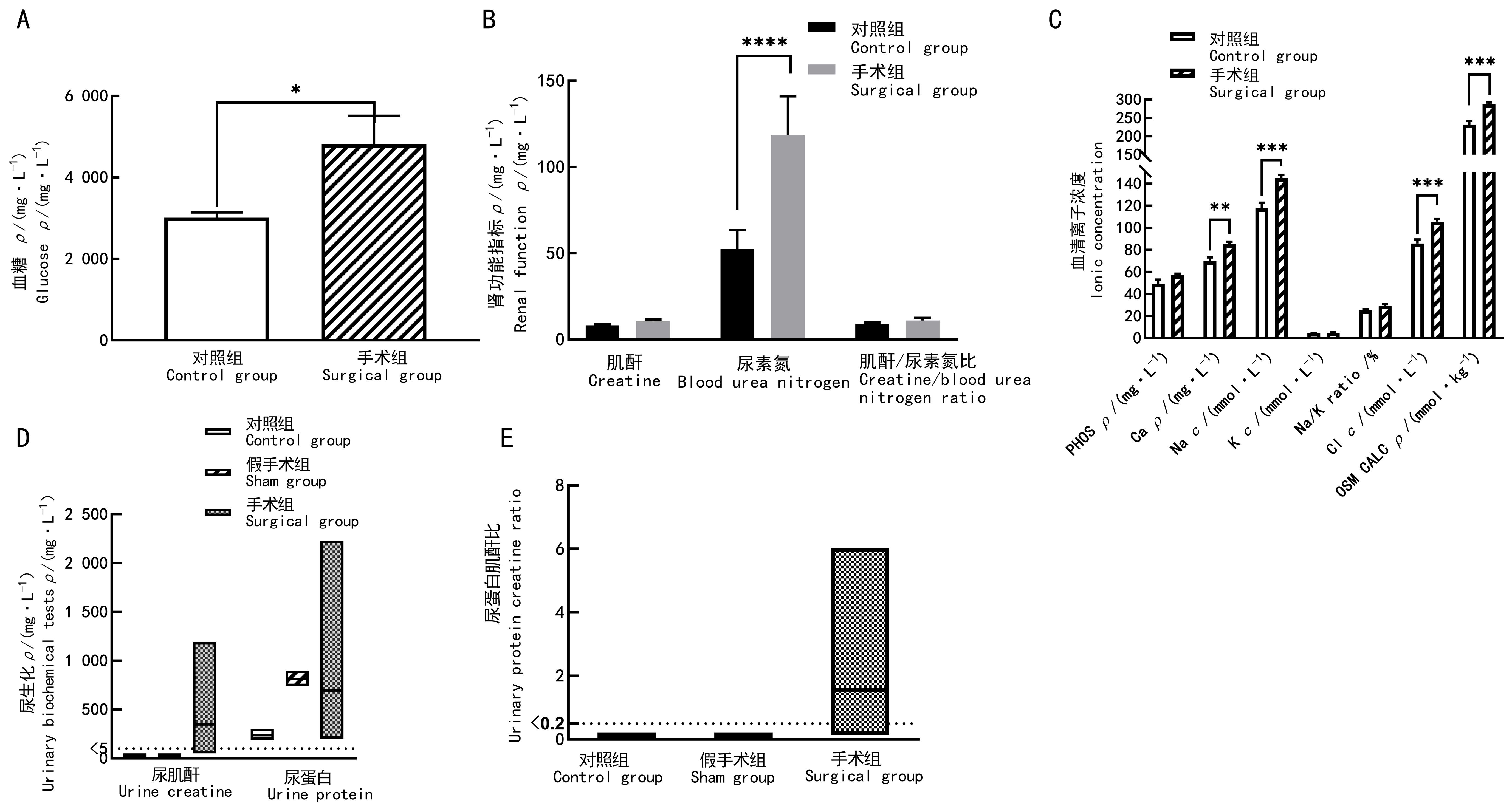
Figure 3 Blood and urinary biochemical results of preeclampsia modeling goat before parturitionNote:A, Blood glucose; B, Renal functional indicators; C, Serum iron indicators (PHOS, phosphate; Ca, calcium; Na, Sodium; K, potassium; Na/K, sodium potassium ratio; Cl, chlorine; OSM CALC, osmotic pressure); D, Urinary creatinine and urinary total protein; E, Creatinine/protein ratio. In the control group (n=11), no surgical operation was done on animals; in sham group (n=4), sham surgery was performed on the 100th day of gestation,to be specific, only their uterine body arteries were exposed and freed;and in surgical group (n=8), preeclampsia modeling was performed by placing silver vascular clamps on one of the uterine body arteries of the animals on the 100th day of gestation to close some of their internal diameters. Comparison between groups, *P<0.05, **P<0.01, ***P<0.001, ****P<0.000 1.
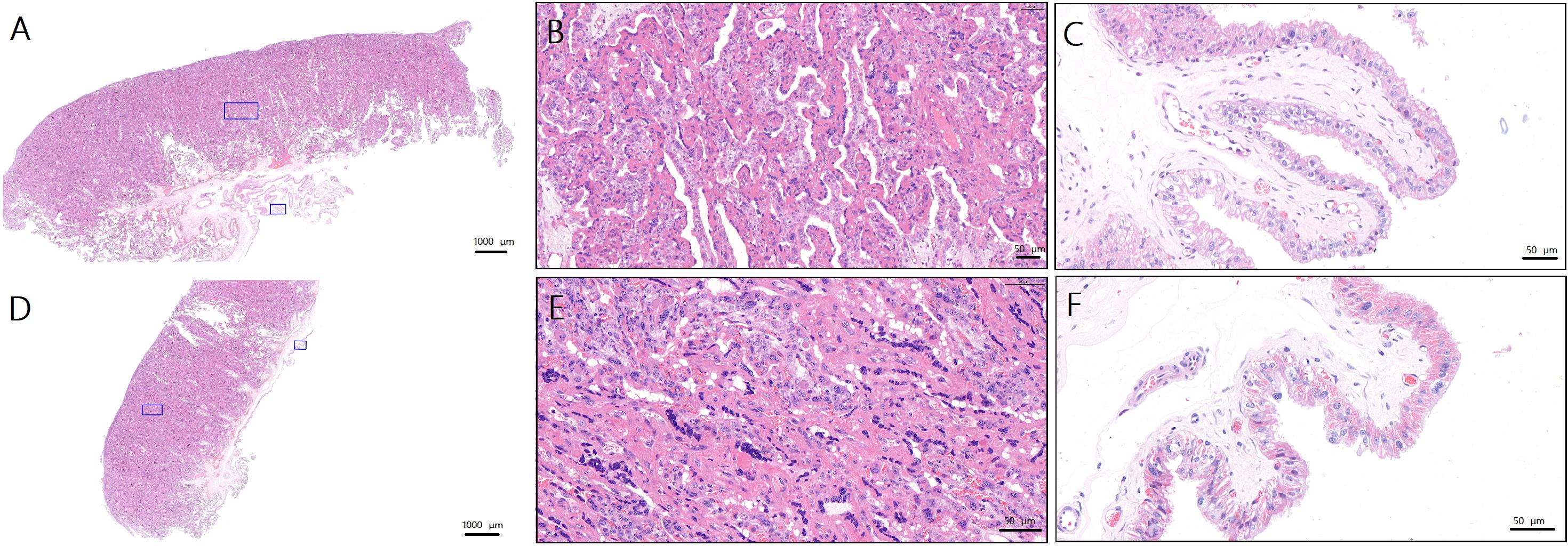
Figure 4 Pathologic changes in the placenta on the operated and non-operated side during parturition in goats after preeclampsia modeling(HE staining)Note:A, Normal placenta of ewes in control group (×1.1, the blue squares are the sampling sites for figure B and C ); B, Trophoblasts of normal placenta in control group (×16.9); C, Villous stroma of normal placenta in control group (×27.2); D, Surgical side placenta of ewes in surgical group (×1.1, the blue squares are the sampling sites for figure E and F ); E, Trophoblasts of surgical side placenta of ewes in surgical group (×28.7); F, Villous stroma of surgical side placenta of ewes in surgical group (×34.3). Scale bars of figure A and D are 1 000 μm. Proportional scales of figure B, C, E and F are 50 μm.
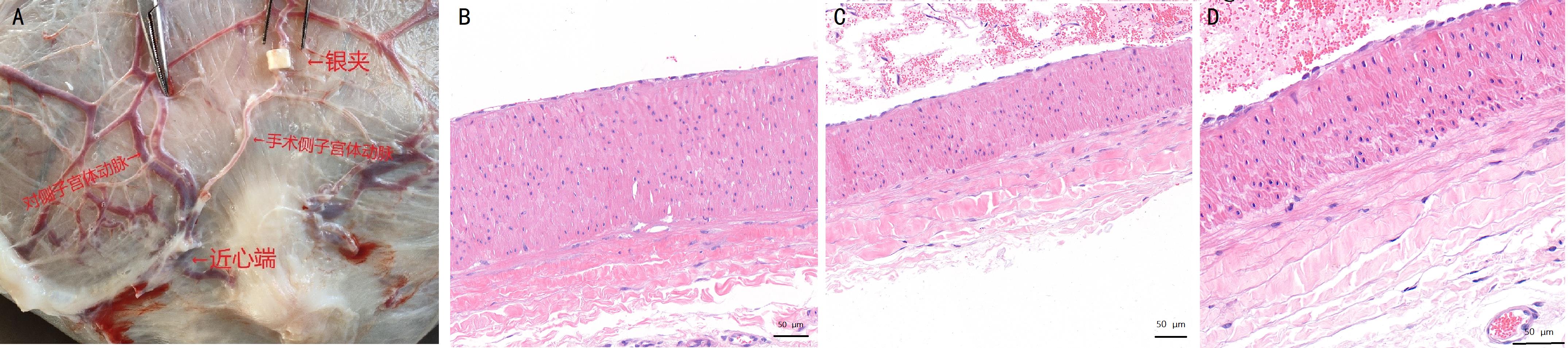
Figure 5 Comparison of the appearance and pathology of the uterine body arteries on surgical and non-surgical side of the uterus at the time of parturition in pregnant goats after preeclampsia modelingNote:A, Schematic diagram of the uterus and uterine arteries of ewes on the 140th day of gestation (silver clips were placed on one side of the uterine body arteries); B-D, HE-stained histopathologic sections of the uterine body arteries (B, uterine arteries of the nonsurgical side; C, uterine arteries proximal to the surgical side; E, uterine arteries distal from the surgical side). The scale bars figure B-D are 50 μm.
| 1 | FILIPEK A, JUREWICZ E. Preeclampsia - a disease of pregnant women[J]. Postepy Biochem, 2018, 64(4):232-229. DOI: 10.18388/pb.2018_146 . |
| 2 | IVES C W, SINKEY R, RAJAPREYAR I, et al. Preeclampsia—pathophysiology and clinical presentations: JACC state-of-the-art review[J]. J Am Coll Cardiol, 2020, 76(14):1690-1702. DOI: 10.1016/j.jacc.2020.08.014 . |
| 3 | CRAICI I M, WAGNER S J, WEISSGERBER T L, et al. Advances in the pathophysiology of pre-eclampsia and related podocyte injury[J]. Kidney Int, 2014, 86(2):275-285. DOI: 10.1038/ki.2014.17 . |
| 4 | ROBERTS J M, KING T L, BARTON J R, et al. Care plan for individuals at risk for preeclampsia: shared approach to education, strategies for prevention, surveillance, and follow-up[J]. Am J Obstet Gynecol, 2023: S0002-9378(23)00260. DOI: 10.1016/j.ajog.2023.04.023 . |
| 5 | GATFORD K L, ANDRAWEERA P H, ROBERTS C T, et al. Animal models of preeclampsia: causes, consequences, and interventions[J]. Hypertension, 2020, 75(6):1363-1381. DOI: 10.1161/HYPERTENSIONAHA.119.14598 . |
| 6 | LI J, LAMARCA B, RECKELHOFF J F. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model[J]. Am J Physiol Heart Circ Physiol, 2012, 303(1): H1-H8. DOI: 10.1152/ajpheart.00117.2012 . |
| 7 | 肖仲琳, 杨青, 张键, 等. 子痫前期动物模型的研究进展[J]. 生物化学与生物物理进展, 2016, 43(6): 563-569. DOI: 10.16476/j.pibb.2016.0011 |
| XIAO Z L, YANG Q, ZHANG J, et al. Research progress of animal models of preeclampsia[J]. Prog Biochem Biophys, 2016, 43(6): 563-569. DOI: 10.16476/j.pibb.2016.0011 . | |
| 8 | UZUN M, GENCER M, TURKON H, et al. Effects of melatonin on blood pressure, oxidative stress and placental expressions of TNFα, IL-6, VEGF and sFlt-1 in RUPP rat model of preeclampsia[J]. Arch Med Res, 2017, 48(7):592-598. DOI: 10.1016/j.arcmed.2017.08.007 . |
| 9 | SHI M T, YANG X F, SUN L, et al. Comparison of different modified operations in the reduced uteroplacental perfusion pressure rat model of preeclampsia[J]. J Reprod Immunol, 2023, 156:103815. DOI: 10.1016/j.jri.2023.103815 . |
| 10 | BAKRANIA B A, GEORGE E M, GRANGER J P. Animal models of preeclampsia: investigating pathophysiology and therapeutic targets[J]. Am J Obstet Gynecol, 2022, 226(2S): S973-S987. DOI: 10.1016/j.ajog.2020.10.025 . |
| 11 | PEAKER M. Gestation period and litter size in the goat[J]. Br Vet J, 1978, 134(4):379-383. DOI: 10.1016/s0007-1935(17)33441-3 . |
| 12 | SMITH M C, SHERMAN D M. Goat Medicine[M]. 2nd ed. Hoboken: Blackwell Pub Professional, 2009. |
| 13 | 吴正一. 实验外科技术: 羊的全身麻醉方法[J]. 上海口腔医学, 2000, 9(1): 42-44. DOI: 10.3969/j.issn.1006-7248.2000.01.015 . |
| WU Z Y. Techniques in experimental surgery—general anesthesia of the goat[J]. Shanghai J Stomatol, 2000, 9(1): 42-44. DOI: 10.3969/j.issn.1006-7248.2000.01.015 . | |
| 14 | PRACTICE A C O O. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists[J]. Int J Gynaecol Obstet, 2002, 77(1):67-75. |
| 15 | JACKSON P, COCKCROFT P. Clinical examination of farm animals[M]. Hoboken: Blackwell Science Ltd, 2002. |
| 16 | 杨怡珂, 漆洪波. 美国妇产科医师学会(ACOG)"妊娠期高血压和子痫前期指南2019版"要点解读(第一部分)[J]. 中国实用妇科与产科杂志, 2019, 35(8):895-899. DOI: 10.19538/j.fk2019080112 . |
| YANG Y K, QI H B. Interpretation of the American college of obstetricians and gynecologists (ACOG) guidelines for pregnancy-induced hypertension and preeclampsia (2019 edition) (part I)[J]. Chin J Pract Gynecol Obstet, 2019, 35(8):895-899. DOI: 10.19538/j.fk2019080112 . | |
| 17 | PHIPPS E A, THADHANI R, BENZING T, et al. Pre-eclampsia: pathogenesis, novel diagnostics and therapies[J]. Nat Rev Nephrol, 2019, 15(5):275-289. DOI: 10.1038/s41581-019-0119-6 . |
| 18 | MILLER D, MOTOMURA K, GALAZ J, et al. Cellular immune responses in the pathophysiology of preeclampsia[J]. J Leukoc Biol, 2021, 111(1):237-260. DOI: 10.1002/JLB.5RU1120-787RR . |
| 19 | JUNG E, ROMERO R, YEO L, et al. The etiology of preeclampsia[J]. Am J Obstet Gynecol, 2022, 226(2S): S844-S866. DOI: 10.1016/j.ajog.2021.11.1356 . |
| 20 | 周晶, 严国锋, 罗章源, 等. 子痫前期大鼠模型建立及相关指标监测[J]. 实验动物与比较医学, 2015, 35(6): 448-452. DOI: 10.3969/j.issn.1674-5817.2015.06.004 . |
| ZHOU J, YAN G F, LUO Z Y, et al. Establishment of preeclampsia model in rat and detection on related indicators[J]. Lab Anim Comp Med, 2015, 35(6): 448-452. DOI: 10.3969/j.issn.1674-5817.2015.06.004 . | |
| 21 | PAAUW N D, JOLES J A, SPRADLEY F T, et al. Exposure to placental ischemia impairs postpartum maternal renal and cardiac function in rats[J]. Am J Physiol Regul Integr Comp Physiol, 2017, 312(5): R664-R670. DOI: 10.1152/ajpregu.00510.2016 . |
| 22 | CORDINA M, BHATTI S, FERNANDEZ M, et al. Maternal hemoglobin at 27-29 weeks' gestation and severity of pre-eclampsia[J]. J Matern Fetal Neonatal Med, 2015, 28(13):1575-1580. DOI: 10.3109/14767058.2014.961006 . |
| 23 | Anon. Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222[J]. Obstet Gynecol, 2020, 135(6): e237-e260. DOI: 10.1097/AOG.0000000000003891 . |
| 24 | HEIDA K Y, BOTS M L, DE GROOT C J, et al. Cardiovascular risk management after reproductive and pregnancy-related disorders: a Dutch multidisciplinary evidence-based guideline[J]. Eur J Prev Cardiol, 2016, 23(17):1863-1879. DOI: 10.1177/2047487316659573 . |
| 25 | RICHARDS C, SESPEREZ K, CHHOR M, et al. Characterisation of cardiac health in the reduced uterine perfusion pressure model and a 3D cardiac spheroid model, of preeclampsia[J]. Biol Sex Differ, 2021, 12(1):31. DOI: 10.1186/s13293-021-00376-1 . |
| [1] | LIU Yayi, JIA Yunfeng, ZUO Yiming, ZHANG Junping, LÜ Shichao. Progress and Evaluation of Animal Model of Heart Qi-Yin Deficiency Syndrome [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 411-421. |
| [2] | ZHAO Xin, WANG Chenxi, SHI Wenqing, LOU Yuefen. Advances in the Application of Zebrafish in the Research of Inflammatory Bowel Disease Mechanisms and Drug Development [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 422-431. |
| [3] | PAN Yicong, JIANG Wenhong, HU Ming, QIN Xiao. Optimization of Surgical Procedure and Efficacy Evaluation of Aortic Calcification Model in Rats with Chronic Kidney Disease [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 279-289. |
| [4] | CHEN Yuhan, CHEN Jinling, LI Xin, OU Yanhua, WANG Si, CHEN Jingyi, WANG Xingyi, YUAN Jiali, DUAN Yuanyuan, YANG Zhongshan, NIU Haitao. Analysis of Animal Models of Myasthenia Gravis Based on Its Clinical Characteristics in Chinese and Western Medicine [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 176-186. |
| [5] | LIAN Hui, JIANG Yanling, LIU Jia, ZHANG Yuli, XIE Wei, XUE Xiaoou, LI Jian. Construction and Evaluation of a Rat Model of Abnormal Uterine Bleeding [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 130-146. |
| [6] | LUO Shixiong, ZHANG Sai, CHEN Hui. Research Progress in Establishment and Evaluation of Common Asthma Animal Models [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 167-175. |
| [7] | WANG Biying, LU Jiashuo, ZAN Guiying, CHEN Ruosong, CHAI Jingrui, LIU Jinggen, WANG Yujun. Establishment Methods and Application Progress of Rodent Models for Drug Addiction [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 158-166. |
| [8] | FEI Bin, GUO Wenke, GUO Jianping. Research Progress on Animal Models for Hernia Diseases and New Hernia Repair Materials [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 55-66. |
| [9] | YANG Jiahao, DING Chunlei, QIAN Fenghua, SUN Qi, JIANG Xusheng, CHEN Wen, SHEN Mengwen. Research Progress on Animal Models of Sepsis-Related Organ Injury [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 636-644. |
| [10] | SUN Xiaorong, SU Dan, GUI Wenjuan, CHEN Yue. Establishment and Evaluation of a Moderate-to-Severe Knee Osteoarthritis Model in Rats Induced by Surgery [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 597-604. |
| [11] | TIAN Fang, PAN Bin, SHI Jiayi, XU Yanyi, LI Weihua. Advances in Development of PM2.5-Exposed Animal Models and Their Application in Reproductive Toxicity Research [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 626-635. |
| [12] | ZHAO Xiaona, WANG Peng, YE Maoqing, QU Xinkai. Establishment of a New Hyperglycemic Obesity Cardiac Dysfunction Mouse Model with Triacsin C [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 605-612. |
| [13] | TU Yingxin, JI Yilan, WANG Fei, YANG Dongming, WANG Dongdong, SUN Zhixin, DAI Yuexin, WANG Yanji, Guanghan KAN, WU Bin, ZHAO Deming, YANG Lifeng. Evaluation of Simulated Weightlessness Model of Hindlimb Unloading Miniature Pigs and Their Tissue Damage [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 475-486. |
| [14] | HUANG Dongyan, WU Jianhui. Establishment Methods and Application Evaluation of Animal Models in Reproductive Toxicology Research [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 550-559. |
| [15] | ZHENG Yiqing, DENG Yasheng, FAN Yanping, LIANG Tianwei, HUANG Hui, LIU Yonghui, NI Zhaobing, LIN Jiang. Application Analysis of Animal Models for Pelvic Inflammatory Disease Based on Data Mining [J]. Laboratory Animal and Comparative Medicine, 2024, 44(4): 405-418. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||