
Laboratory Animal and Comparative Medicine ›› 2022, Vol. 42 ›› Issue (4): 284-293.DOI: 10.12300/j.issn.1674-5817.2021.147
• Animal Experimental Techniques and Methods • Previous Articles Next Articles
Yiru WANG1( )(
)( ), Xiaoying JIANG1, Ruoxi DONG1, Yibin PAN1, Xianghui HAN2, Yongqing CAO1(
), Xiaoying JIANG1, Ruoxi DONG1, Yibin PAN1, Xianghui HAN2, Yongqing CAO1( )(
)( )
)
Received:2021-09-02
Revised:2022-03-20
Online:2022-08-25
Published:2022-08-25
Contact:
Yongqing CAO
CLC Number:
Yiru WANG,Xiaoying JIANG,Ruoxi DONG,et al. Modified Method for Inducing Acute Intestinal Fibrosis in Rats Using 2,4,6-Trinitrobenzene Sulfonic Acid[J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 284-293. DOI: 10.12300/j.issn.1674-5817.2021.147.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2021.147
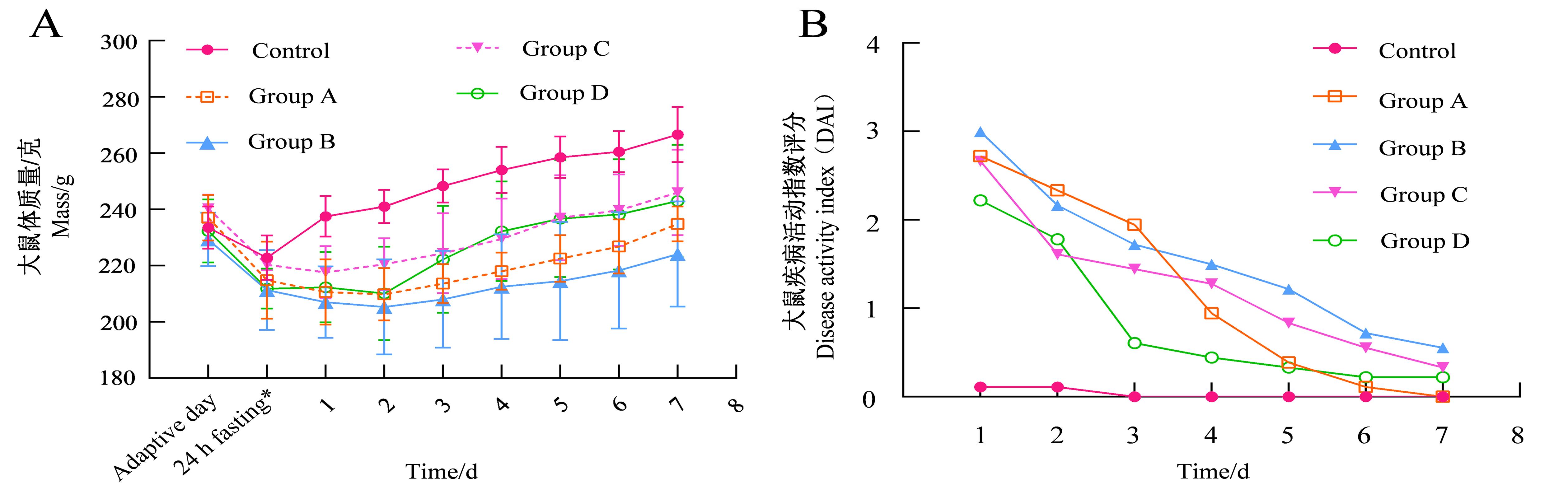
Figure 2 Changes in rat body mass (A) and disease activity index (B) after model establishmentNote:*shows the modeling day. The control group was induced by normal saline enema;Groups A to D were model groups: group A was induced by 5% TNBS + 50% ethanol solution(1∶1 v/v) enema,group B was induced by 5% TNBS+75% ethanol solution (1∶1 v/v) enema,group C was induced by 5% TNBS+100% ethanol solution (1∶1 v/v) enema,group D was induced by 5%TNBS+50% ethanol solution (2∶1 v/v) enema. There're 6 rats contained in each group, but one of which died on days 2 and 6 in group C and two of which died on day 3 in group D after modeling.
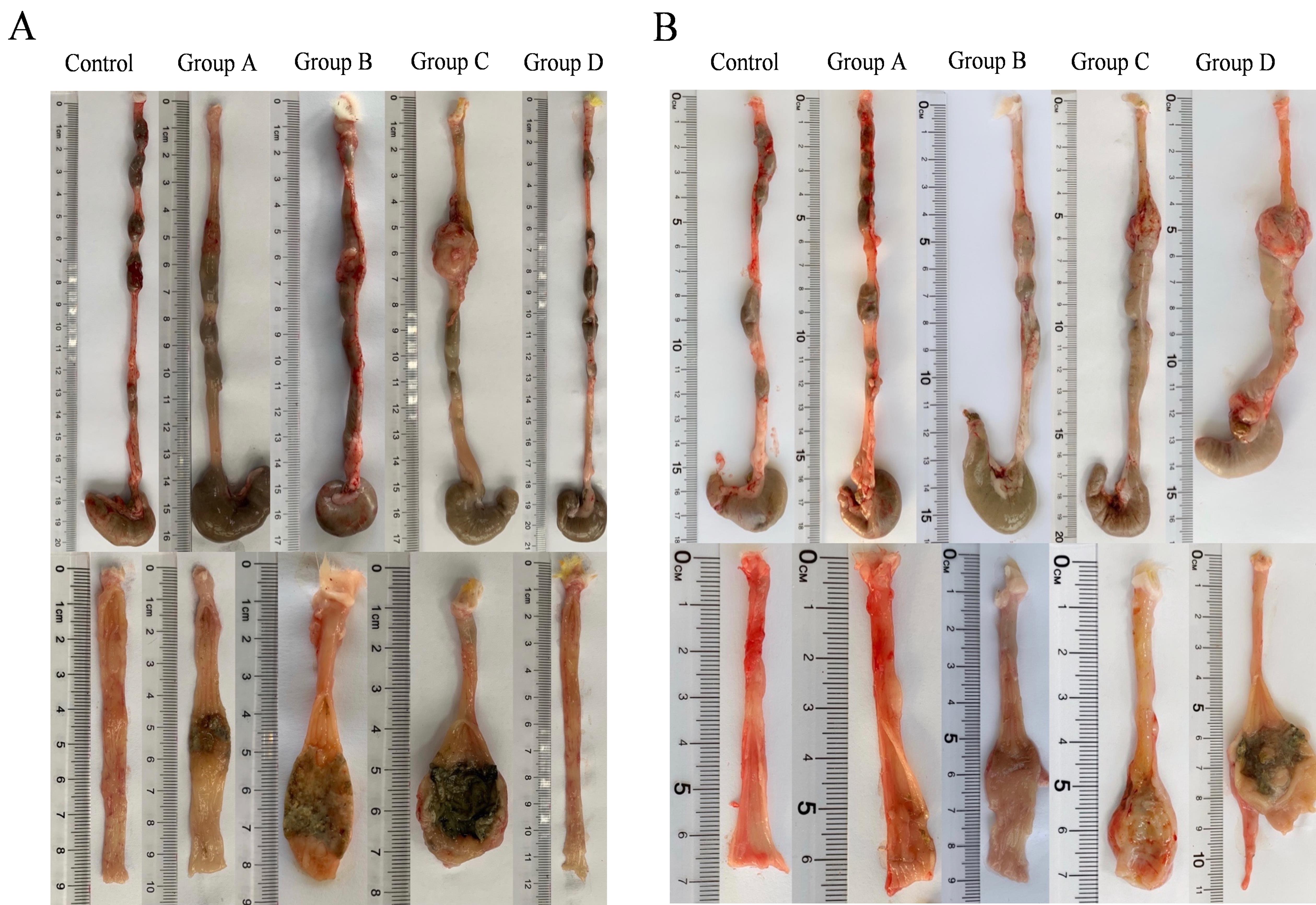
Figure 3 Colonic tissue of rats after model establishmentNote: (A) Seven days after model establishment. (B) Fourteen days after model establishment. The upper panel shows the overall appearance of the whole colon and the lower panel shows the longitudinal section of the colon model site. The control group was induced by normal saline enema;Groups A to D were model groups: group A was induced by 5% TNBS + 50% ethanol solution(1∶1 v/v) enema,group B was induced by 5% TNBS+75% ethanol solution (1∶1 v/v) enema,group C was induced by 5% TNBS+100% ethanol solution (1∶1 v/v) enema,group D was induced by 5%TNBS+50% ethanol solution (2∶1 v/v) enema.
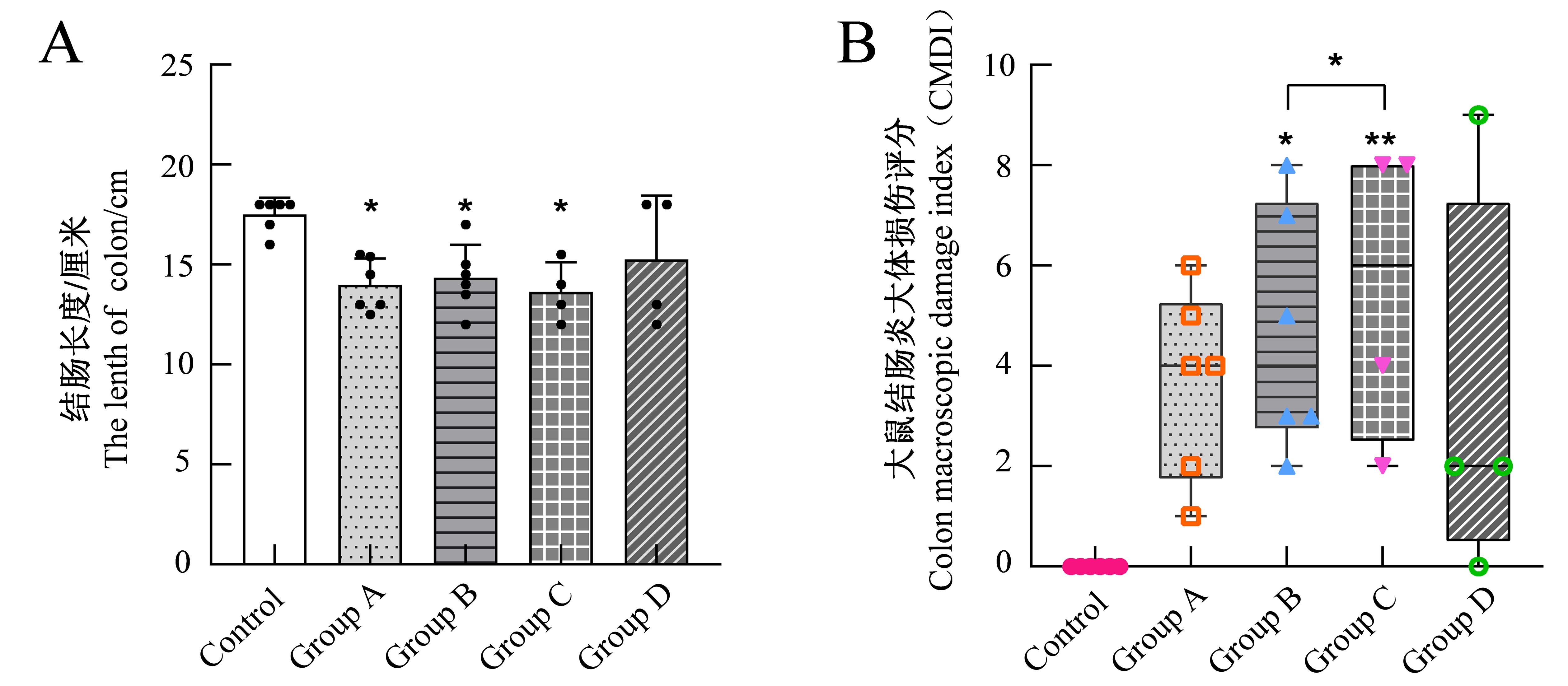
Figure 4 The length of rat colon after model establishment (A) and colon macroscopic damage index (B) in rats after model establishmentNote: The control group was induced by normal saline enema;Groups A to D were model groups: group A was induced by 5% TNBS + 50% ethanol solution(1∶1 v/v) enema,group B was induced by 5% TNBS+75% ethanol solution (1∶1 v/v) enema,group C was induced by 5% TNBS+100% ethanol solution (1∶1 v/v) enema,group D was induced by 5%TNBS+50% ethanol solution (2∶1 v/v) enema. There're 6 rats contained in each group, but one of which died on days 2 and 6 in group C and two of which died on day 3 in group D after modeling. Compared with the control group, *P < 0.05, **P < 0.01.
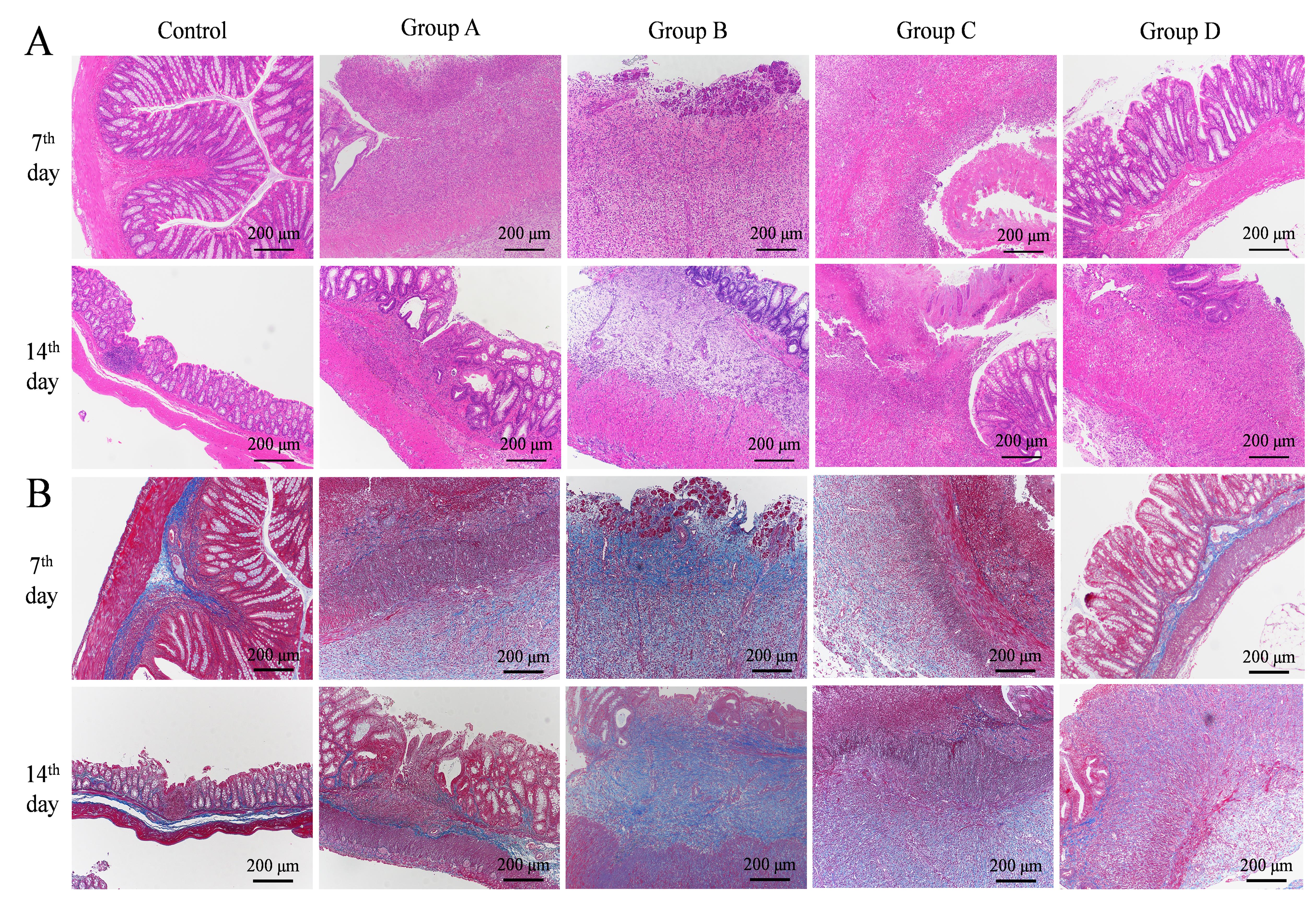
Figure 5 HE staining (A) and Masson staining (B) of rats’ colons after model establishment (×100)Note:The control group was induced by normal saline enema;Groups A to D were model groups: group A was induced by 5% TNBS + 50% ethanol solution(1∶1 v/v) enema,group B was induced by 5% TNBS+75% ethanol solution (1∶1 v/v) enema,group C was induced by 5% TNBS+100% ethanol solution (1∶1 v/v) enema,group D was induced by 5%TNBS+50% ethanol solution (2∶1 v/v) enema.
| 1 | RIEDER F, BETTENWORTH D, MA C, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn's disease[J]. Aliment Pharmacol Ther, 2018, 48(3):347-357. DOI:10.1111/apt.14853 . |
| 2 | SCHMOYER C J, SAIDMAN J, BOHL J L, et al. The pathogenesis and clinical management of stricturing crohn disease[J]. Inflamm Bowel Dis, 2021, 27(11):1839-1852. DOI:10.1093/ibd/izab038 . |
| 3 | ROGLER G, HAUSMANN M. Factors promoting development of fibrosis in Crohn's disease[J]. Front Med (Lausanne), 2017, 4:96. DOI:10.3389/fmed.2017.00096 . |
| 4 | KUEMMERLE J F. Murine trinitrobenzoic acid-induced colitis as a model of Crohn's disease[J]. Methods Mol Biol, 2016, 1422:243-252. DOI:10.1007/978-1-4939-3603-8_22 . |
| 5 | MORRIS G P, BECK P L, HERRIDGE M S, et al. Hapten-induced model of chronic inflammation and ulceration in the rat colon[J]. Gastroenterology, 1989, 96(2):795-803. DOI:10.1016/S0016-5085(89)80079-4 . |
| 6 | MURANO M, MAEMURA K, HIRATA I, et al. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis[J]. Clin Exp Immunol, 2000, 120(1):51-58. DOI:10.1046/j.1365-2249.2000.01183.x . |
| 7 | BUTZNER J D, PARMAR R, BELL C J, et al. Butyrate enema therapy stimulates mucosal repair in experimental colitis in the rat[J]. Gut, 1996, 38(4):568-573. DOI:10.1136/gut.38.4.568 . |
| 8 | RIEDER F, KESSLER S, SANS M, et al. Animal models of intestinal fibrosis: new tools for the understanding of pathogenesis and therapy of human disease[J]. Am J Physiol Gastrointest Liver Physiol, 2012, 303(7): G786-G801. DOI:10.1152/ajpgi.00059.2012 . |
| 9 | DE SALVO C, RAY S, PIZARRO T T. Mechanisms and models for intestinal fibrosis in IBD[J]. Dig Dis, 2014, 32():26-34. DOI:10.1159/000367822 . |
| 10 | ELSON C O, SARTOR R B, TENNYSON G S, et al. Experimental models of inflammatory bowel disease[J]. Gastroenterology, 1995, 109(4):1344-1367. DOI:10.1016/0016-5085(95)90599-5 . |
| 11 | 甘华田. 正确选择炎症性肠病实验研究的动物模型[J]. 胃肠病学, 2007, 12(3):132-134. |
| GAN H T. Select the correct animal model for experimental study of inflammatory bowel disease[J]. Chin J Gastroenterol, 2007, 12(3):132-134. | |
| 12 | ALFREDSSON J, WICK M J. Mechanism of fibrosis and stricture formation in Crohn's disease[J]. Scand J Immunol, 2020, 92(6): e12990. DOI:10.1111/sji.12990 . |
| 13 | 刘登瑞, 哈小琴, 高明太. 炎症性肠病动物模型的研究进展[J]. 中国比较医学杂志, 2008, 18(1):77-80. DOI:10.3969/j.issn.1008-7125.2009.09.012 . |
| LIU D R, HA X Q, GAO M T. Progress on animal model of inflammatory bowel disease[J]. Chin J Comp Med, 2008, 18(1):77-80. DOI:10.3969/j.issn.1008-7125.2009.09.012 . | |
| 14 | LI C, FLYNN R S, GRIDER J R, et al. Increased activation of latent TGF-β1 by αVβ3 in human Crohn's disease and fibrosis in TNBS colitis can be prevented by cilengitide[J]. Inflamm Bowel Dis, 2013, 19(13):2829-2839. DOI:10.1097/MIB.0b013e3182a8452e . |
| 15 | LAWRANCE I C, WU F, LEITE A Z A, et al. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-κB[J]. Gastroenterology, 2003, 125(6):1750-1761. DOI:10.1053/j.gastro.2003.08.027 . |
| 16 | D'ALESSIO S, UNGARO F, NOVIELLO D, et al. Revisiting fibrosis in inflammatory bowel disease: the gut thickens[J]. Nat Rev Gastroenterol Hepatol, 2022, 19(3):169-184. DOI:10.1038/s41575-021-00543-0 . |
| 17 | ZIDAR N, LANGNER C, JERALA M, et al. Pathology of fibrosis in Crohn's disease-contribution to understanding its pathogenesis[J]. Front Med (Lausanne), 2020, 7:167. DOI:10.3389/fmed.2020.00167 . |
| 18 | 方磊, 乔立超, 顾一帆, 等. 克罗恩病大小鼠动物模型研究进展[J]. 中国实验动物学报, 2020, 28(5):688-694. |
| FANG L, QIAO L C, GU Y F, et al. Progress on animal model of Crohn's disease in rats and mice[J]. Acta Lab Animalis Sci Sin, 2020, 28(5):688-694.[知网] | |
| 19 | SARTOR R B. Microbial influences in inflammatory bowel diseases[J]. Gastroenterology, 2008, 134(2):577-594. DOI:10.1053/j.gastro.2007.11.059 . |
| 20 | SCHEIFFELE F, FUSS I J. Induction of TNBS colitis in mice[J]. Curr Protoc Immunol, 2002, Chapter 15: Unit 15.19. DOI:10.1002/0471142735.im1519s49 . |
| 21 | 李球, 丁健. 小鼠灌肠方法的总结与优化[J]. 实验动物科学, 2020, 37(2):70-72. DOI:10.3969/j.issn.1006-6179.2020.02.012 . |
| LI Q, DING J. The summary and improvement of mice's Enema method[J]. Lab Animal Sci, 2020, 37(2):70-72. DOI:10.3969/j.issn.1006-6179.2020.02.012 . |
| [1] | ZHAO Xin, WANG Chenxi, SHI Wenqing, LOU Yuefen. Advances in the Application of Zebrafish in the Research of Inflammatory Bowel Disease Mechanisms and Drug Development [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 422-431. |
| [2] | WU Zhihao, CAO Shuyang, ZHOU Zhengyu. Establishment of an Intestinal Fibrosis Model Associated with Inflammatory Bowel Disease in VDR-/- Mice Induced by Helicobacter hepaticus Infection and Mechanism Exploration [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 37-46. |
| [3] | Xiaorui ZHANG, Jing CAO, Qianqian WU, Jijun LIU, Guoyuan CHEN, Baojin WU. Effects of Probucol Formulations on Mesenteric Lymphatic Trans-port Efficiency and Pharmacokinetics in Rats [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 275-283. |
| [4] | Sijia ZHAO, Xinyu HE, Quan JING, Lin MA, Chunlan GUO, Kuo WAN. Evaluation of Pain in Acute Pulpitis Hyperalgesia Model Rats [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 333-341. |
| [5] | Xiaorui ZHANG, Jing CAO, Qianqian WU, Kang KANG, Guoyuan CHEN, Baojin WU. A Preliminary Method for Continuous Drainage of Mesenteric Lymph Fluid in Rats [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 267-274. |
| [6] | Dingshan FENG, Yeyu HUANG, Xiaoxin ZHANG, Aiqin WU, Zhan WANG, Linliang SU. Effects of Storage Time on Electrolyte Content and pH Value in Rat Serum Samples [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 301-305. |
| [7] | ZHOU Yani, LIU Dan. Therapeutic Effect of Anti-complement 5a Receptor Antibody Combined with Allicin on Induced Inflammatory Bowel Disease In Rats [J]. Laboratory Animal and Comparative Medicine, 2020, 40(4): 328-. |
| [8] | YUAN Xiao-hong, HE Feng, JIANG Ze-hui, ZHAO He, YE Chao, WU Shao-ming, YU Hai-chuan, LI Chun-gen. Establishment and Evaluation of Rat Model of Neurogenic Bladder after Spinal Cord Transection Injury [J]. Laboratory Animal and Comparative Medicine, 2016, 36(6): 423-427. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||