













实验动物与比较医学 ›› 2025, Vol. 45 ›› Issue (6): 688-704.DOI: 10.12300/j.issn.1674-5817.2025.104
收稿日期:2025-07-01
修回日期:2025-10-11
出版日期:2025-12-25
发布日期:2025-12-19
通讯作者:
刘竞男(1974—),女,博士,副研究员,研究方向:动物分子生理学。E-mail:jnliu@shnu.edu.cn。ORCID:0000-0002-4325-1796
作者简介:陈浩田(2004—),男,本科生,研究方向:生物科学。E-mail:1529646524@qq.com
基金资助:Received:2025-07-01
Revised:2025-10-11
Published:2025-12-25
Online:2025-12-19
Contact:
LIU Jingnan (ORCID: 0000-0002-4325-1796), E-mail: jnliu@shnu.edu.cn摘要:
肥胖是一种由能量摄入与消耗长期失衡引起的慢性代谢性疾病,该病可显著增加心血管疾病、2型糖尿病、多种癌症及早衰等风险。世界卫生组织最新统计结果显示,全球成年人超重与肥胖率持续攀升,肥胖已成为亟待精准防治的公共卫生难题。本综述系统总结了黑腹果蝇(Drosophila melangaster)作为模式生物在肥胖代谢研究中的独特价值与应用进展。果蝇因其生命周期短、饲养成本低、器官功能保守且与人类疾病相关基因高度同源,以及遗传工具完善,已成为解析肥胖与代谢紊乱的高效模式生物。通过给果蝇饲喂高糖/高脂饮食可稳定复制脂质蓄积、胰岛素抵抗、心脏代谢功能受损与寿命缩短等典型肥胖相关表型,并可与组织或细胞类型特异性遗传的操控结合,用于靶点发现与机制验证。在器官层面,果蝇脂肪体是储能、代谢感应和内分泌调节的中枢;绛色细胞参与脂质、固醇与超长链脂肪酸代谢及饥饿应答;中肠通过区域化吸收与肠内分泌的功能来整合来自营养物质与肠道微生物的信号;马氏管除排泄功能外,还通过调控能量感应信号通路的表达与葡萄糖转运体的膜定位,调控自身重吸收水平并影响代谢和生长发育;肌肉是果蝇主要的能量消耗器官,其中飞行肌的能量需求最旺盛,以血淋巴中的海藻糖和葡萄糖作为主要的能量供给,同时可动员糖原与脂肪酸参与能量代谢,并通过肌源性因子调节全身体内代谢稳态。此外,昼夜节律与进食时间可重塑外周时钟-代谢耦合,缓解饮食诱导的代谢紊乱。在跨器官内分泌调控网络中,脑部类胰岛素样肽生成细胞通过分泌胰岛素样肽降低血糖,从而促进代谢平衡;心侧体通过分泌脂动激素升高血糖并促进脂解,这两种内分泌系统相互拮抗,共同构成了果蝇体内关键的代谢稳态调控轴。脂肪体与肠道通过释放非配对蛋白2、限制素及类脂联素样因子,按营养状态调节胰岛素样肽的分泌,形成“肠-脂肪体-脑-外周器官”的多层次反馈环。综上所述,果蝇凭借其在器官功能与代谢通路的高度保守性,以及强大的遗传操作优势,为解析肥胖病因学机制、阐明跨组织信号网络、发掘潜在的转化靶点与评估营养/药物干预策略提供了高效、可拓展的实验平台。
中图分类号:
陈浩田,刘竞男. 果蝇在肥胖及相关代谢性疾病研究中的应用与进展[J]. 实验动物与比较医学, 2025, 45(6): 688-704. DOI: 10.12300/j.issn.1674-5817.2025.104.
CHEN Haotian,LIU Jingnan. Applications and Advances of Drosophila in Research of Obesity and Its Related Metabolic Diseases[J]. Laboratory Animal and Comparative Medicine, 2025, 45(6): 688-704. DOI: 10.12300/j.issn.1674-5817.2025.104.
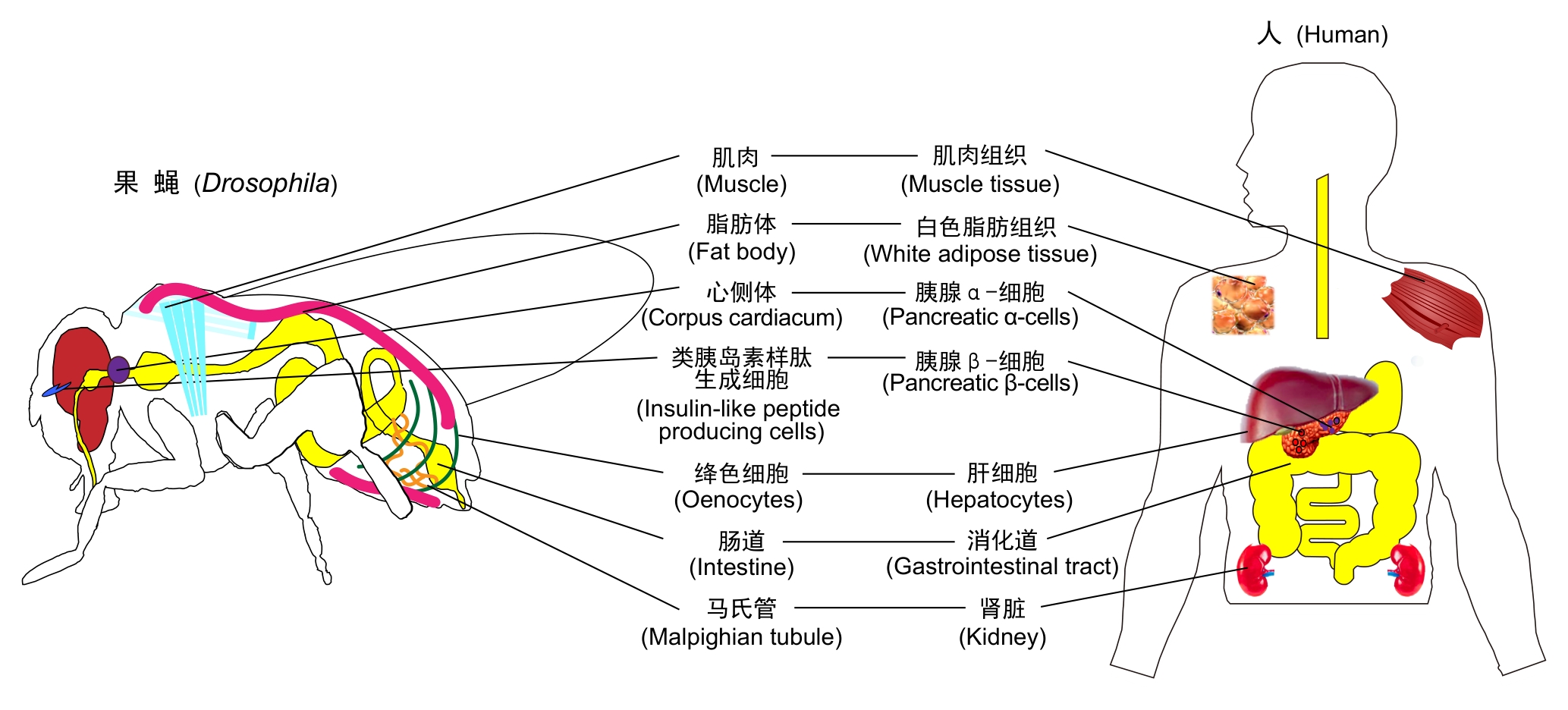
图1 果蝇与哺乳动物(以人类为例)同源的主要能量代谢组织或器官的对应示意图
Figure 1 Homologous major energy metabolism tissues/organs between Drosophila and mammals (taking humans as an example)
果蝇的组织或器官 Drosophila tissues or organs | 能量代谢调控 Functions in energy metabolism | 人类对应的组织或器官 Human counterpart tissues or organs |
|---|---|---|
脂肪体 Fat body | (1) 糖类、脂质和氨基酸的代谢中枢 (2) 能量储存、营养动员与代谢状态感应 (3) 分泌Upd2、抗菌肽,调节胰岛素信号与免疫应答 | 肝脏和白色脂肪组织 |
绛色细胞 Oenocytes | (1) 参与脂质合成、固醇代谢和解毒反应 (2) 在饥饿状态下,诱导脂滴的聚积 (3) 合成表皮烃,并参与信息素的调控 | 肝细胞 |
中肠 Midgut | (1) 参与营养消化吸收、代谢与肠道免疫联动(识别共生菌/病原并启动局部免疫,同时通过肠–脑–脂肪体轴影响全身代谢) (2) 参与内分泌的调控(如分泌NPF、CCHa2等神经肽) | 小肠 |
肌肉 Muscle | (1) 运动/飞行时能量需求高,主要利用糖原(由葡萄糖聚合)与脂肪酸β-氧化供能 (2) 肌源信号影响脂肪体/胰岛素信号通路,并协调能量分配 | 骨骼肌 |
马氏管 Malpighian tubule | (1) 参与排泄与渗透压调节 (2) 通过营养与能量感应(AMPK/TOR与胰岛素样信号)调节转运、蛋白质合成/自噬与代谢适应 (3) 应激状态下促进脂质与ROS的清除,缓冲代谢压力 | 肾脏的肾小管 |
类胰岛素样肽生成细胞 Insulin-like peptide producing cells | (1) 可分泌Dilps (2) 感知能量状态变化,系统调节全身糖脂代谢 | 胰腺β细胞 |
心侧体 Corpora cardiaca | (1) 分泌AKH(类似胰高血糖素) (2) 饥饿时,促进糖原分解和糖异生,并动员脂质供能,以维持血糖稳定,保障生存需求 | 胰腺α细胞 |
表1 果蝇重要代谢组织或器官的功能及与哺乳动物(以人类为例)相应组织或器官的对照表
Table 1 Functional comparison of principal metabolic tissues/organs in Drosophila and homologous tissues/organs in mammals (taking humans as an example)
果蝇的组织或器官 Drosophila tissues or organs | 能量代谢调控 Functions in energy metabolism | 人类对应的组织或器官 Human counterpart tissues or organs |
|---|---|---|
脂肪体 Fat body | (1) 糖类、脂质和氨基酸的代谢中枢 (2) 能量储存、营养动员与代谢状态感应 (3) 分泌Upd2、抗菌肽,调节胰岛素信号与免疫应答 | 肝脏和白色脂肪组织 |
绛色细胞 Oenocytes | (1) 参与脂质合成、固醇代谢和解毒反应 (2) 在饥饿状态下,诱导脂滴的聚积 (3) 合成表皮烃,并参与信息素的调控 | 肝细胞 |
中肠 Midgut | (1) 参与营养消化吸收、代谢与肠道免疫联动(识别共生菌/病原并启动局部免疫,同时通过肠–脑–脂肪体轴影响全身代谢) (2) 参与内分泌的调控(如分泌NPF、CCHa2等神经肽) | 小肠 |
肌肉 Muscle | (1) 运动/飞行时能量需求高,主要利用糖原(由葡萄糖聚合)与脂肪酸β-氧化供能 (2) 肌源信号影响脂肪体/胰岛素信号通路,并协调能量分配 | 骨骼肌 |
马氏管 Malpighian tubule | (1) 参与排泄与渗透压调节 (2) 通过营养与能量感应(AMPK/TOR与胰岛素样信号)调节转运、蛋白质合成/自噬与代谢适应 (3) 应激状态下促进脂质与ROS的清除,缓冲代谢压力 | 肾脏的肾小管 |
类胰岛素样肽生成细胞 Insulin-like peptide producing cells | (1) 可分泌Dilps (2) 感知能量状态变化,系统调节全身糖脂代谢 | 胰腺β细胞 |
心侧体 Corpora cardiaca | (1) 分泌AKH(类似胰高血糖素) (2) 饥饿时,促进糖原分解和糖异生,并动员脂质供能,以维持血糖稳定,保障生存需求 | 胰腺α细胞 |
疾病类型 Types of diseases | 模型构建方法 Model construction methods | 主要表型/表型指标 Major phenotypic/phenotypic traits |
|---|---|---|
肥胖 Obesity | (1)食物诱导(长期高脂饮食、高糖高脂复合饮食,增加能量摄入) (2)基因突变/失活(脂解相关基因如bmm等发生突变或下调;过表达脂质合成和储存基因) (3)代谢激素通路相关因子的缺失或减弱(如akh基因缺失或其功能受损) (4)神经/行为层面(特定神经元或神经肽基因的过表达或敲除,引起摄食行为的显著增强) | (1)全身与组织中的TAG含量显著升高,糖原储备量增加 (2)脂肪体、肠道等组织细胞中脂滴的体积和数量增加 (3)果蝇体重增加,腹部膨大,活动能力下降 (4)胰岛素信号失衡(如p-Akt的水平下降),出现胰岛素抵抗样表型 (5)耐饥饿能力增强,可能伴随寿命延长或应激耐受性的变化(根据模型而定) |
2型糖尿病 Type 2 diabetes | (1)食物诱导(高糖饮食、高糖+高脂饮食,长期喂养导致慢性高血糖与胰岛素抵抗) (2)胰岛素通路基因的突变、过表达或缺失(如基因InR/chico突变,基因FOXO过表达,基因Upd2缺失) | (1)血淋巴循环糖(以海藻糖为主,部分为葡萄糖)的浓度升高; (2)胰岛素抵抗(Akt激酶的表达下调,FOXO的核定位增加),外源胰岛素信号的激活反应减弱;TAG和脂滴水平异常(如脂肪过度积累或动员紊乱),伴随氧化应激水平升高 (3)果蝇的生长缓慢、体型变小,发育时间延长以及繁殖能力下降 |
1型糖尿病 Type 1 diabetes | (1)消除/损伤IPCs(利用在IPCs中特异性表达的Dilp2-GAL4驱动rpr/hid表达,以选择性消除IPCs;抑制IPCs的活性,阻断Dilps的分泌) (2)基因Dilp1~5基因簇敲除或组合突变,导致系统性的胰岛素缺乏 (3)调控因子的突变(调控IPCs形成和功能的转录因子或信号通路的缺失,使IPCs的数量严重减少) | (1)Dilps的缺失或显著降低导致高血糖,血淋巴中海藻糖浓度显著升高 (2)果蝇体型显著变小,生长严重滞缓,发育延迟或停滞 (3)脂质与糖原储备减少,对饥饿和应激反应更为敏感 (4)寿命明显缩短,运动与繁殖能力下降 (5)对外源胰岛素样信号或遗传“救援”操作高度敏感,相关表型可被部分恢复 |
心血管疾病 Cardiovascular diseases | (1)食物诱导(高糖、高脂饮食长期饲喂诱导心肌脂肪异常沉积、能量代谢紊乱和心律失常) (2)心肌相关基因突变体[利用易导致心律失常和心肌结构改变的基因(如Eas、srl、Ork1等)构建突变果蝇模型] | (1)心律失常(心跳节律紊乱,心周期延长或缩短)、心肌舒缩异常(舒张和收缩末期直径均增大,缩短分数下降,提示心脏泵血功能减弱)、扩张型心肌病样表型(心腔扩大、缩短分数下降,泵血功能减弱)、心肌细胞凋亡增加,并出现退行性改变 (2)全身性功能减退(应激耐受下降、运动能力减弱、寿命缩短, |
肿瘤 Tumors | (1)在组织特异性过表达原癌基因,可诱导组织细胞过度增殖并产生类肿瘤表型 (2)RasV12与scrib-/- 等极性基因突变组合,可形成高度侵袭性肿瘤 (3)果蝇中眼盘、肠道等组织中Hippo通路抑癌因子的功能缺失或Yki的过度激活,均会导致组织过度增殖,形成肿瘤样团块 (4)将原位诱导的肿瘤组织移植到受体果蝇成虫或幼虫体内,建立全身性肿瘤负荷与转移模型 (5)协同调控多个抑癌基因的表达,构建具有不同分子特征的肿瘤亚型模型 | (1)局部或全身范围内的细胞异常增殖,器官体积显著增大,形成肉眼可见的肿瘤团块 (2)细胞极性丧失、上皮结构破坏、基底膜降解,肿瘤细胞可浸润周围组织,甚至侵入血淋巴循环 (3)可视化的GFP标记肿瘤块,实时追踪体内肿瘤生长、侵袭与转移样过程 (4)机体整体表现为体重下降、脂肪体和肌肉消耗、代谢重塑,最终导致个体死亡 |
表2 常见代谢疾病的果蝇模型
Table 2 Canonical Drosophila models of metabolic diseases
疾病类型 Types of diseases | 模型构建方法 Model construction methods | 主要表型/表型指标 Major phenotypic/phenotypic traits |
|---|---|---|
肥胖 Obesity | (1)食物诱导(长期高脂饮食、高糖高脂复合饮食,增加能量摄入) (2)基因突变/失活(脂解相关基因如bmm等发生突变或下调;过表达脂质合成和储存基因) (3)代谢激素通路相关因子的缺失或减弱(如akh基因缺失或其功能受损) (4)神经/行为层面(特定神经元或神经肽基因的过表达或敲除,引起摄食行为的显著增强) | (1)全身与组织中的TAG含量显著升高,糖原储备量增加 (2)脂肪体、肠道等组织细胞中脂滴的体积和数量增加 (3)果蝇体重增加,腹部膨大,活动能力下降 (4)胰岛素信号失衡(如p-Akt的水平下降),出现胰岛素抵抗样表型 (5)耐饥饿能力增强,可能伴随寿命延长或应激耐受性的变化(根据模型而定) |
2型糖尿病 Type 2 diabetes | (1)食物诱导(高糖饮食、高糖+高脂饮食,长期喂养导致慢性高血糖与胰岛素抵抗) (2)胰岛素通路基因的突变、过表达或缺失(如基因InR/chico突变,基因FOXO过表达,基因Upd2缺失) | (1)血淋巴循环糖(以海藻糖为主,部分为葡萄糖)的浓度升高; (2)胰岛素抵抗(Akt激酶的表达下调,FOXO的核定位增加),外源胰岛素信号的激活反应减弱;TAG和脂滴水平异常(如脂肪过度积累或动员紊乱),伴随氧化应激水平升高 (3)果蝇的生长缓慢、体型变小,发育时间延长以及繁殖能力下降 |
1型糖尿病 Type 1 diabetes | (1)消除/损伤IPCs(利用在IPCs中特异性表达的Dilp2-GAL4驱动rpr/hid表达,以选择性消除IPCs;抑制IPCs的活性,阻断Dilps的分泌) (2)基因Dilp1~5基因簇敲除或组合突变,导致系统性的胰岛素缺乏 (3)调控因子的突变(调控IPCs形成和功能的转录因子或信号通路的缺失,使IPCs的数量严重减少) | (1)Dilps的缺失或显著降低导致高血糖,血淋巴中海藻糖浓度显著升高 (2)果蝇体型显著变小,生长严重滞缓,发育延迟或停滞 (3)脂质与糖原储备减少,对饥饿和应激反应更为敏感 (4)寿命明显缩短,运动与繁殖能力下降 (5)对外源胰岛素样信号或遗传“救援”操作高度敏感,相关表型可被部分恢复 |
心血管疾病 Cardiovascular diseases | (1)食物诱导(高糖、高脂饮食长期饲喂诱导心肌脂肪异常沉积、能量代谢紊乱和心律失常) (2)心肌相关基因突变体[利用易导致心律失常和心肌结构改变的基因(如Eas、srl、Ork1等)构建突变果蝇模型] | (1)心律失常(心跳节律紊乱,心周期延长或缩短)、心肌舒缩异常(舒张和收缩末期直径均增大,缩短分数下降,提示心脏泵血功能减弱)、扩张型心肌病样表型(心腔扩大、缩短分数下降,泵血功能减弱)、心肌细胞凋亡增加,并出现退行性改变 (2)全身性功能减退(应激耐受下降、运动能力减弱、寿命缩短, |
肿瘤 Tumors | (1)在组织特异性过表达原癌基因,可诱导组织细胞过度增殖并产生类肿瘤表型 (2)RasV12与scrib-/- 等极性基因突变组合,可形成高度侵袭性肿瘤 (3)果蝇中眼盘、肠道等组织中Hippo通路抑癌因子的功能缺失或Yki的过度激活,均会导致组织过度增殖,形成肿瘤样团块 (4)将原位诱导的肿瘤组织移植到受体果蝇成虫或幼虫体内,建立全身性肿瘤负荷与转移模型 (5)协同调控多个抑癌基因的表达,构建具有不同分子特征的肿瘤亚型模型 | (1)局部或全身范围内的细胞异常增殖,器官体积显著增大,形成肉眼可见的肿瘤团块 (2)细胞极性丧失、上皮结构破坏、基底膜降解,肿瘤细胞可浸润周围组织,甚至侵入血淋巴循环 (3)可视化的GFP标记肿瘤块,实时追踪体内肿瘤生长、侵袭与转移样过程 (4)机体整体表现为体重下降、脂肪体和肌肉消耗、代谢重塑,最终导致个体死亡 |
| [1] | CHATTERJEE N, PERRIMON N. What fuels the fly: Energy metabolism in Drosophila and its application to the study of obesity and diabetes[J]. Sci Adv, 2021, 7(24): 1-14. DOI:10.1126/sciadv.abg4336 . |
| [2] | BARNES A I, WIGBY S, BOONE J M, et al. Feeding, fecundity and lifespan in female Drosophila melanogaster [J]. Proc Biol Sci, 2008, 275(1643):1675-1683. DOI:10.1098/rspb.2008.0139 . |
| [3] | HOUSDEN B E, VALVEZAN A J, KELLEY C, et al. Identification of potential drug targets for tuberous sclerosis complex by synthetic screens combining CRISPR-based knockouts with RNAi[J]. Sci Signal, 2015, 8(393): 1-22. DOI:10.1126/scisignal.aab3729 . |
| [4] | HARDY C M, BIRSE R T, WOLF M J, et al. Obesity-associated cardiac dysfunction in starvation-selected Drosophila melanogaster [J]. Am J Physiol Regul Integr Comp Physiol, 2015, 309(6): R658-R667. DOI:10.1152/ajpregu.00160.2015 . |
| [5] | ADAMS M D, CELNIKER S E, HOLT R A, et al. The genome sequence of Drosophila melanogaster [J]. Science, 2000, 287(5461):2185-2195. DOI:10.1126/science.287.5461.2185 . |
| [6] | REITER L T, POTOCKI L, CHIEN S, et al. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster [J]. Genome Res, 2001, 11(6):1114-1125. DOI:10.1101/gr.169101 . |
| [7] | AGRAWAL N, LAWLER K, DAVIDSON C M, et al. Predicting novel candidate human obesity genes and their site of action by systematic functional screening in Drosophila [J]. PLoS Biol, 2021, 19(11): 1-22. DOI:10.1371/journal.pbio.3001255 . |
| [8] | DOANE W W. Developmental physiology of the mutant female sterile(2)adipose of Drosophila melanogaster. Ⅰ. Adult morphology, longevity, egg production, and egg lethality[J]. J Exp Zool, 1960, 145(1):1-21. DOI:10.1002/jez.1401450102 . |
| [9] | HÄDER T, MÜLLER S, AGUILERA M, et al. Control of triglyceride storage by a WD40/TPR-domain protein[J]. EMBO Rep, 2003, 4(5):511-516. DOI:10.1038/sj.embor.embor837 . |
| [10] | KRUDE H, BIEBERMANN H, LUCK W, et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans[J]. Nat Genet, 1998, 19(2):155-157. DOI:10.1038/509 . |
| [11] | SAEED S, BONNEFOND A, MANZOOR J, et al. Genetic variants in LEP, LEPR, and MC4R explain 30% of severe obesity in children from a consanguineous population[J]. Obesity, 2015, 23(8):1687-1695. DOI:10.1002/oby.21142 . |
| [12] | FRAYLING T M, TIMPSON N J, WEEDON M N, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity[J]. Science, 2007, 316(5826):889-894. DOI:10.1126/science. 1141634 . |
| [13] | YEO G S, FAROOQI I S, AMINIAN S, et al. A frameshift mutation in MC4R associated with dominantly inherited human obesity[J]. Nat Genet, 1998, 20(2):111-112. DOI:10.1038/2404 . |
| [14] | CIABRELLI F, ATINBAYEVA N, PANE A, et al. Epigenetic inheritance and gene expression regulation in early Drosophila embryos[J]. EMBO Rep, 2024, 25(10):4131-4152. DOI:10.1038/s44319-024-00245-z . |
| [15] | GLASER-SCHMITT A, PARSCH J. Dynamics and stage-specificity of between-population gene expression divergence in the Drosophila melanogaster larval fat body[J]. PLoS Genet, 2023, 19(4): 1-25. DOI:10.1371/journal.pgen.10 10730 . |
| [16] | LIU J N, ZHANG Y B, ZHOU Y F, et al. Cytoophidia coupling adipose architecture and metabolism[J]. Cell Mol Life Sci, 2022, 79(10):534. DOI:10.1007/s00018-022-04567-w . |
| [17] | LIU J N, ZHANG Y B, WANG Q Q, et al. Fat body-specific reduction of CTPS alleviates HFD-induced obesity[J]. eLife, 2023, 12: 1-22. DOI:10.7554/eLife.85293 . |
| [18] | HONG J Y, LI J M, ZHANG Y B, et al. Integrative role of CTPS cytoophidia in polyploid tissue growth and nutrient adaptation[J]. Insect Sci, 2025. DOI:10.1111/1744-7917.70060. DOI:10.1111/1744-7917.70060 . |
| [19] | PARK J E, JI L, KWAN K, et al. Flightless-I controls fat storage in Drosophila [J]. Mol Cells, 2018, 41(6):603-611. DOI:10.14348/molcells.2018.0120 . |
| [20] | DING L, YANG X, TIAN H, et al. Seipin regulates lipid homeostasis by ensuring calcium-dependent mitochondrial metabolism[J]. EMBO J, 2018, 37(17): e97572. DOI:10.15252/embj.201797572 . |
| [21] | INGARAMO M C, SÁNCHEZ J A, PERRIMON N, et al. Fat body p53 regulates systemic insulin signaling and autophagy under nutrient stress via Drosophila Upd2 repression[J]. Cell Rep, 2020, 33(4):1-34. DOI:10.1016/j.celrep.2020.108321 . |
| [22] | GHOSH A C, TATTIKOTA S G, LIU Y F, et al. Drosophila PDGF/VEGF signaling from muscles to hepatocyte-like cells protects against obesity[J]. eLife, 2020, 9: 1-29. DOI:10.7554/eLife.56969 . |
| [23] | KIM B, KANAI M I, OH Y, et al. Response of the microbiome-gut-brain axis in Drosophila to amino acid deficit[J]. Nature, 2021, 593(7860):570-574. DOI:10.1038/s41586-021-03522-2 . |
| [24] | CHEN J T, NOUZOVÁ M, NORIEGA F G, et al. Gut-to-brain regulation of Drosophila aging through neuropeptide F, insulin, and juvenile hormone[J]. Proc Natl Acad Sci USA, 2024, 121(43): 1-11. DOI:10.1073/pnas.2411987121 . |
| [25] | PALM W, SAMPAIO J L, BRANKATSCHK M, et al. Lipoproteins in Drosophila melanogaster: assembly, function, and influence on tissue lipid composition[J]. PLoS Genet, 2012, 8(7): 1-18. DOI:10.1371/journal.pgen.1002828 . |
| [26] | OHLSTEIN B, SPRADLING A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells[J]. Nature, 2006, 439(7075):470-474. DOI:10.1038/nature04333 . |
| [27] | WONG A C N, VANHOVE A S, WATNICK P I. The interplay between intestinal bacteria and host metabolism in health and disease: lessons from Drosophila melanogaster [J]. Dis Model Mech, 2016, 9(3):271-281. DOI:10.1242/dmm.023408 . |
| [28] | LIVELO C, GUO Y M, ABOU DAYA F, et al. Time-restricted feeding promotes muscle function through purine cycle and AMPK signaling in Drosophila obesity models[J]. Nat Commun, 2023, 14(1):949. DOI:10.1038/s41467-023-36474-4 . |
| [29] | DARK C, ALI N, GOLENKINA S, et al. Mitochondrial fusion and altered beta-oxidation drive muscle wasting in a Drosophila cachexia model[J]. EMBO Rep, 2024, 25(4):1835-1858. DOI:10.1038/s44319-024-00102-z . |
| [30] | GAMBERI C, HIPFNER D R, TRUDEL M, et al. Bicaudal C mutation causes myc and TOR pathway up-regulation and polycystic kidney disease-like phenotypes in Drosophila [J]. PLoS Genet, 2017, 13(4): 1-20. DOI:10.1371/journal.pgen.1006694 . |
| [31] | LIN Z W, HE Z H, GUO J F, et al. Xgr is involved in body size control in Drosophila through promoting glucose uptake in the Malpighian tubules[J]. J Genet Genomics, 2025: S1673-S8527(25)00151-1. DOI:10.1016/j.jgg.2025.05.007 . |
| [32] | LI X X, ROMMELAERE S, KONDO S, et al. Renal purge of hemolymphatic lipids prevents the accumulation of ROS-induced inflammatory oxidized lipids and protects Drosophila from tissue damage[J]. Immunity, 2020, 52(2):374-387,e1-e6. DOI:10.1016/j.immuni.2020.01.008 . |
| [33] | LIESSEM S, HELD M, BISEN R S, et al. Behavioral state-dependent modulation of insulin-producing cells in Drosophila [J]. Curr Biol, 2023, 33(3):449-463,e1-e5. DOI:10.1016/j.cub.2022.12.005 . |
| [34] | HUGHSON B N. The glucagon-like adipokinetic hormone in Drosophila melanogaster–biosynthesis and secretion[J]. Front Physiol, 2021, 12:1-15. DOI:10.3389/fphys.2021.710652 . |
| [35] | OH Y, LAI J S, MILLS H J, et al. A glucose-sensing neuron pair regulates insulin and glucagon in Drosophila [J]. Nature, 2019, 574(7779):559-564. DOI:10.1038/s41586-019-1675-4 . |
| [36] | KRÉNEISZ O, CHEN X N, FRIDELL Y C, et al. Glucose increases activity and Ca2+ in insulin-producing cells of adult Drosophila [J]. Neuroreport, 2010, 21(17):1116-1120. DOI:10.1097/WNR.0b013e3283409200 . |
| [37] | BROGIOLO W, STOCKER H, IKEYA T, et al. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control[J]. Curr Biol, 2001, 11(4):213-221. DOI:10.1016/s0960-9822(01)00068-9 . |
| [38] | BROUGHTON S J, PIPER M D W, IKEYA T, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands[J]. Proc Natl Acad Sci USA, 2005, 102(8):3105-3110. DOI:10.1073/pnas.0405775102 . |
| [39] | IKEYA T, GALIC M, BELAWAT P, et al. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila [J]. Curr Biol, 2002, 12(15):1293-1300. DOI:10.1016/s0960-9822(02)01043-6 . |
| [40] | BRENT A E, RAJAN A. Insulin and leptin/Upd2 exert opposing influences on synapse number in fat-sensing neurons[J]. Cell Metab, 2020, 32(5):786-800,e1-e7. DOI:10.1016/j.cmet. 2020.08.017 . |
| [41] | ALFA R W, PARK S, SKELLY K R, et al. Suppression of insulin production and secretion by a decretin hormone[J]. Cell Metab, 2015, 21(2):323-334. DOI:10.1016/j.cmet.2015.01.006 . |
| [42] | KAPAN N, LUSHCHAK O V, LUO J N, et al. Identified peptidergic neurons in the Drosophila brain regulate insulin-producing cells, stress responses and metabolism by coex-pressed short neuropeptide F and corazonin[J]. Cell Mol Life Sci, 2012, 69(23):4051-4066. DOI:10.1007/s00018-012-1097-z . |
| [43] | COLOMBANI J, ANDERSEN D S, LÉOPOLD P. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing[J]. Science, 2012, 336(6081):582-585. DOI:10.1126/science.1216689 . |
| [44] | HELD M, BISEN R S, ZANDAWALA M, et al. Aminergic and peptidergic modulation of insulin-producing cells in Drosophila [J]. eLife, 2025, 13: 1-31. DOI:10.7554/eLife.99548 . |
| [45] | MIYAMOTO T, AMREIN H. Diverse roles for the Drosophila fructose sensor Gr43a[J]. Fly, 2014, 8(1):19-25. DOI:10.4161/fly.27241 . |
| [46] | MIYAMOTO T, SLONE J, SONG X Y, et al. A fructose receptor functions as a nutrient sensor in the Drosophila brain[J]. Cell, 2012, 151(5):1113-1125. DOI:10.1016/j.cell.2012.10.024 . |
| [47] | WATANABE K, KANAOKA Y, MIZUTANI S, et al. Interspecies comparative analyses reveal distinct carbohydrate-responsive systems among Drosophila species[J]. Cell Rep, 2019, 28(10):2594-2607.e7. DOI:10.1016/j.celrep.2019.08.030 . |
| [48] | KIM S K, RULIFSON E J. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells[J]. Nature, 2004, 431(7006):316-320. DOI:10.1038/nature02897 . |
| [49] | BHARUCHA K N, TARR P, ZIPURSKY S L. A glucagon-like endocrine pathway in Drosophila modulates both lipid and carbohydrate homeostasis[J]. J Exp Biol, 2008, 211(Pt 19):3103-3110. DOI:10.1242/jeb.016451 . |
| [50] | LEE K S, KWON O Y, LEE J H, et al. Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling[J]. Nat Cell Biol, 2008, 10(4):468-475. DOI:10.1038/ncb1710 . |
| [51] | YOSHINARI Y, KOSAKAMOTO H, KAMIYAMA T, et al. The sugar-responsive enteroendocrine neuropeptide F regulates lipid metabolism through glucagon-like and insulin-like hormones in Drosophila melanogaster [J]. Nat Commun, 2021, 12:4818. DOI:10.1038/s41467-021-25146-w . |
| [52] | SANO H, NAKAMURA A, TEXADA M J, et al. The nutrient-responsive hormone CCHamide-2 controls growth by regulating insulin-like peptides in the brain of Drosophila melanogaster [J]. PLoS Genet, 2015, 11(5): 1-26. DOI:10.1371/journal.pgen.1005209 . |
| [53] | SONG W, VEENSTRA J A, PERRIMON N. Control of lipid metabolism by tachykinin in Drosophila [J]. Cell Rep, 2020, 30(7):2461. DOI:10.1016/j.celrep.2020.02.011 . |
| [54] | YAPICI N, ZIMMER M, DOMINGOS A I. Cellular and molecular basis of decision-making[J]. EMBO Rep, 2014, 15(10):1023-1035. DOI:10.15252/embr.201438993 . |
| [55] | YARMOLINSKY D A, ZUKER C S, RYBA N J P. Common sense about taste: from mammals to insects[J]. Cell, 2009, 139(2):234-244. DOI:10.1016/j.cell.2009.10.001 . |
| [56] | DUNIPACE L, MEISTER S, MCNEALY C, et al. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system[J]. Curr Biol, 2001, 11(11):822-835. DOI:10.1016/s0960-9822(01)00258-5 . |
| [57] | SCHWARTZ M W, WOODS S C, PORTE D Jr, et al. Central nervous system control of food intake[J]. Nature, 2000, 404(6778):661-671. DOI:10.1038/35007534 . |
| [58] | STERNSON S M, ATASOY D. Agouti-related protein neuron circuits that regulate appetite[J]. Neuroendocrinology, 2014, 100(2-3):95-102. DOI:10.1159/000369072 . |
| [59] | AUGUSTINE V, LEE S J, OKA Y. Neural control and modulation of thirst, sodium appetite, and hunger[J]. Cell, 2020, 180(1):25-32. DOI:10.1016/j.cell.2019.11.040 . |
| [60] | AL-ANZI B, SAPIN V, WATERS C, et al. Obesity-blocking neurons in Drosophila [J]. Neuron, 2009, 63(3):329-341. DOI:10.1016/j.neuron.2009.07.021 . |
| [61] | LEE G, PARK J H. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster [J]. Genetics, 2004, 167(1):311-323. DOI:10.1534/genetics.167.1.311 . |
| [62] | WU Q, WEN T Q, LEE G, et al. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system[J]. Neuron, 2003, 39(1):147-161. DOI:10.1016/s0896-6273(03)00396-9 . |
| [63] | INAGAKI H K, BEN-TABOU DE-LEON S, WONG A M, et al. Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing[J]. Cell, 2012, 148(3):583-595. DOI:10.1016/j.cell.2011.12.022 . |
| [64] | MARELLA S, MANN K, SCOTT K. Dopaminergic modulation of sucrose acceptance behavior in Drosophila [J]. Neuron, 2012, 73(5):941-950. DOI:10.1016/j.neuron.2011.12.032 . |
| [65] | LIU J, LI T, YANG D, et al. Synphilin-1 alters metabolic homeostasis in a novel Drosophila obesity model[J]. Int J Obes, 2012, 36(12):1529-1536. DOI:10.1038/ijo.2012.111 . |
| [66] | RAJAN A, PERRIMON N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion[J]. Cell, 2012, 151(1):123-137. DOI:10.1016/j.cell.2012.08.019 . |
| [67] | DUS M, LAI J S, GUNAPALA K M, et al. Nutrient sensor in the brain directs the action of the brain-gut axis in Drosophila [J]. Neuron, 2015, 87(1):139-151. DOI:10.1016/j.neuron.2015.05.032 . |
| [68] | MUSSELMAN L P, FINK J L, BARANSKI T J. Similar effects of high-fructose and high-glucose feeding in a Drosophila model of obesity and diabetes[J]. PLoS One, 2019, 14(5): 1-13. DOI:10.1371/journal.pone.0217096 . |
| [69] | EICKELBERG V, LÜERSEN K, STAATS S, et al. Phenotyping of Drosophila melanogaster-a nutritional perspective[J]. Bio-molecules, 2022, 12(2):221. DOI:10.3390/biom12020221 . |
| [70] | JUNG J, KIM D I, HAN G Y, et al. The effects of high fat diet-induced stress on olfactory sensitivity, behaviors, and transcriptional profiling in Drosophila melanogaster [J]. Int J Mol Sci, 2018, 19(10):2855. DOI:10.3390/ijms19102855 . |
| [71] | VON FRIELING J, FAISAL M N, SPORN F, et al. A high-fat diet induces a microbiota-dependent increase in stem cell activity in the Drosophila intestine[J]. PLoS Genet, 2020, 16(5): 1-23. DOI:10.1371/journal.pgen.1008789 . |
| [72] | GUIDA M C, BIRSE R T, DALL'AGNESE A, et al. Intergenerational inheritance of high fat diet-induced cardiac lipotoxicity in Drosophila [J]. Nat Commun, 2019, 10(1):193. DOI:10.1038/s41467-018-08128-3 . |
| [73] | MELKANI G C, PANDA S. Time-restricted feeding for prevention and treatment of cardiometabolic disorders[J]. J Physiol, 2017, 595(12):3691-3700. DOI:10.1113/JP273094 . |
| [74] | CHAIX A, ZARRINPAR A, PANDA S. The circadian coordination of cell biology[J]. J Cell Biol, 2016, 215(1):15-25. DOI:10.1083/jcb.201603076 . |
| [75] | GUO Y M, ABOU DAYA F, LE H D, et al. Diurnal expression of Dgat2 induced by time-restricted feeding maintains cardiac health in the Drosophila model of circadian disruption[J]. Aging Cell, 2024, 23(7): 1-14. DOI:10.1111/acel.14169 . |
| [76] | CROCKER A, SEHGAL A. Octopamine regulates sleep in Drosophila through protein kinase A-dependent mechanisms[J]. J Neurosci, 2008, 28(38):9377-9385. DOI:10.1523/JNEUROSCI.3072-08a.2008 . |
| [77] | CROCKER A, SHAHIDULLAH M, LEVITAN I B, et al. Identification of a neural circuit that underlies the effects of octopamine on sleep: wake behavior[J]. Neuron, 2010, 65(5):670-681. DOI:10.1016/j.neuron.2010.01.032 . |
| [78] | BRACO J T, NELSON J M, SAUNDERS C J, et al. Modulation of metabolic hormone signaling via a circadian hormone and biogenic amine in Drosophila melanogaster [J]. Int J Mol Sci, 2022, 23(8):4266. DOI:10.3390/ijms23084266 . |
| [79] | BÖHNI R, RIESGO-ESCOVAR J, OLDHAM S, et al. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4[J]. Cell, 1999, 97(7):865-875. DOI:10.1016/s0092-8674(00)80799-0 . |
| [80] | SONG W, REN D C, LI W J, et al. SH2B regulation of growth, metabolism, and longevity in both insects and mammals[J]. Cell Metab, 2010, 11(5):427-437. DOI:10.1016/j.cmet.20 10.04.002 . |
| [81] | NA J B, MUSSELMAN L P, PENDSE J, et al. A Drosophila model of high sugar diet-induced cardiomyopathy[J]. PLoS Genet, 2013, 9(1): 1-14. DOI:10.1371/journal.pgen.1003175 . |
| [82] | MORRIS S N S, COOGAN C, CHAMSEDDIN K, et al. Development of diet-induced insulin resistance in adult Drosophila melanogaster [J]. Biochim Biophys Acta, 2012, 1822(8):1230-1237. DOI:10.1016/j.bbadis.2012.04.012 . |
| [83] | TSUDA M, KOBAYASHI T, MATSUO T, et al. Insulin-degrading enzyme antagonizes insulin-dependent tissue growth and Abeta-induced neurotoxicity in Drosophila [J]. FEBS Lett, 2010, 584(13):2916-2920. DOI:10.1016/j.febslet.2010.05.010 . |
| [84] | RULIFSON E J, KIM S K, NUSSE R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes[J]. Science, 2002, 296(5570):1118-1120. DOI:10.1126/science. 1070058 . |
| [85] | ZHANG H, LIU J N, LI C R, et al. Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities[J]. Proc Natl Acad Sci USA, 2009, 106(46):19617-19622. DOI:10.1073/pnas.0905083106 . |
| [86] | HASELTON A, SHARMIN E, SCHRADER J, et al. Partial ablation of adult Drosophila insulin-producing neurons modulates glucose homeostasis and extends life span without insulin resistance[J]. Cell Cycle, 2010, 9(15):3063-3071. DOI:10.4161/cc.9.15.12458 . |
| [87] | SOUIDI A, JAGLA K. Drosophila Heart as a model for cardiac development and diseases[J]. Cells, 2021, 10(11):3078. DOI:10.3390/cells10113078 . |
| [88] | BIRSE R T, CHOI J, REARDON K, et al. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila [J]. Cell Metab, 2010, 12(5):533-544. DOI:10.1016/j.cmet.2010.09.014 . |
| [89] | DIOP S B, BODMER R. Drosophila as a model to study the genetic mechanisms of obesity-associated heart dysfunction[J]. J Cell Mol Med, 2012, 16(5):966-971. DOI:10.1111/j.1582-4934.2012.01522.x . |
| [90] | LIM H Y, WANG W D, WESSELLS R J, et al. Phospholipid homeostasis regulates lipid metabolism and cardiac function through SREBP signaling in Drosophila [J]. Genes Dev, 2011, 25(2):189-200. DOI:10.1101/gad.1992411 . |
| [91] | GERSHMAN B, PUIG O, HANG L L, et al. High-resolution dynamics of the transcriptional response to nutrition in Drosophila: a key role for dFOXO[J]. Physiol Genomics, 2007, 29(1):24-34. DOI:10.1152/physiolgenomics.00061.2006 . |
| [92] | HANAHAN D, WEINBERG R A. Hallmarks of cancer: the next generation[J]. Cell, 2011, 144(5):646-674. DOI:10.1016/j.cell. 2011.02.013 . |
| [93] | JOHNSTON D ST. The art and design of genetic screens: Drosophila melanogaster [J]. Nat Rev Genet, 2002, 3(3):176-188. DOI:10.1038/nrg751 . |
| [94] | LEE T, LUO L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis[J]. Neuron, 1999, 22(3):451-461. DOI:10.1016/s0896-6273(00)80701-1 . |
| [95] | BRUMBY A M, RICHARDSON H E. Scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila [J]. EMBO J, 2003, 22(21):5769-5779. DOI:10.1093/emboj/cdg548 . |
| [96] | PAGLIARINI R A, XU T. A genetic screen in Drosophila for metastatic behavior[J]. Science, 2003, 302(5648):1227-1231. DOI:10.1126/science.1088474 . |
| [97] | WU M, PASTOR-PAREJA J C, XU T. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion[J]. Nature, 2010, 463(7280):545-548. DOI:10.1038/nature08702 . |
| [98] | DILLARD C, REIS J G T, RUSTEN T E. RasV12; scrib–/– tumors: a cooperative oncogenesis model fueled by tumor/host interactions[J]. Int J Mol Sci, 2021, 22(16):8873. DOI:10.3390/ijms22168873 . |
| [99] | BILDER D, ONG K, HSI T C, et al. Tumour-host interactions through the lens of Drosophila [J]. Nat Rev Cancer, 2021, 21(11):687-700. DOI:10.1038/s41568-021-00387-5 . |
| [100] | LEE J H, BASSEL-DUBY R, OLSON E N. Heart- and muscle-derived signaling system dependent on MED13 and Wingless controls obesity in Drosophila [J]. Proc Natl Acad Sci USA, 2014, 111(26):9491-9496. DOI:10.1073/pnas.1409427111 . |
| [101] | HUANG J B, WU S A, BARRERA J, et al. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP[J]. Cell, 2005, 122(3):421-434. DOI:10.1016/j.cell.20 05.06.007 . |
| [102] | GILLOOLY J F, GOMEZ J P, MAVRODIEV E V. A broad-scale comparison of aerobic activity levels in vertebrates: endotherms versus ectotherms[J]. Proc R Soc B, 2017, 284(1849):20162328. DOI:10.1098/rspb.2016.2328 . |
| [103] | WEHR M C, HOLDER M V, GAILITE I, et al. Salt-inducible kinases regulate growth through the Hippo signalling pathway in Drosophila [J]. Nat Cell Biol, 2013, 15(1):61-71. DOI:10.1038/ncb2658 . |
| [1] | 王晗玥, 陈嘉玮, 高湘滨, 罗威, 刘素宁. 黑腹果蝇心侧体功能的研究概述[J]. 实验动物与比较医学, 2025, 45(6): 705-718. |
| [2] | 王明珠, 高英豪, 谭霜霜, 吴薇. UAS-Irk3-EGFP转基因果蝇品系的构建与鉴定[J]. 实验动物与比较医学, 2025, 45(6): 656-662. |
| [3] | 王也, 王露. 黑腹果蝇转座子的特性、调控及其在基因组进化中的作用[J]. 实验动物与比较医学, 2025, 45(6): 676-687. |
| [4] | 邓贤铭, 汪菲. 果蝇电子显微镜连接组数据库及相关神经环路功能解析的研究进展[J]. 实验动物与比较医学, 2025, 45(6): 663-675. |
| [5] | 罗一凡, 张臻玮, 梅璐, 史叶萍, 邢艺彤, 张泽奇, 李楚欣, 韩春霞, 杨平顺, 陈秋生. 特络细胞介导肥胖症大鼠膏摩寡肽中草药复合制剂的减肥作用及其机制[J]. 实验动物与比较医学, 2025, 45(5): 551-560. |
| [6] | 韦炎冶, 申果, 张鹏飞, 石松平, 胡家豪, 张绪哲, 花慧源, 花冠洋, 陆宏正, 曾勇, 季风, 韦祝梅. 肥胖食蟹猴一般身体指标、血糖、血脂的动态监测及相关性分析[J]. 实验动物与比较医学, 2025, 45(1): 30-36. |
| [7] | 徐龙梅, 沈如凌, 范春, 吴薇. 基于ΦC31整合酶和载体质粒pUASTattB的12株果蝇转基因阴性对照品系的建立[J]. 实验动物与比较医学, 2023, 43(5): 541-547. |
| [8] | 李晗, 张笑瑞, 张成芳. 间歇禁食法在改善奥氮平诱导小鼠代谢紊乱中的机制研究[J]. 实验动物与比较医学, 2023, 43(1): 3-10. |
| [9] | 吕晓君, 吴森, 张菊, 徐小玲, 潘望平, 李厚钢, 王平慧, 何开勇. 用高热量饲料建立大鼠肥胖模型的实验研究[J]. 实验动物与比较医学, 2020, 40(5): 374-. |
| [10] | 邸亚男, 朱丽英, 钱雯, 潘卫. 斑马鱼作为模式动物在人类眼睛疾病研究中的应用[J]. 实验动物与比较医学, 2020, 40(5): 440-. |
| [11] | 唐润东, 宋思远, 吴薇. 饮食中糖分控制对雌黑腹果蝇寿命和中肠干细胞的影响[J]. 实验动物与比较医学, 2019, 39(2): 118-123. |
| [12] | 盛译萱, 曾国威, 姚亮亮, 李冰涛, 姜丽, 张启云, 徐国良. 肥胖大鼠的胰岛素抵抗指数与血脂的相关性观测[J]. 实验动物与比较医学, 2018, 38(6): 417-421. |
| [13] | 池骏, 龚慧, 何玥炜, 万颖寒, 费俭, 匡颖. 瘦素基因敲除小鼠模型的建立及表型分析[J]. 实验动物与比较医学, 2017, 37(5): 344-351. |
| [14] | 张云丽, 娄淑杰. 高脂膳食和跑台运动对雄性大鼠腓肠肌腺苷酸活化蛋白激酶 /乙酰辅酶A羧化酶信号通路和膜蛋白脂肪酸转位酶蛋白含量的影响[J]. 实验动物与比较医学, 2015, 35(6): 441-447. |
| [15] | 韦祝梅, 杨波, 李振明, 韩银华, 苏科龙, 杨继红. 肥胖及糖尿病食蟹猴全天血糖、胰岛素值及相关生理指标观测[J]. 实验动物与比较医学, 2015, 35(5): 394-397. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

