













实验动物与比较医学 ›› 2023, Vol. 43 ›› Issue (1): 21-29.DOI: 10.12300/j.issn.1674-5817.2022.128
收稿日期:2022-08-17
修回日期:2022-10-30
出版日期:2023-02-25
发布日期:2023-02-25
通讯作者:
崔立(1971—),女,博士,研究员,研究方向:人兽共患病与比较医学。E-mail: lcui@sjtu.edu.cn。ORCID: 0000-0001-5202-9671;作者简介:杨 平(1986—),男,硕士研究生,执业兽医师,研究方向:临床前药物药理药效评价。E-mail:pingyang@sjtu.edu.cn
基金资助:
Ping YANG1, Li CUI1( )(
)( ), Cheng YU2, Zhiyue WEN1
), Cheng YU2, Zhiyue WEN1
Received:2022-08-17
Revised:2022-10-30
Published:2023-02-25
Online:2023-02-25
Contact:
CUI Li (ORCID: 0000-0001-5202-9671), E-mail: lcui@sjtu.edu.cn摘要:
目的 通过观察贝伐珠单抗注射液对食蟹猴背部皮肤伤口愈合速度及损伤皮肤组织中CD34表达的影响,验证贝伐珠单抗注射液对食蟹猴伤口愈合的延迟作用,为临床肿瘤手术患者使用贝伐珠单抗治疗时提供剂量和频次的用药参考。 方法 6只雄性食蟹猴采用背部全层皮肤切除术建立伤口愈合评估模型,随机选取3只食蟹猴作为生理盐水组,其余3只食蟹猴为贝伐珠单抗组。贝伐珠单抗组食蟹猴在手术后第0、4、8、11天静脉注射30 mg/kg 贝伐珠单抗注射液,生理盐水组静脉注射等量生理盐水;术后进行血常规检测,并观察各组食蟹猴的伤口愈合情况,Image J软件分析伤口愈合率,用评分法对伤口状态的严重程度进行评价。4周后对6只食蟹猴伤口愈合皮肤取材,通过免疫组织化学法检查皮肤组织中CD34的表达水平。 结果 成功构建食蟹猴伤口愈合评估模型。术后第3天的食蟹猴血液中白细胞、中性粒细胞数量明显升高(P<0.05),第7天后白细胞数量逐渐恢复正常,而红细胞、血红蛋白、红细胞压积没有明显变化,提示动物营养状态良好。与生理盐水组相比,贝伐珠单抗组的伤口愈合率在术后7 d、28 d明显降低(P<0.01,P<0.001),愈合皮肤组织中CD34表达水平明显降低(P<0.01)。 结论 贝伐珠单抗注射给药可能通过抑制食蟹猴伤口新生皮肤组织中的微血管生成,延缓伤口愈合。临床中患者手术后使用贝伐珠单抗注射液时应该权衡利弊,科学合理地选择干预时间点、剂量、频次。
中图分类号:
杨平,崔立,俞诚,等. 贝伐珠单抗注射液对食蟹猴皮肤伤口愈合的影响[J]. 实验动物与比较医学, 2023, 43(1): 21-29. DOI: 10.12300/j.issn.1674-5817.2022.128.
Ping YANG,Li CUI,Cheng YU,et al. Effects of Bevacizumab Injection on the Skin Wound Healing in Cynomolgus Monkeys[J]. Laboratory Animal and Comparative Medicine, 2023, 43(1): 21-29. DOI: 10.12300/j.issn.1674-5817.2022.128.
| 项目 Item | 得分Score | 描述 Description |
|---|---|---|
伤口周围的皮肤颜色(距离伤口2 cm) Skin color around the wound (2 cm from the wound) | 0 | 正常颜色或粉红色 |
| 1 | 淡红色或有压之褪色的发红 | |
| 2 | 白色或灰白色 | |
| 3 | 深红色或压之不褪色的发红 | |
| 4 | 黑色或色素沉着过度 | |
伤口周围组织水肿 Edema in wound surrounding tissue | 0 | 无水肿或肿胀 |
| 1 | 伤口周围非凹陷水肿范围<2 cm | |
| 2 | 伤口周围非凹陷水肿范围≥2 cm | |
| 3 | 伤口周围凹陷水肿范围<2 cm | |
| 4 | 伤口周围凹陷水肿范围≥2 cm | |
渗出液类型 Type of exudate | 0 | 无渗出液 |
| 1 | 红色 | |
| 2 | 粉红色 | |
| 3 | 薄而透明,水性 | |
| 4 | 黄色或绿色,气味难闻 | |
渗出量 Exudate amount | 0 | 无渗出液 |
| 1 | 伤口组织微湿,但无法测量 | |
| 2 | 伤口组织湿润,浸泡<25%的敷料 | |
| 3 | 伤口组织湿润,浸泡敷料的25%~75% | |
| 4 | 伤口组织湿润,浸泡>75%的敷料 |
表1 食蟹猴背部伤口评分表
Table 1 The back wound score in cynomolgus monkeys
| 项目 Item | 得分Score | 描述 Description |
|---|---|---|
伤口周围的皮肤颜色(距离伤口2 cm) Skin color around the wound (2 cm from the wound) | 0 | 正常颜色或粉红色 |
| 1 | 淡红色或有压之褪色的发红 | |
| 2 | 白色或灰白色 | |
| 3 | 深红色或压之不褪色的发红 | |
| 4 | 黑色或色素沉着过度 | |
伤口周围组织水肿 Edema in wound surrounding tissue | 0 | 无水肿或肿胀 |
| 1 | 伤口周围非凹陷水肿范围<2 cm | |
| 2 | 伤口周围非凹陷水肿范围≥2 cm | |
| 3 | 伤口周围凹陷水肿范围<2 cm | |
| 4 | 伤口周围凹陷水肿范围≥2 cm | |
渗出液类型 Type of exudate | 0 | 无渗出液 |
| 1 | 红色 | |
| 2 | 粉红色 | |
| 3 | 薄而透明,水性 | |
| 4 | 黄色或绿色,气味难闻 | |
渗出量 Exudate amount | 0 | 无渗出液 |
| 1 | 伤口组织微湿,但无法测量 | |
| 2 | 伤口组织湿润,浸泡<25%的敷料 | |
| 3 | 伤口组织湿润,浸泡敷料的25%~75% | |
| 4 | 伤口组织湿润,浸泡>75%的敷料 |
指标及组别 Index and group | 手术后时间 Time after surgery/d | ||||
|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 28 | |
| WBC/(109·L-1) | |||||
| 生理盐水组 Saline group | 13.20±0.08 | 31.00±1.05△ | 14.65±0.10 | 15.64±0.08 | 18.70±0.69 |
| 贝伐珠单抗组 Bevacizumab group | 12.15±0.09 | 25.25±0.08△ | 13.48±0.04 | 15.72±0.50 | 17.14±0.23 |
| RBC/(1012·L-1) | |||||
| 生理盐水组 Saline group | 5.80±0.21 | 6.01±0.48 | 6.22±0.13 | 5.88±0.35 | 5.99±0.34 |
| 贝伐珠单抗组Bevacizumab group | 6.10±0.16 | 5.84±0.43 | 5.97±0.15 | 5.72±0.11 | 5.84±0.18 |
| NEU/% | |||||
| 生理盐水组 Saline group | 44.03±2.23 | 81.80±3.98△ | 50.97±4.83 | 61.57±6.75 | 60.73±17.91 |
| 贝伐珠单抗组 Bevacizumab group | 59.67±4.58 | 66.50±10.38 | 61.6±4.85 | 58.57±7.83 | 74.40±4.97 |
| NEU/(109·L-1) | |||||
| 生理盐水组 Saline group | 5.81±0.30 | 25.41±1.93△△ | 7.46±0.66 | 9.61±1.01 | 11.53±3.54 |
| 贝伐珠单抗组 Bevacizumab group | 7.26±0.59 | 16.80±2.62△ | 8.31±0.66 | 9.25±1.39 | 12.74±0.83 |
| LYM/% | |||||
| 生理盐水组 Saline group | 50.37±2.92 | 14.53±3.75 | 43.17±3.76 | 30.97±6.64 | 30.37±14.77 |
| 贝伐珠单抗组 Bevacizumab group | 33.30±4.35 | 28.43±9.75*△ | 33.93±5.04 | 34.53±7.23 | 20.30±4.66 |
| LYM/(109·L-1) | |||||
| 生理盐水组 Saline group | 6.65±0.38 | 4.44±1.08 | 6.33±0.59 | 4.86±1.06 | 5.52±2.52 |
| 贝伐珠单抗组 Bevacizumab group | 4.05±0.52 | 7.18±2.48 | 4.58±0.68 | 5.40±1.05 | 3.48±0.78 |
| HG1B ρ/(g·L-1) | |||||
| 生理盐水组 Saline group | 140.00±3.06 | 147.00±7.00 | 149.33±5.84 | 144.33±7.17 | 139.00±3.61 |
| 贝伐珠单抗组 Bevacizumab group | 150.00±5.57 | 142.33±7.06 | 142.67±7.84 | 140.67±2.73 | 140.33±3.84 |
| HCT/% | |||||
| 生理盐水组 Saline group | 45.93±1.13 | 47.27±2.20 | 47.93±2.15 | 45.73±2.22 | 44.97±1.90 |
| 贝伐珠单抗组 Bevacizumab group | 48.70±1.65 | 45.40±2.39 | 45.93±2.05 | 45.87±1.22 | 45.30±1.56 |
| EOS/% | |||||
| 生理盐水组 Saline group | 0.87±0.29 | 0.43±0.34 | 1.23±0.29 | 2.13±0.95 | 3.50±3.06 |
| 贝伐珠单抗组Bevacizumab group | 0.83±0.38 | 0.47±0.20 | 0.27±0.03 | 1.57±0.75 | 0.73±0.35 |
| EOS/(109·L-1) | |||||
| 生理盐水组 Saline group | 0.12±0.04 | 0.14±0.10 | 0.18±0.04 | 0.33±0.15 | 0.62±0.54 |
| 贝伐珠单抗组 Bevacizumab group | 0.10±0.05 | 0.11±0.05 | 0.03±0.01 | 0.24±0.11 | 0.13±0.06 |
表2 食蟹猴血常规检测结果
Table 2 Blood routine examination results of cynomolgus monkeys
指标及组别 Index and group | 手术后时间 Time after surgery/d | ||||
|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 28 | |
| WBC/(109·L-1) | |||||
| 生理盐水组 Saline group | 13.20±0.08 | 31.00±1.05△ | 14.65±0.10 | 15.64±0.08 | 18.70±0.69 |
| 贝伐珠单抗组 Bevacizumab group | 12.15±0.09 | 25.25±0.08△ | 13.48±0.04 | 15.72±0.50 | 17.14±0.23 |
| RBC/(1012·L-1) | |||||
| 生理盐水组 Saline group | 5.80±0.21 | 6.01±0.48 | 6.22±0.13 | 5.88±0.35 | 5.99±0.34 |
| 贝伐珠单抗组Bevacizumab group | 6.10±0.16 | 5.84±0.43 | 5.97±0.15 | 5.72±0.11 | 5.84±0.18 |
| NEU/% | |||||
| 生理盐水组 Saline group | 44.03±2.23 | 81.80±3.98△ | 50.97±4.83 | 61.57±6.75 | 60.73±17.91 |
| 贝伐珠单抗组 Bevacizumab group | 59.67±4.58 | 66.50±10.38 | 61.6±4.85 | 58.57±7.83 | 74.40±4.97 |
| NEU/(109·L-1) | |||||
| 生理盐水组 Saline group | 5.81±0.30 | 25.41±1.93△△ | 7.46±0.66 | 9.61±1.01 | 11.53±3.54 |
| 贝伐珠单抗组 Bevacizumab group | 7.26±0.59 | 16.80±2.62△ | 8.31±0.66 | 9.25±1.39 | 12.74±0.83 |
| LYM/% | |||||
| 生理盐水组 Saline group | 50.37±2.92 | 14.53±3.75 | 43.17±3.76 | 30.97±6.64 | 30.37±14.77 |
| 贝伐珠单抗组 Bevacizumab group | 33.30±4.35 | 28.43±9.75*△ | 33.93±5.04 | 34.53±7.23 | 20.30±4.66 |
| LYM/(109·L-1) | |||||
| 生理盐水组 Saline group | 6.65±0.38 | 4.44±1.08 | 6.33±0.59 | 4.86±1.06 | 5.52±2.52 |
| 贝伐珠单抗组 Bevacizumab group | 4.05±0.52 | 7.18±2.48 | 4.58±0.68 | 5.40±1.05 | 3.48±0.78 |
| HG1B ρ/(g·L-1) | |||||
| 生理盐水组 Saline group | 140.00±3.06 | 147.00±7.00 | 149.33±5.84 | 144.33±7.17 | 139.00±3.61 |
| 贝伐珠单抗组 Bevacizumab group | 150.00±5.57 | 142.33±7.06 | 142.67±7.84 | 140.67±2.73 | 140.33±3.84 |
| HCT/% | |||||
| 生理盐水组 Saline group | 45.93±1.13 | 47.27±2.20 | 47.93±2.15 | 45.73±2.22 | 44.97±1.90 |
| 贝伐珠单抗组 Bevacizumab group | 48.70±1.65 | 45.40±2.39 | 45.93±2.05 | 45.87±1.22 | 45.30±1.56 |
| EOS/% | |||||
| 生理盐水组 Saline group | 0.87±0.29 | 0.43±0.34 | 1.23±0.29 | 2.13±0.95 | 3.50±3.06 |
| 贝伐珠单抗组Bevacizumab group | 0.83±0.38 | 0.47±0.20 | 0.27±0.03 | 1.57±0.75 | 0.73±0.35 |
| EOS/(109·L-1) | |||||
| 生理盐水组 Saline group | 0.12±0.04 | 0.14±0.10 | 0.18±0.04 | 0.33±0.15 | 0.62±0.54 |
| 贝伐珠单抗组 Bevacizumab group | 0.10±0.05 | 0.11±0.05 | 0.03±0.01 | 0.24±0.11 | 0.13±0.06 |
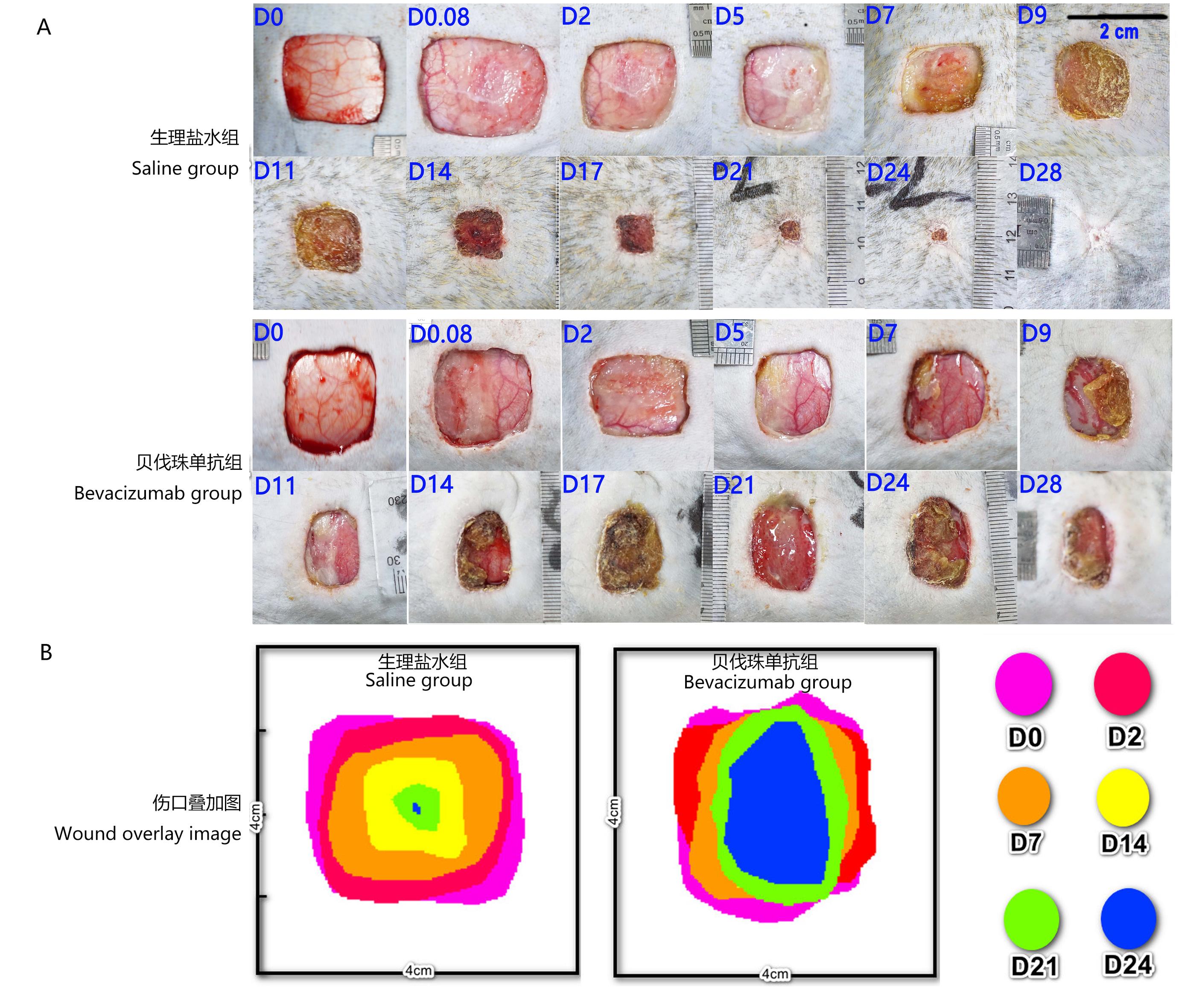
图 1 食蟹猴背部伤口状态注:A,生理盐水组与贝伐珠单抗组的代表性伤口,切除皮肤面积为4 cm×4 cm;B,生理盐水组与贝伐珠单抗组的伤口愈合叠加图,各时间点的叠加图以伤口中心4 cm×4 cm区域获得。D0、D0.08、D2、D5、D7、D9、D11、D14、D17、D21、D24、D28分别表示全层皮肤切除手术后即刻、第2小时,以及第2、5、7、9、11、14、17、21、24和28天。
Figure 1 The picture of back wound state in cynomolgus monkeysNote:A, Representative wound pictures in saline group and Bevacizumab group, the resection area of skin was 4 cm×4 cm; B, Wound healing overlap diagrams in saline group and Bevacizumab group, which the 4 cm×4 cm images of the wound center were obtained at each superimposed time point. D0, D0.08, D2, D5, D7, D9, D11, D14, D17, D21, D24, and D28 represent minute 2, hour 2, day 2, day 5, day 7, day 9, day 11, day 14, day 17, day 21, day 24 and day 28 after full-thickness skin resection, respectively.
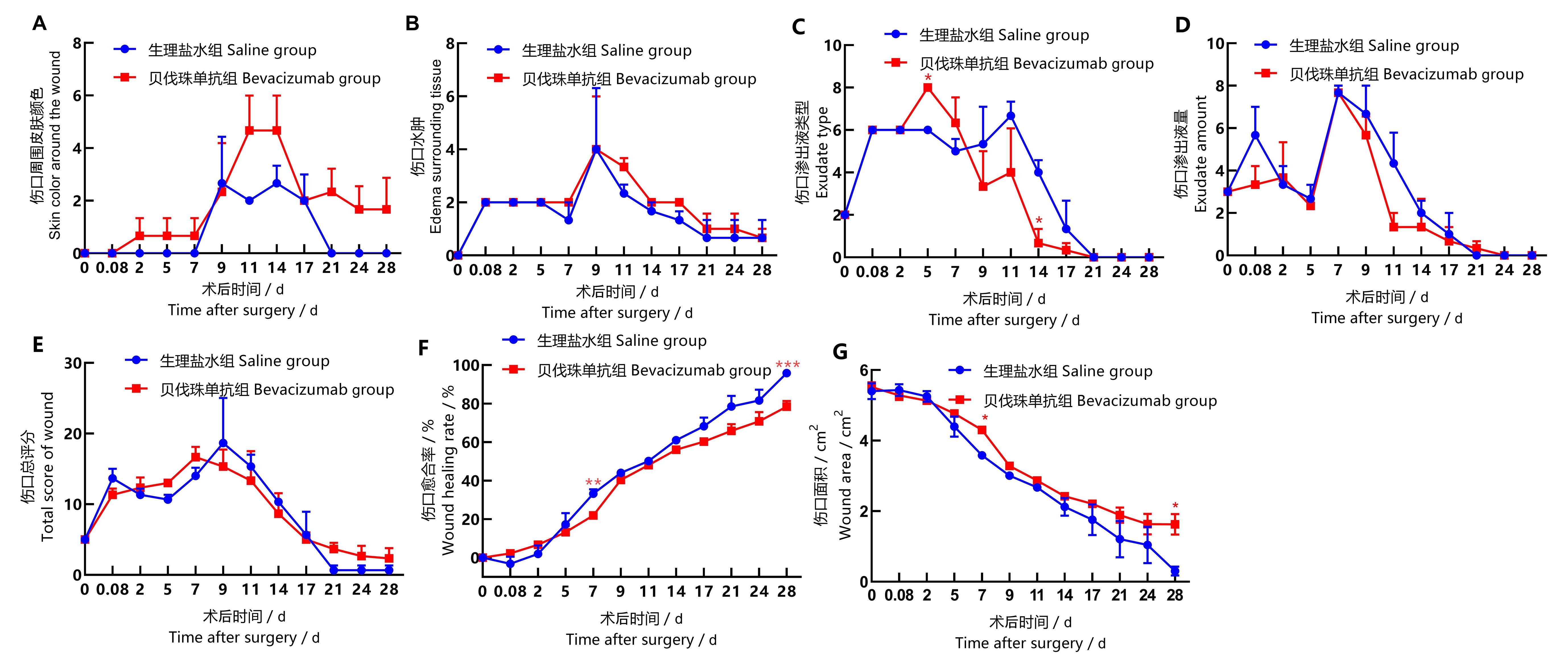
图2 食蟹猴背部伤口状态评分及愈合指标的统计图注:同一个时间点贝伐珠单抗组与生理盐水组比较,*P<0.05,**P<0.01,***P<0.001。每组3只食蟹猴(n=3)。
Figure 2 Statistical chart of back wound score and wound healing index in back of cynomolgus monkeysNote:T-test was used to compare Bevacizumab group with saline group at the same time point, *P<0.05, **P<0.01, ***P<0.001, n=3.
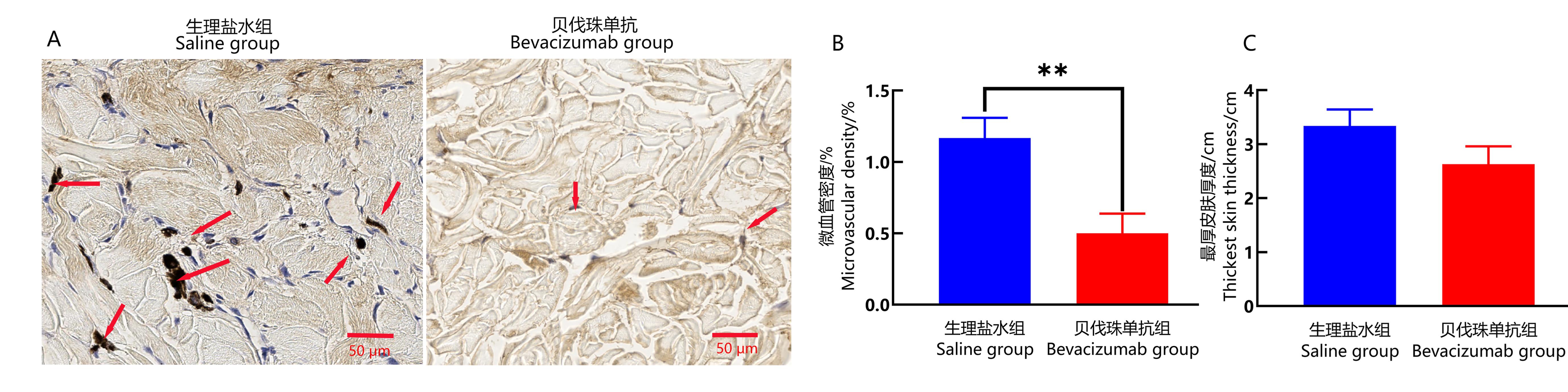
图3 食蟹猴伤口愈合部位皮肤组织中CD34免疫组织化学染色和皮肤厚度分析注:A为生理盐水组和贝伐珠单抗组伤口愈合皮肤组织中CD34免疫组织化学染色(DAB,×400),图中箭头指示微细血管内皮细胞内CD34被染色为棕黄色或者棕褐色颗粒,标尺为50 μm。B为两组伤口愈合皮肤组织中,CD34阳性表达的微血管面积占选区面积的百分比(即微血管密度)(**P<0.01, n=3)。C为两组新生皮肤最厚部位的厚度。
Figure 3 Immunohistochemical staining of CD34 and skin thickness analysis in the wound healing site of cynomolgus monkeysNote:A, CD34 immunohistochemical staining in the wound skin tissues of saline group and Bevacizumab group, showing skin characteristics of each group under 400× microscope with a scale bar of 50 μm. The arrow in the figure indicates that the CD34 in the cytoplasm of microvascular endothelial cells is stained with tan or brown particles.B, Comparison of percentage of microvessel area of CD34 positive expression (showing microvessel density) in the wound healing skin tissues between the two groups (**P<0.01, n=3). C, The thickness of the thickest part of newborn skin in the two groups.
| 1 | GUAN Z Z, XU J M, LUO R C, et al. Efficacy and safety of Bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: a randomized phaseⅢ ARTIST trial[J]. Chin J Cancer, 2011, 30(10):682-689. DOI:10.5732/cjc.011.10188 . |
| 2 | NARITA Y. Bevacizumab for glioblastoma[J]. Ther Clin Risk Manag, 2015:1759. DOI:10.2147/tcrm.s58289 . |
| 3 | MAK D Y, TJONG M C, LOUIE A V. Usage of radiotherapy with osimertinib plus Bevacizumab versus osimertinib for untreated patients with nonsquamous NSCLC Harboring EGFR mutations[J]. J Thoracic Oncol, 2023, 18(1): e3-e4. DOI:https://doi.org/10.1016/j.jtho.2022.10.010 . |
| 4 | 寿建忠, 马建辉. 肾癌的靶向治疗现状与进展[J]. 中国新药杂志, 2010, 19(17):1539-1546. |
| SHOU J Z, MA J H. Current status and advances in targeted therapy for renal cell carcinoma[J]. Chin J New Drugs, 2010, 19(17):1539-1546. | |
| 5 | 唐欣颖, 匡泽民. «接受贝伐珠单抗治疗的卵巢癌和宫颈癌患者血压管理专家共识»: 2019英国专家建议解读[J]. 中国合理用药探索, 2020, 17(1):11-15. |
| TANG X Y, KUANG Z M. Interpretation of 2019 UK expert recommendations on the management of hypertension in patients with ovarian and cervical cancer receiving Bevacizumab[J]. Chin J Ration Drug Use, 2020, 17(1):11-15. | |
| 6 | ZHANG H, HUANG Z, ZOU X, et al. Bevacizumab and wound-healing complications: A systematic review and meta-analysis of randomized controlled trials[J]. Oncotarget, 2016, 7(50): 82473-82481. DOI: 10.18632/oncotarget.12666 . |
| 7 | PRZEKORA A. A concise review on tissue engineered artificial skin grafts for chronic wound treatment: can we reconstruct functional skin tissue in vitro?[J]. Cells, 2020, 9(7):1622. DOI:10.3390/cells9071622 . |
| 8 | LAURENS N, KOOLWIJK P, DE MAAT M P M. Fibrin structure and wound healing[J]. J Thromb Haemost, 2006, 4(5):932-939. DOI:10.1111/j.1538-7836.2006.01861.x . |
| 9 | DE OLIVEIRA R C, WILSON S E. Fibrocytes, wound healing, and corneal fibrosis[J]. Invest Ophthalmol Vis Sci, 2020, 61(2):28. DOI:10.1167/iovs.61.2.28 . |
| 10 | URCIUOLO F, CASALE C, IMPARATO G, et al. Bioengineered skin substitutes: the role of extracellular matrix and vascularization in the healing of deep wounds[J]. J Clin Med, 2019, 8(12): E2083. DOI:10.3390/jcm8122083 . |
| 11 | VIG K, CHAUDHARI A, TRIPATHI S, et al. Advances in skin regeneration using tissue engineering[J]. Int J Mol Sci, 2017, 18(4): E789. DOI:10.3390/ijms18040789 . |
| 12 | HUGHES M R, CANALS HERNAEZ D, CAIT J, et al. A sticky wicket: defining molecular functions for CD34 in hematopoietic cells[J]. Exp Hematol, 2020, 86:1-14. DOI:10.1016/j.exphem.2020.05.004 . |
| 13 | KORNTNER S, LEHNER C, GEHWOLF R, et al. Limiting angiogenesis to modulate scar formation[J]. Adv Drug Deliv Rev, 2019, 146:170-189. DOI:10.1016/j.addr.2018.02.010 . |
| 14 | SAMI D G, HEIBA H H, ABDELLATIF A. Wound healing models: a systematic review of animal and non-animal models[J]. Wound Med, 2019, 24(1):8-17. DOI: 10.1016/j.wndm.2018.12.001 . |
| 15 | BYUN H S, LEE S J, LEE J I, et al. Effects of chitosan on wound healing in monkeys[J]. J Vet Clin, 2013, 30(4):241-246. |
| 16 | 中国药品评审中心. GPT1-1: 治疗用生物制品非临床安全性技术审评一般原则 [S]. 2007. |
| China Drug Review Center, GPT1-1: Guidelines for the non-clinical safety evaluation of therapeutic biological products [S]. 2007. | |
| 17 | 李劲锋, 于佳玥, 戴小宇, 等. 贝伐珠单抗类似药SMMU-13对食蟹猴的毒性[J]. 上海交通大学学报(农业科学版), 2018, 36(1):76-80. DOI:10.3969/J.ISSN.1671-9964.2018.01.013 . |
| LI J F, YU J Y, DAI X Y, et al. Toxicitystudy of SMMU-13, a biosimilar of Bevacizumab, in cynomolgus monkeys[J]. J Shanghai Jiao Tong Univ Agric Sci, 2018, 36(1):76-80. DOI:10.3969/J.ISSN.1671-9964.2018.01.013 . | |
| 18 | JIA Y, ZHAO G, JIA J. Preliminary evaluation: The effects of Aloe ferox Miller and Aloe arborescens Miller on wound healing[J]. J Ethnopharmacol, 2008, 120(2):181-189. DOI: 10.1016/j.jep.2008.08.008 . |
| 19 | APTE R S, CHEN D S, FERRARA N. VEGF in signaling and disease: Beyond discovery and development [J]. Cell, 2019, 176(6): 1248-1264. DOI:10.1016/j.cell.2019.01.021 . |
| 20 | GOLAS A R, BOYKO T, SCHWARTZ T H, et al. Prophylactic plastic surgery closure of neurosurgical scalp incisions reduces the incidence of wound complications in previously-operated patients treated with Bevacizumab (Avastin®) and radiation[J]. J Neurooncol, 2014, 119(2):327-331. DOI: 10.1007/s11060-014-1482-6 . |
| 21 | RIOS J E S, ALMEIDA F M, LIMONGI R M, et al. The effect of Bevacizumab, 5-fluorouracil, and triamcinolone on the healing modulation of surgical wounds in rats[J]. Histol Histopathol, 2023: 18583. DOI:10.14670/HH-18-583 . |
| 22 | ALMADANI Y H, VORSTENBOSCH J, DAVISON P G, et al. Wound healing: A comprehensive review[J]. Seminars in plastic surgery,2021, 35(3):141-144. DOI:10.1055/s-0041-1731791 . |
| 23 | 黄丽霞, 刘基华, 刘雪莹, 等. 贝伐珠单抗生物类似药与原研药疗效及不良反应的回顾性分析比较[J]. 中国处方药, 2022, 20(8):1-4. DOI: 10.3969/j.issn.1671-945X.2022.08.002 . |
| HUANG L X, LIU J H, LIU X Y, et al. Comparison of efficacy and adverse reactions between Bevacizumab biosimilar and the reference listed drug[J]. J China Prescr Drug, 2022, 20(8):1-4. DOI: 10.3969/j.issn.1671-945X.2022.08.002 . | |
| 24 | RECK M, WEHLER T, ORLANDI F, et al. Safety and patient-reported outcomes of atezolizumab plus chemotherapy with or without Bevacizumab versus Bevacizumab plus chemotherapy in non-small-cell lung cancer[J]. J Clin Oncol, 2020, 38(22):2530-2542. DOI:10.1200/jco.19.03158 . |
| 25 | FINN R S, QIN S, IKEDA M, et al. Atezolizumab plus Bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382(20):1894-1905. DOI:10.1056/nejmoa1915745 . |
| 26 | AHN J W, SHALABI D, CORREA-SELM L M, et al. Impaired wound healing secondary to Bevacizumab[J]. Int Wound J, 2019, 16(4):1009-1012. DOI:10.1111/iwj.13139 . |
| 27 | 冯芬, 胡斌, 招丽蓉, 等. 血清VEGF水平与贝伐珠单抗联合化疗治疗转移性结直肠癌患者疗效的关系研究[J]. 现代生物医学进展, 2017, 17(31):6136-6139. DOI:10.13241/j.cnki.pmb.2017.31.032.FENG F , |
| HU B, ZHAO L R, et al. Metastasis colorectal cancer: effects of serum VEGF and bevacizumab combined with chemotherapy[J]. Prog Mod Biomed, 2017, 17(31):6136-6139. DOI: 10.13241/j.cnki.pmb.2017.31.032 . | |
| 28 | 张万虎, 何亚茹, 宋瑜, 等. 康柏西普与贝伐单抗玻璃体腔注射治疗新生血管性老年性黄斑变性疗效对比观察[J]. 当代医学, 2018, 24(8):12-15. DOI:10.3969/j.issn.1009-4393.2018.08.004 . |
| ZHANG W H, HE Y R, SONG Y, et al. Efficacy of conbercept versus Bevacizumab in intramuscular injection for neovascular age-related macular degeneration[J]. Contemp Med, 2018, 24(8):12-15. DOI: 10.3969/j.issn.1009-4393.2018.08.004 . | |
| 29 | SIMONDS J, MILLER F, MANDEL J, et al. The effect of Bevacizumab (Avastin) treatment on epistaxis in hereditary hemorrhagic telangiectasia[J]. Laryngoscope, 2009, 119(5):988-992. DOI:10.1002/lary.20159 . |
| 30 | KWAK D H, BAE T H, KIM W S, et al. Anti-vascular endothelial growth factor (Bevacizumab) therapy reduces hypertrophic scar formation in a rabbit ear wounding model[J]. Arch Plast Surg, 2016, 43(6):491-497. DOI:10.5999/aps.2016.43.6.491 . |
| [1] | 王晨娟, 杨玲焰, 王立鹏, 孙雪萍, 李静文, 郭连香, 荣荣, 时长军. 2019年某实验猴养殖场食蟹猴犬瘟热暴发的诊断[J]. 实验动物与比较医学, 2025, 45(3): 360-367. |
| [2] | 韦炎冶, 申果, 张鹏飞, 石松平, 胡家豪, 张绪哲, 花慧源, 花冠洋, 陆宏正, 曾勇, 季风, 韦祝梅. 肥胖食蟹猴一般身体指标、血糖、血脂的动态监测及相关性分析[J]. 实验动物与比较医学, 2025, 45(1): 30-36. |
| [3] | 卞新彦, 陆勇, 王燕, 孙强. 食蟹猴分娩行为分析[J]. 实验动物与比较医学, 2023, 43(4): 355-362. |
| [4] | 韦祝梅, 申果, 李振明, 曾勇, 季风, 杨继红. 不同年龄段食蟹猴骨矿含量与骨密度测定分析[J]. 实验动物与比较医学, 2022, 42(5): 409-415. |
| [5] | 高仕平, 李锋, 查思凡. 高脂饮食诱导的非酒精性脂肪肝病食蟹猴模型[J]. 实验动物与比较医学, 2020, 40(2): 123-127. |
| [6] | 王成舟. 食蟹猴急性心肌梗死模型的建立及护理[J]. 实验动物与比较医学, 2019, 39(4): 314-318. |
| [7] | 李良顺, 潘世友, 支元芳, 白帅州, 孙建华, 宫丽崑, 任进. 一例食蟹猴肠套叠诊断与治疗[J]. 实验动物与比较医学, 2019, 39(3): 236-238. |
| [8] | 韦毅, 符明泰, 阳明, 黄卫宣, 蓝娇, 覃喜军, 玉宁. 正常成年雄性食蟹猴部分免疫功能指标观测[J]. 实验动物与比较医学, 2018, 38(3): 236-238. |
| [9] | 李伟, 金益, 邢正弘, 陈国强. 单笼与群居饲养对雌性食蟹猴情绪的影响[J]. 实验动物与比较医学, 2017, 37(1): 46-49. |
| [10] | 李伟, 金益, 柏熊, 王晓东, 邢正弘, 王校权. 单笼与群居饲养对雌性食蟹猴生理指标的影响[J]. 实验动物与比较医学, 2016, 36(5): 369-373. |
| [11] | 邢正弘. 湿帘降温原理及其在食蟹猴饲育设施中的初步应用[J]. 实验动物与比较医学, 2016, 36(2): 141-145. |
| [12] | 韦祝梅, 杨波, 李振明, 韩银华, 苏科龙, 杨继红. 肥胖及糖尿病食蟹猴全天血糖、胰岛素值及相关生理指标观测[J]. 实验动物与比较医学, 2015, 35(5): 394-397. |
| [13] | 杨波, 韦祝梅, 邓信宁, 何乃知, 李振明, 杨继红. 利用MRI检查建立食蟹猴腓肠肌体积检测方法[J]. 实验动物与比较医学, 2014, 34(4): 314-318. |
| [14] | 多海刚, 桑传兰, 陈子亮, 梁兴林, 李比海, 金石军, 饶军华. 食蟹猴麻疹胎传抗体及其仔猴初次接种麻疹疫苗免疫效果的研究[J]. 实验动物与比较医学, 2013, 33(5): 347-350. |
| [15] | 韦祝梅, 谢莉萍, 朱琳, 李振明, 梁福东, 杨继红. 双酚A和4-壬基酚对未成年雌性食蟹猴的生殖内分泌毒性作用[J]. 实验动物与比较医学, 2013, 33(5): 351-353. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||