
Laboratory Animal and Comparative Medicine ›› 2024, Vol. 44 ›› Issue (5): 531-542.DOI: 10.12300/j.issn.1674-5817.2024.031
• Animal Models of Human Diseases • Previous Articles Next Articles
QI Longju1( ), CHEN Shiyuan1,3, LIAO Zehua1,3, SHI Yuanhu1,3, SUN Yuyu1, WANG Qinghua2(
), CHEN Shiyuan1,3, LIAO Zehua1,3, SHI Yuanhu1,3, SUN Yuyu1, WANG Qinghua2( )(
)( )
)
Received:2024-02-27
Revised:2024-08-06
Online:2024-10-25
Published:2024-10-25
Contact:
WANG Qinghua
CLC Number:
QI Longju,CHEN Shiyuan,LIAO Zehua,et al. Transcriptomic Analysis of Menstrual Blood-Derived Stem Cells Transplantation Combined with Exercise Training in Promoting Spinal Cord Injury Recovery in Rats[J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 531-542. DOI: 10.12300/j.issn.1674-5817.2024.031.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2024.031
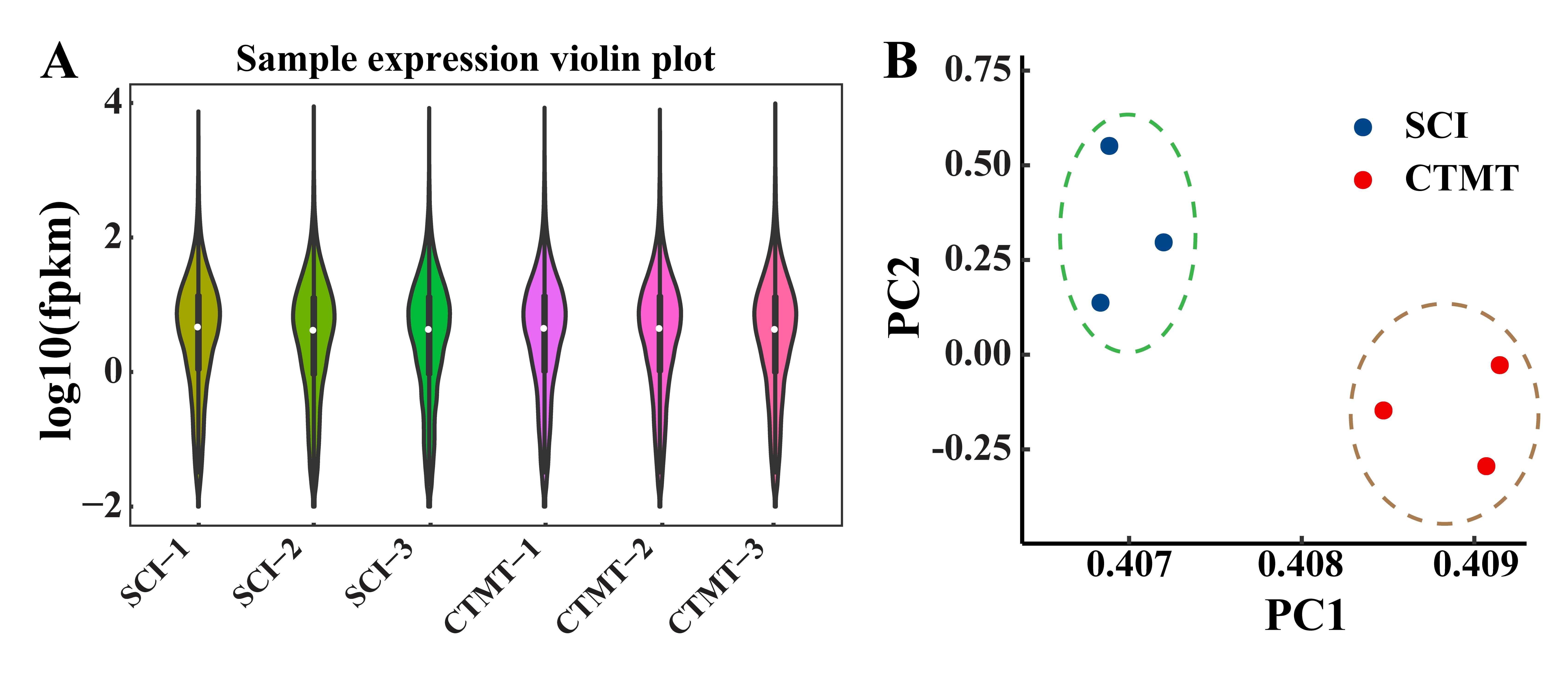
Figure 1 Distribution of gene expression and principal component analysis of spinal cord tissue samples in the two groups of ratsNote:A showed the distribution of sample expression levels (FPKM, fragments per kilobase of exon model per million mapped fragments); in the figure, the median, interquartile range and extreme values of the samples in SCI (spinal cord injury, as the control, n=3) group and the CTMT (the transplantation of menstrual blood-derived stem cells combined with treadmill training after SCI surgery, n=3) group were relatively close, which indicated that the sequencing results were more homogeneous within the group and the sequencing data quality was high. B showed the principal components analysis (PC1, principal component 1; PC2, principal component 2), and the samples from the same group were clustered together with close proximity, and the differences between samples mainly arose from intergroup differences.
表达上调基因 Up-regulated gene | log2(差异倍数) log2 (FoldChange) | t值 t value | P值 P value | 表达下调基因 Down-regulated gene | log2(差异倍数) log2 (FoldChange) | t值 t value | P值 P value | |
|---|---|---|---|---|---|---|---|---|
| Psme3 | 2.542 363 895 | 2.672 366 157 | 0.040 381 267 | Ptpdc1 | -0.941 169 616 | -2.823 151 331 | 0.033 418 310 | |
| Fgd2 | 1.870 409 798 | 5.277 683 881 | 0.002 476 179 | RT1-N2 | -0.961 937 408 | -3.508 946 559 | 0.014 746 432 | |
| Sdc4 | 1.591 559 898 | 2.530 079 050 | 0.048 412 378 | Cd79al | -1.007 349 648 | -3.292 831 274 | 0.018 934 378 | |
| Cthrc1 | 1.259 963 833 | 3.177 793 692 | 0.021 693 595 | Six4 | -1.071 889 332 | -15.482 205 520 | 0.000 009 930 | |
| RGD1302996 | 1.205 943 515 | 12.811 980 370 | 0.000 027 300 | Matn2 | -1.137 472 728 | -3.927 529 073 | 0.009 277 287 | |
| Rabepk | 1.201 118 420 | 2.962 815 910 | 0.028 125 989 | Clec4a3 | -1.273 541 156 | -2.512 270 495 | 0.049 532 946 | |
| Bdnf | 1.141 863 432 | 3.525 110 815 | 0.001 447 754 | AABR07051551.2 | -1.308 692 094 | -2.825 531 707 | 0.033 319 465 | |
| Pkig | 1.036 827 364 | 6.255 767 217 | 0.001 095 780 | Cmtm3 | -1.324 596 882 | -5.371 453 966 | 0.002 279 345 | |
| AABR07065925.1 | 0.970 924 295 | 6.151 591 228 | 0.001 189 396 | Dpy19l3 | -1.623 739 520 | -3.419 084 313 | 0.016 347 174 | |
| Ehmt1 | 0.944 670 184 | 6.578 534 619 | 0.000 855 702 | Cd74 | -2.034 312 055 | -2.712 776 259 | 0.038 372 308 | |
| AABR07062152.1 | 0.939 321 669 | 6.344 499 009 | 0.001 022 741 | Cxcl13 | -2.697 259 120 | -3.378 916 162 | 0.017 124 825 | |
| Ctbp1 | 0.898 198 041 | 5.961 511 123 | 0.001 385 227 | RGD1563231 | -3.151 695 504 | -2.603 665 774 | 0.044 063 014 | |
| Akr1b10 | 0.859 136 478 | 3.868 507 287 | 0.009 887 528 | Jchain | -3.390 915 739 | -2.827 200 269 | 0.033 250 368 | |
| Ssh1 | 0.818 982 469 | 5.813 203 431 | 0.001 564 263 | Ighm | -3.673 986 054 | -3.369 414 954 | 0.017 314 754 | |
| Piga | 0.785 438 853 | 3.303 450 635 | 0.018 700 031 | AABR07060872.1 | -5.052 108 514 | -3.355 872 330 | 0.017 589 546 |
Table 1 Significantly up-regulated and down-regulated genes in spinal cord tissues of spinal cord injury rats after transplantation of menstrual blood-derived stem cells combined with exercise training
表达上调基因 Up-regulated gene | log2(差异倍数) log2 (FoldChange) | t值 t value | P值 P value | 表达下调基因 Down-regulated gene | log2(差异倍数) log2 (FoldChange) | t值 t value | P值 P value | |
|---|---|---|---|---|---|---|---|---|
| Psme3 | 2.542 363 895 | 2.672 366 157 | 0.040 381 267 | Ptpdc1 | -0.941 169 616 | -2.823 151 331 | 0.033 418 310 | |
| Fgd2 | 1.870 409 798 | 5.277 683 881 | 0.002 476 179 | RT1-N2 | -0.961 937 408 | -3.508 946 559 | 0.014 746 432 | |
| Sdc4 | 1.591 559 898 | 2.530 079 050 | 0.048 412 378 | Cd79al | -1.007 349 648 | -3.292 831 274 | 0.018 934 378 | |
| Cthrc1 | 1.259 963 833 | 3.177 793 692 | 0.021 693 595 | Six4 | -1.071 889 332 | -15.482 205 520 | 0.000 009 930 | |
| RGD1302996 | 1.205 943 515 | 12.811 980 370 | 0.000 027 300 | Matn2 | -1.137 472 728 | -3.927 529 073 | 0.009 277 287 | |
| Rabepk | 1.201 118 420 | 2.962 815 910 | 0.028 125 989 | Clec4a3 | -1.273 541 156 | -2.512 270 495 | 0.049 532 946 | |
| Bdnf | 1.141 863 432 | 3.525 110 815 | 0.001 447 754 | AABR07051551.2 | -1.308 692 094 | -2.825 531 707 | 0.033 319 465 | |
| Pkig | 1.036 827 364 | 6.255 767 217 | 0.001 095 780 | Cmtm3 | -1.324 596 882 | -5.371 453 966 | 0.002 279 345 | |
| AABR07065925.1 | 0.970 924 295 | 6.151 591 228 | 0.001 189 396 | Dpy19l3 | -1.623 739 520 | -3.419 084 313 | 0.016 347 174 | |
| Ehmt1 | 0.944 670 184 | 6.578 534 619 | 0.000 855 702 | Cd74 | -2.034 312 055 | -2.712 776 259 | 0.038 372 308 | |
| AABR07062152.1 | 0.939 321 669 | 6.344 499 009 | 0.001 022 741 | Cxcl13 | -2.697 259 120 | -3.378 916 162 | 0.017 124 825 | |
| Ctbp1 | 0.898 198 041 | 5.961 511 123 | 0.001 385 227 | RGD1563231 | -3.151 695 504 | -2.603 665 774 | 0.044 063 014 | |
| Akr1b10 | 0.859 136 478 | 3.868 507 287 | 0.009 887 528 | Jchain | -3.390 915 739 | -2.827 200 269 | 0.033 250 368 | |
| Ssh1 | 0.818 982 469 | 5.813 203 431 | 0.001 564 263 | Ighm | -3.673 986 054 | -3.369 414 954 | 0.017 314 754 | |
| Piga | 0.785 438 853 | 3.303 450 635 | 0.018 700 031 | AABR07060872.1 | -5.052 108 514 | -3.355 872 330 | 0.017 589 546 |
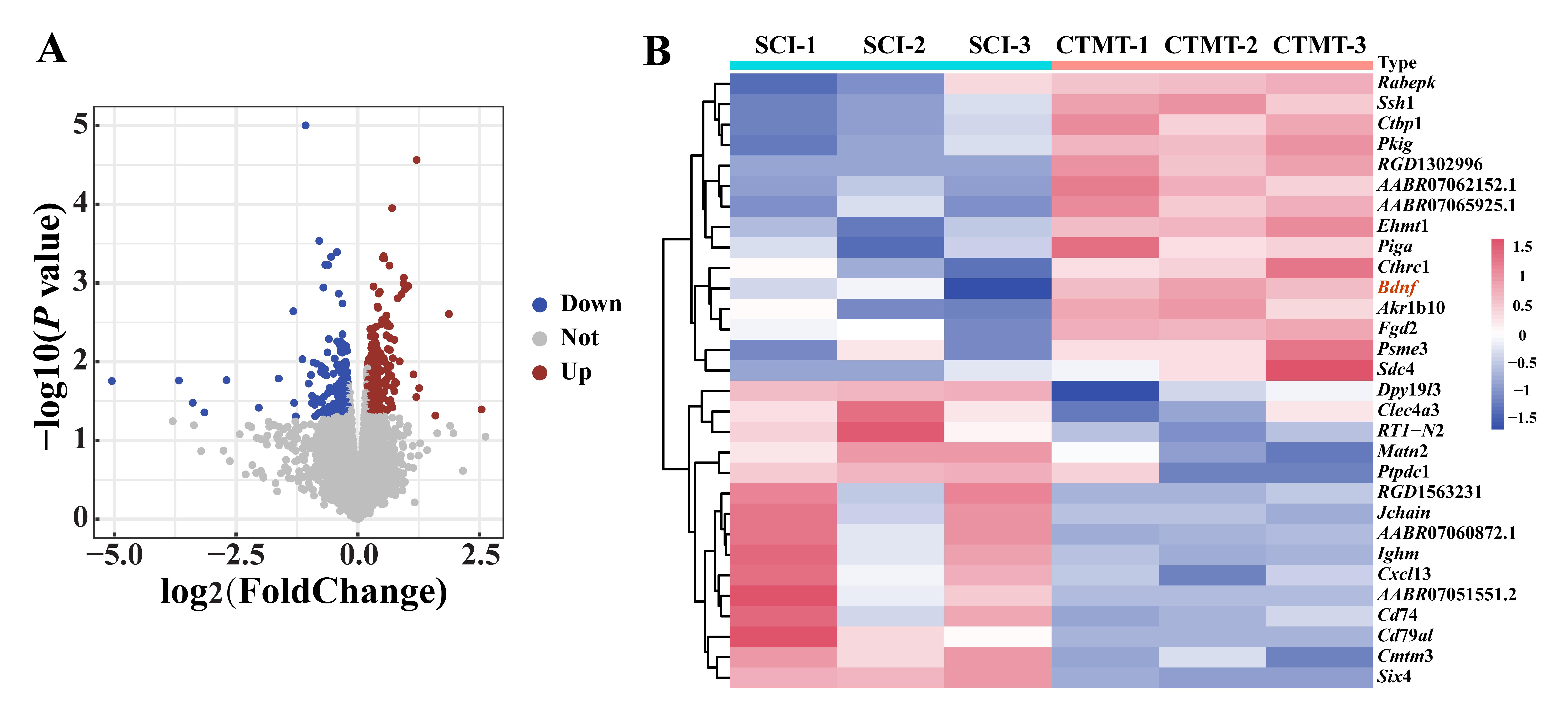
Figure 2 Volcano plot and clustered heatmap of differentially expressed genes in spinal cord tissues between the two groups of ratsNote: A, in volcano plot, horizontal axis indicated the fold change in the gene expression, the vertical axis indicated the statistical significance of differential gene expression profile, the red and blue dots indicated the upregulated and downregulated genes, respectively, and the gray dots indicated the non-DEGs; B, in clustered heatmap, the horizontal axis indicated the sample names, the vertical axis indicated gene names, the pink indicated highly expressed genes, and the blue indicated low expressed genes. SCI was the control group (spinal cord injury, as the control, n=3), and CTMT was the cell and treadmill training group (the transplantation of menstrual blood-derived stem cells combined with treadmill training after SCI surgery, n=3).
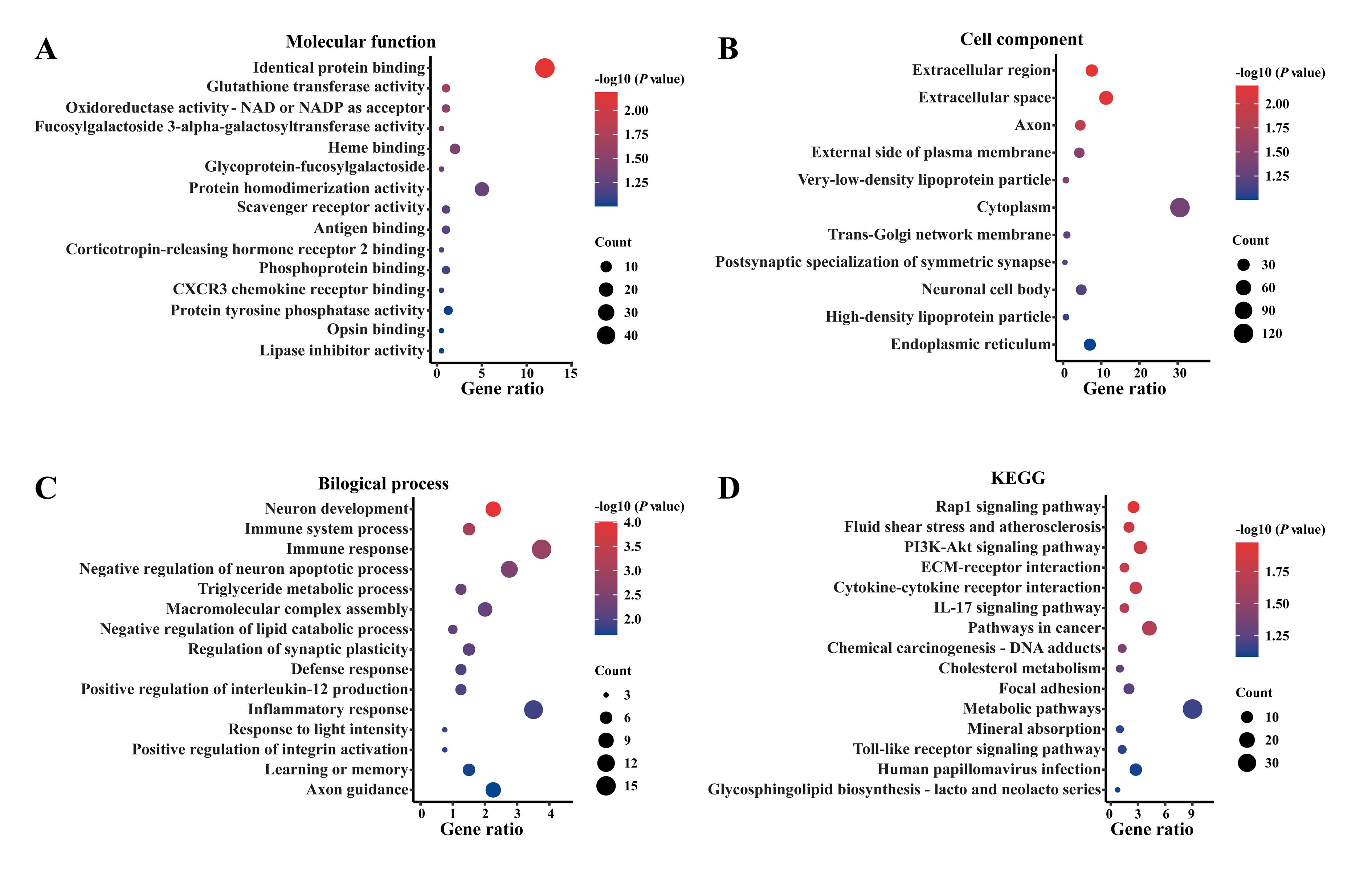
Figure 3 GO enrichment and KEGG pathway enrichment analyses of differentially expressed genes in spinal cord tissues of the two groups of ratsNote:A-C indicated the 3 analysis ontologies for Gene Ontology (GO) enrichment analysis, including molecular function (A), cellular components (B) and biological processes (C), respectively. D represented the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. The X-axis represented the fold enrichment, the Y-axis represented the enrichment items, and the bubble size indicated the number of genes enriched on each item. The larger the bubble, the greater the number of enriched genes. The color corresponded to P-value. The darker the red color, the more significant the difference.
信号通路名 Term of signal pathway | 计数 Count | 富集系数 Gene ratio | P值 P value | 基因名 Genes |
|---|---|---|---|---|
| Rap1 signaling pathway | 10 | 2.506 265 664 | 0.010 546 665 | Angpt1, Rassf5、Vegfb、Lpar1、Rapgef6、Efna4、Met、Bcar1、Rap1gap、Adcy6 |
Fluid shear stress and atherosclerosis | 8 | 2.005 012 531 | 0.014 625 259 | Prkaa1、Mef2c、Sdc4、Gsta4、Il1r2、Gstt3、Hmox1 |
PI3K-Akt signaling pathway | 13 | 3.258 145 363 | 0.014 714 234 | Prkaa1、Itga4、Angpt1、Bdnf、 Lama4、Lpar1、Vegfb、Ppp2r3b、Efna4、Gng7、Spp1、Col9a3、Met |
ECM-receptor interaction | 6 | 1.503 759 398 | 0.016 252 600 | Sdc4、Itga4、Rgd1565355、Lama4、Spp1、Col9a3 |
Cytokine-cytokine receptor interaction | 11 | 2.756 892 231 | 0.016 582 561 | Cxcl10、Cntf、Il25、Tnfsf15、Tnfrsf9、Il1r2、Il21r、Ccr7、Ltb、Cxcl13、Ccl17 |
| IL-17 signaling pathway | 6 | 1.503 759 398 | 0.020 227 194 | Cxcl10、Il25、Traf3、Mmp3、Lcn2、Ccl17 |
| Pathways in cancer | 17 | 4.260 651 629 | 0.020 758 687 | Ctbp1、Lama4、Lpar1、Vegfb、Gstt3、Adcy6、Dll4、Gsta4、Peg12、Traf3、Gng7、Rassf5、Ccdc6、Hmox1、E2f3、Met |
Chemical carcinogenesis- DNA adducts | 5 | 1.253 132 832 | 0.040 451 734 | Cyp3a9、Hpgds、Gsta4、Gstt3 |
| Cholesterol metabolism | 4 | 1.002 506 266 | 0.053 168 933 | Rgd1565355、Apoh、Apoc1、Apoc3 |
| Focal adhesion | 8 | 2.005 012 531 | 0.054 767 437 | Itga4、Lama4、Vegfb、Spp1、Col9a3、 Pip5k1c、Met、Bcar1 |
Metabolic pathway | 36 | 9.022 556 391 | 0.068 256 357 | Mtmr2、Gldc、Mtmr14、Pde3b、Gstt3、Hexd、Ehmt1、Adcy6、Abo、Selenbp1、Cyp3a9、Hpgds、Neu4、Spp1、Hmox1、St3gal6、Ca8、Pip5k1c、Csgalnact1、Car1、Uros、Qrsl1、 Coq7、Sirt3、Nt5c3b、Akr1b10、 Cyp2u1、Gsta4、Akr1c15、P4ha3、Piga、Cat、Acot1、Idnk |
| Mineral absorption | 4 | 1.002 506 266 | 0.072 932 376 | Atp7b、Slc40a1、Hmox1、Slc39a4 |
Toll-like receptor signaling pathway | 5 | 1.253 132 832 | 0.074 076 217 | Cxcl10、Irf3、Traf3、Spp1、Tlr3 |
Human papillomavirus infection | 11 | 2.756 892 231 | 0.079 092 324 | Irf3、Itga4、Traf3、Rt1-N2、Lama4、Spp1、Col9a3、Ppp2r3b、Rt1-T24-1、Tlr3、Llgl1 |
Glycosphingolipid biosynthesis | 3 | 0.751 879 699 | 0.081 761 102 | St3gal6、Abo |
Table 2 Results of KEGG pathway enrichment analysis of differentially expressed genes in spinal cord tissues of the two groups of rats
信号通路名 Term of signal pathway | 计数 Count | 富集系数 Gene ratio | P值 P value | 基因名 Genes |
|---|---|---|---|---|
| Rap1 signaling pathway | 10 | 2.506 265 664 | 0.010 546 665 | Angpt1, Rassf5、Vegfb、Lpar1、Rapgef6、Efna4、Met、Bcar1、Rap1gap、Adcy6 |
Fluid shear stress and atherosclerosis | 8 | 2.005 012 531 | 0.014 625 259 | Prkaa1、Mef2c、Sdc4、Gsta4、Il1r2、Gstt3、Hmox1 |
PI3K-Akt signaling pathway | 13 | 3.258 145 363 | 0.014 714 234 | Prkaa1、Itga4、Angpt1、Bdnf、 Lama4、Lpar1、Vegfb、Ppp2r3b、Efna4、Gng7、Spp1、Col9a3、Met |
ECM-receptor interaction | 6 | 1.503 759 398 | 0.016 252 600 | Sdc4、Itga4、Rgd1565355、Lama4、Spp1、Col9a3 |
Cytokine-cytokine receptor interaction | 11 | 2.756 892 231 | 0.016 582 561 | Cxcl10、Cntf、Il25、Tnfsf15、Tnfrsf9、Il1r2、Il21r、Ccr7、Ltb、Cxcl13、Ccl17 |
| IL-17 signaling pathway | 6 | 1.503 759 398 | 0.020 227 194 | Cxcl10、Il25、Traf3、Mmp3、Lcn2、Ccl17 |
| Pathways in cancer | 17 | 4.260 651 629 | 0.020 758 687 | Ctbp1、Lama4、Lpar1、Vegfb、Gstt3、Adcy6、Dll4、Gsta4、Peg12、Traf3、Gng7、Rassf5、Ccdc6、Hmox1、E2f3、Met |
Chemical carcinogenesis- DNA adducts | 5 | 1.253 132 832 | 0.040 451 734 | Cyp3a9、Hpgds、Gsta4、Gstt3 |
| Cholesterol metabolism | 4 | 1.002 506 266 | 0.053 168 933 | Rgd1565355、Apoh、Apoc1、Apoc3 |
| Focal adhesion | 8 | 2.005 012 531 | 0.054 767 437 | Itga4、Lama4、Vegfb、Spp1、Col9a3、 Pip5k1c、Met、Bcar1 |
Metabolic pathway | 36 | 9.022 556 391 | 0.068 256 357 | Mtmr2、Gldc、Mtmr14、Pde3b、Gstt3、Hexd、Ehmt1、Adcy6、Abo、Selenbp1、Cyp3a9、Hpgds、Neu4、Spp1、Hmox1、St3gal6、Ca8、Pip5k1c、Csgalnact1、Car1、Uros、Qrsl1、 Coq7、Sirt3、Nt5c3b、Akr1b10、 Cyp2u1、Gsta4、Akr1c15、P4ha3、Piga、Cat、Acot1、Idnk |
| Mineral absorption | 4 | 1.002 506 266 | 0.072 932 376 | Atp7b、Slc40a1、Hmox1、Slc39a4 |
Toll-like receptor signaling pathway | 5 | 1.253 132 832 | 0.074 076 217 | Cxcl10、Irf3、Traf3、Spp1、Tlr3 |
Human papillomavirus infection | 11 | 2.756 892 231 | 0.079 092 324 | Irf3、Itga4、Traf3、Rt1-N2、Lama4、Spp1、Col9a3、Ppp2r3b、Rt1-T24-1、Tlr3、Llgl1 |
Glycosphingolipid biosynthesis | 3 | 0.751 879 699 | 0.081 761 102 | St3gal6、Abo |
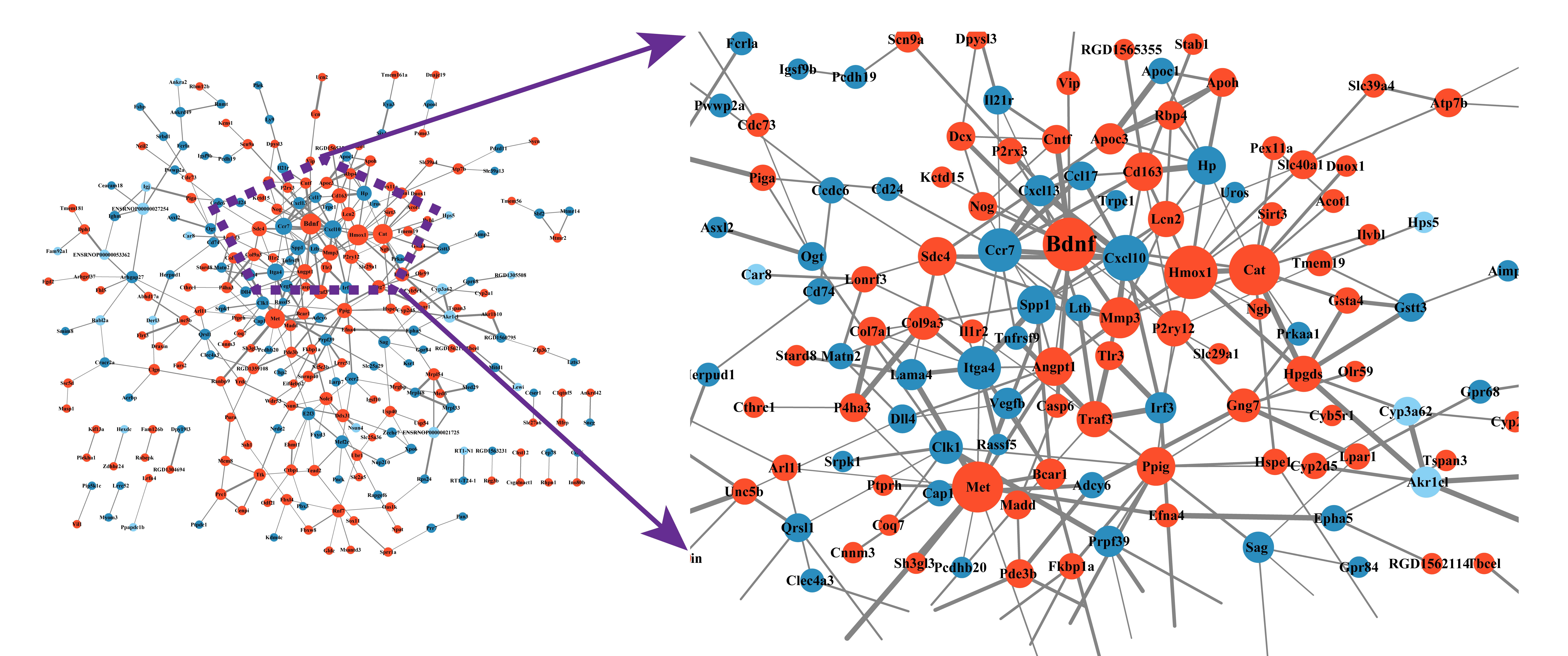
Figure 4 Protein-protein interaction network of differentially expressed genes in spinal cord tissues of the two groups of ratsNote:The size of the dots represented the node and degree, the node with more connections had a larger size; the red color in the dots represented the up-regulation of gene expression in this node, and the blue color represented the down-regulation of gene expression.
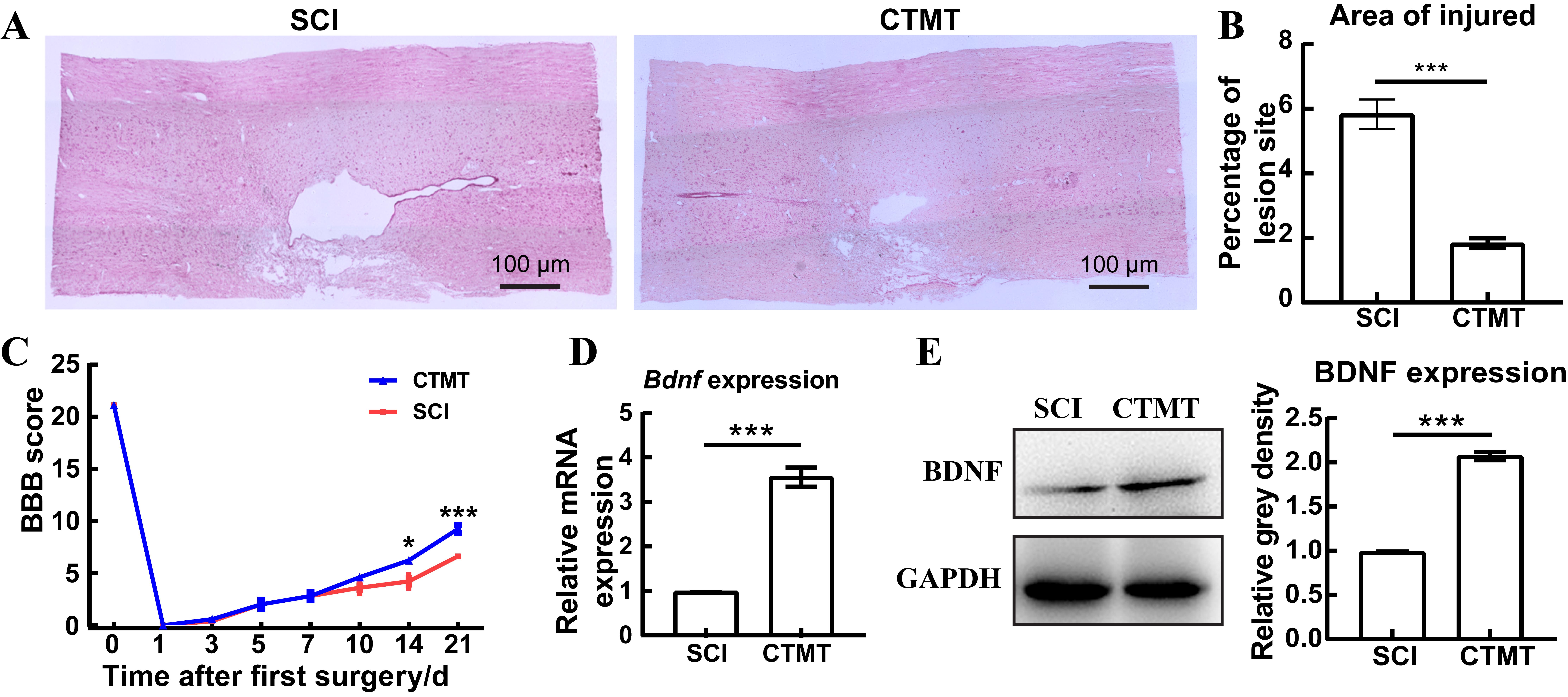
Figure 5 Menstrual blood-derived stem cells transplantation combined with treadmill training promotes motor function recovery and BDNF expression in spinal cord injury ratsNote:A showed the local structural pathological changes of the spinal cord injury in the SCI group (spinal cord injury, as the control, n=3) and the CTMT group (the transplantation of menstrual blood-derived stem cells combined with treadmill training after SCI surgery, n=3), observed by hematoxylin-eosin staining (magnified 40 times). B, statistical results of the lesion area in Figure A (***P<0.001, n=3). C, BBB motor score, a higher score indicated a better recovery of motor function. D, Real-time fluorescence quantitative PCR was performed to detect the changes in the transcriptional level of Bdnf gene in the injured spinal cord in rats of SCI and CTMT groups (***P<0.001, n=5). E, Western blotting analysis was performed to detect the changes in the protein level of BDNF in SCI and CTMT groups (***P<0.001, n=5).
| 1 | COURTINE G, SOFRONIEW M V. Spinal cord repair: advances in biology and technology[J]. Nat Med, 2019, 25(6):898-908. DOI: 10.1038/s41591-019-0475-6 . |
| 2 | O'SHEA T M, BURDA J E, SOFRONIEW M V. Cell biology of spinal cord injury and repair[J]. J Clin Invest, 2017, 127(9):3259-3270. DOI: 10.1172/JCI90608 . |
| 3 | ZHOU X, WAHANE S, FRIEDL M S, et al. Microglia and macrophages promote corralling, wound compaction and recovery after spinal cord injury via Plexin-B2[J]. Nat Neurosci, 2020, 23(3):337-350. DOI: 10.1038/s41593-020-0597-7 . |
| 4 | ASSINCK P, DUNCAN G J, HILTON B J, et al. Cell transplantation therapy for spinal cord injury[J]. Nat Neurosci, 2017, 20(5):637-647. DOI: 10.1038/nn.4541 . |
| 5 | JONES I, NOVIKOVA L N, NOVIKOV L N, et al. Regenerative effects of human embryonic stem cell-derived neural crest cells for treatment of peripheral nerve injury[J]. J Tissue Eng Regen Med, 2018, 12(4): e2099-e2109. DOI: 10.1002/term.2642 . |
| 6 | CAO S X, MA J, ZHANG J Y, et al. Reprogramming of one human induced pluripotent stem cell line from healthy donor[J]. Stem Cell Res, 2021, 57:102613. DOI: 10.1016/j.scr. 2021.102613 . |
| 7 | FENG P H, LI P P, TAN J C. Human menstrual blood-derived stromal cells promote recovery of premature ovarian insufficiency via regulating the ECM-dependent FAK/AKT signaling[J]. Stem Cell Rev Rep, 2019, 15(2):241-255. DOI: 10.1007/s12015-018-9867-0 . |
| 8 | LI Y, LI X N, ZHAO H X, et al. Efficient induction of pluripotent stem cells from menstrual blood[J]. Stem Cells Dev, 2013, 22(7):1147-1158. DOI: 10.1089/scd.2012.0428 . |
| 9 | SHIRIAN S, EBRAHIMI-BAROUGH S, SABERI H, et al. Comparison of capability of human bone marrow mesenchymal stem cells and endometrial stem cells to differentiate into motor neurons on electrospun poly(ε-caprolactone) scaffold[J]. Mol Neurobiol, 2016, 53(8):5278-5287. DOI: 10.1007/s12035-015-9442-5 . |
| 10 | ZEMEL'KO V I, KOZHUKHAROVA I V, KOVALEVA Z V, et al. [BDNF secretion in human mesenchymal stem cells isolated from bone marrow, endometrium and adipose tissue].[J]. Tsitologiia, 2014, 56(3):204-211. DOI: 10.1134/S1990519X1404 0129 . |
| 11 | WU Q F, WANG Q H, LI Z J, et al. Human menstrual blood-derived stem cells promote functional recovery in a rat spinal cord hemisection model[J]. Cell Death Dis, 2018, 9(9):882. DOI: 10.1038/s41419-018-0847-8 . |
| 12 | RONG Y L, LIU W, WANG J X, et al. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy[J]. Cell Death Dis, 2019, 10(5):340. DOI: 10.1038/s41419-019-1571-8 . |
| 13 | SHARIF K, WATAD A, BRAGAZZI N L, et al. Physical activity and autoimmune diseases: get moving and manage the disease[J]. Autoimmun Rev, 2018, 17(1):53-72. DOI: 10.1016/j.autrev.2017.11.010 . |
| 14 | BOOTH F W, ROBERTS C K, LAYE M J. Lack of exercise is a major cause of chronic diseases[J]. Compr Physiol, 2012, 2(2):1143-1211. DOI: 10.1002/cphy.c110025 . |
| 15 | ZBOGAR D, ENG J J, MILLER W C, et al. Physical activity outside of structured therapy during inpatient spinal cord injury rehabilitation[J]. J Neuroeng Rehabil, 2016, 13(1):99. DOI: 10.1186/s12984-016-0208-8 . |
| 16 | BEHRMAN A L, ARDOLINO E M, HARKEMA S J. Activity-based therapy: from basic science to clinical application for recovery after spinal cord injury[J]. J Neurol Phys Ther, 2017, 41 Suppl 3(Suppl 3 IV STEP Spec Iss): S39-S45. DOI: 10.1097/NPT.0000000000000184 . |
| 17 | UNGERLEIDER L G, DOYON J, KARNI A. Imaging brain plasticity during motor skill learning[J]. Neurobiol Learn Mem, 2002, 78(3):553-564. DOI: 10.1006/nlme.2002.4091 . |
| 18 | SUN T S, YE C Q, WU J, et al. Treadmill step training promotes spinal cord neural plasticity after incomplete spinal cord injury[J]. Neural Regen Res, 2013, 8(27):2540-2547. DOI: 10.3969/j.issn.1673-5374.2013.27.005 . |
| 19 | WANG H X, LIU N K, ZHANG Y P, et al. Treadmill training induced lumbar motoneuron dendritic plasticity and behavior recovery in adult rats after a thoracic contusive spinal cord injury[J]. Exp Neurol, 2015, 271:368-378. DOI: 10.1016/j.expneurol.2015.07.004 . |
| 20 | 殷睿安, 王双燕, 王培, 等. 重复经颅磁刺激叠加运动训练对脊髓损伤大鼠运动功能和神经元可塑性的影响[J]. 中国康复医学杂志, 2021, 36(7):774-778, 792. DOI: 10.3969/j.issn.1001-1242.2021.07.002 . |
| YIN R A, WANG S Y, WANG P, et al. Effects of combined rTMS and exercise training on locomotor function and neuronal plasticity in rats with spinal cord injury[J]. Chin J Rehabil Med, 2021, 36(7):774-778, 792. DOI: 10.3969/j.issn.1001-1242.2021.07.002 . | |
| 21 | HE W H, ZHANG X X, LI X Z, et al. A decellularized spinal cord extracellular matrix-gel/GelMA hydrogel three-dimensional composite scaffold promotes recovery from spinal cord injury via synergism with human menstrual blood-derived stem cells[J]. J Mater Chem B, 2022, 10(30):5753-5764. DOI: 10.1039/d2tb00792d . |
| 22 | HWANG D H, SHIN H Y, KWON M J, et al. Survival of neural stem cell grafts in the lesioned spinal cord is enhanced by a combination of treadmill locomotor training via insulin-like growth factor-1 signaling[J]. J Neurosci, 2014, 34(38):12788-12800. DOI: 10.1523/JNEUROSCI.5359-13.2014 . |
| 23 | DE MIRANDA L, LEVI R, DIVANOGLOU A. Tapping into the unimpossible: Philosophical health in lives with spinal cord injury[J]. J Eval Clin Pract, 2023, 29(7):1203-1210. DOI: 10.1111/jep.13874 . |
| 24 | WANG M H, CORDELL H J, VAN STEEN K. Statistical methods for genome-wide association studies[J]. Semin Cancer Biol, 2019, 55:53-60. DOI: 10.1016/j.semcancer. 2018.04.008 . |
| 25 | LI Y, CHEN Y, LI X, et al. RNA sequencing screening of differentially expressed genes after spinal cord injury[J]. Neural Regen Res, 2019, 14(9):1583-1593. DOI: 10.4103/1673-5374.255994 . |
| 26 | 唐丹, 王先斌, 杨香莲, 等. 跑台运动训练对脊髓损伤后大鼠肺损伤及HMGB1/TLR4/NF-κB信号通路表达的影响[J]. 中国康复医学杂志, 2023, 38(2):159-166. DOI: 10.3969/j.issn.1001-1242.2023.02.004 . |
| TANG D, WANG X B, YANG X L, et al. Effects of treadmill training on lung injury and HMGB1/TLR-4/NF-κB signaling pathway after spinal cord injury in rats[J]. Chin J Rehabil Med, 2023, 38(2):159-166. DOI: 10.3969/j.issn.1001-1242.2023.02.004 . | |
| 27 | MITRE M, MARIGA A, CHAO M V. Neurotrophin signalling: novel insights into mechanisms and pathophysiology[J]. Clin Sci (Lond), 2017, 131(1):13-23. DOI: 10.1042/CS20160044 . |
| 28 | WEISHAUPT N, BLESCH A, FOUAD K. BDNF: the career of a multifaceted neurotrophin in spinal cord injury[J]. Exp Neurol, 2012, 238(2):254-264. DOI: 10.1016/j.expneurol.2012.09.001 . |
| 29 | KEEFE K M, SHEIKH I S, SMITH G M. Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury[J]. Int J Mol Sci, 2017, 18(3):548. DOI: 10.3390/ijms18030548 . |
| 30 | WU Q F, CAO Y N, DONG C M, et al. Neuromuscular interaction is required for neurotrophins-mediated locomotor recovery following treadmill training in rat spinal cord injury[J]. PeerJ, 2016, 4: e2025. DOI: 10.7717/peerj.2025 . |
| 31 | HERNANDEZ-TORRES V, GRANSEE H M, MANTILLA C B, et al. BDNF effects on functional recovery across motor behaviors after cervical spinal cord injury[J]. J Neurophysiol, 2017, 117(2):537-544. DOI: 10.1152/jn.00654.2016 . |
| 32 | SAHELI M, KHORAMIPOUR K, VOSOUGH M, et al. Athletes' mesenchymal stem cells could be the best choice for cell therapy in Omicron-infected patients[J]. Cells, 2022, 11(12):1926. DOI: 10.3390/cells11121926 . |
| 33 | TAKAHASHI A, NAKAJIMA H, KUBOTA A, et al. Adipose-derived mesenchymal stromal cell transplantation for severe spinal cord injury: functional improvement supported by angiogenesis and neuroprotection[J]. Cells, 2023, 12(11):1470. DOI: 10.3390/cells12111470 . |
| 34 | WU R, GUO Y P, ZHANG L Y, et al. Physical exercise promotes integration of grafted cells and functional recovery in an acute stroke rat model[J]. Stem Cell Reports, 2022, 17(2):276-288. DOI: 10.1016/j.stemcr.2021.12.006 . |
| 35 | SUN X, HUANG L Y, PAN H X, et al. Bone marrow mesenchymal stem cells and exercise restore motor function following spinal cord injury by activating PI3K/AKT/mTOR pathway[J]. Neural Regen Res, 2023, 18(5):1067-1075. DOI: 10.4103/1673-5374.355762 . |
| [1] | QIN Chao, LI Shuangxing, ZHAO Tingting, JIANG Chenchen, ZHAO Jing, YANG Yanwei, LIN Zhi, WANG Sanlong, WEN Hairuo. Study on the 90-day Feeding Experimental Background Data of SD Rats for Drug Safety Evaluation [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 439-448. |
| [2] | LIU Liyu, JI Bo, LIU Xiaoxuan, FANG Yang, ZHANG Ling, GUO Tingting, QUAN Ye, LI Hewen, LIU Yitian. Exploration of Rat Fetal Lung Tissue Fixation Methods [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 432-438. |
| [3] | LIU Zhiwei, YANG Ran, LIAN Hao, ZHANG Yu, JIN Lilun. Cartilage Protection and Anti-Inflammatory Effects of Fraxetin on Monosodium Iodoacetate-Induced Rat Model of Osteoarthritis [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 259-268. |
| [4] | JIANG Meng, HAO Shulan, TONG Liguo, ZHONG Qiming, GAO Zhenfei, WANG Yonghui, WANG Xixing, JI Haijie. Dynamic Evaluation of Vinorelbine-Induced Phlebitis of Dorsalis Pedis Vein in a Rat Model [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 251-258. |
| [5] | PAN Yicong, JIANG Wenhong, HU Ming, QIN Xiao. Optimization of Surgical Procedure and Efficacy Evaluation of Aortic Calcification Model in Rats with Chronic Kidney Disease [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 279-289. |
| [6] | LIAN Hui, JIANG Yanling, LIU Jia, ZHANG Yuli, XIE Wei, XUE Xiaoou, LI Jian. Construction and Evaluation of a Rat Model of Abnormal Uterine Bleeding [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 130-146. |
| [7] | XU Qiuyu, YAN Guofeng, FU Li, FAN Wenhua, ZHOU Jing, ZHU Lian, QIU Shuwen, ZHANG Jie, WU Ling. A Mouse Model of Polycystic Ovary Syndrome Established Through Subcutaneous Administration of Letrozole Sustained-Release Pellets and Hepatic Transcriptome Analysis [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 119-129. |
| [8] | YIN Yulian, MA Lina, TU Siyuan, CHEN Ling, YE Meina, CHEN Hongfeng. Establishment and Evaluation of a Rat Model of Non-Puerperal Mastitis [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 587-596. |
| [9] | YANG Jin, YU Shiya, LIN Nan, FANG Yongchao, ZHAO Hu, QIU Jinwei, LIN Hongming, CHEN Huiyan, WANG Yu, WU Weihang. Effect of Modified Duodenal Exclusion Surgery on Glucose Metabolism in Rats with Type 2 Diabetes Mellitus [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 523-530. |
| [10] | ZHANG Naiqun, YUAN Piaopiao, CAO Linrong, YING Na, YANG Taotao. Application of PNR Detection in the Diagnosis and Drug-efficacy Evaluation of Diabetic Kidney Disease in Rats [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 543-549. |
| [11] | ZHENG Yiqing, DENG Yasheng, FAN Yanping, LIANG Tianwei, HUANG Hui, LIU Yonghui, NI Zhaobing, LIN Jiang. Application Analysis of Animal Models for Pelvic Inflammatory Disease Based on Data Mining [J]. Laboratory Animal and Comparative Medicine, 2024, 44(4): 405-418. |
| [12] | XIAO Pan, WANG Hongyi, LU Lu, ZHANG Mei, CHEN Keming, SHEN Dongshuai, NIU Tingxian. Screening of Hypoxia-Sensitive and Hypoxia-Tolerant Wistar Rats and Preliminary Exploration of Hypoxia Sensitivity in Their G1 Generation [J]. Laboratory Animal and Comparative Medicine, 2024, 44(4): 374-383. |
| [13] | Xiaoyu ZHU, Hantao YUAN, Sibo LI. MicroRNA-887-3p Inhibited MDM4 Expression and Proliferation but Promoted Apoptosis of Intervertebral Disc Annulus Fibrosus Cells in Rats [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 270-278. |
| [14] | Jinhua HU, Jingjie HAN, Min JIN, Bin HU, Yuefen LOU. Effects of Puerarin on Bone Density in Rats and Mice: A Meta-analysis [J]. Laboratory Animal and Comparative Medicine, 2024, 44(2): 149-161. |
| [15] | Liya ZHAO, Liju NI, Caiqin ZHANG, Jianping TANG, Yangzheng YAO, Yanyan NIE, Xiaoxue GU, Ying ZHAO. Establishing a Genetic Detection Protocol of Single Nucleotide Polymorphisms Panels in Inbred Rats Based on Multiplex PCR-LDR [J]. Laboratory Animal and Comparative Medicine, 2023, 43(5): 548-558. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||