
Laboratory Animal and Comparative Medicine ›› 2022, Vol. 42 ›› Issue (3): 194-200.DOI: 10.12300/j.issn.1674-5817.2021.140
• Animal Models of Human Diseases • Previous Articles Next Articles
·Ablimiti Merika1( )(
)( ), ·Abibule Aishan1, Shen SHI1, ·Abdu-reyimu Abdu-salamu2, ··Taleb Enivar3
), ·Abibule Aishan1, Shen SHI1, ·Abdu-reyimu Abdu-salamu2, ··Taleb Enivar3
Received:2021-03-16
Revised:2021-07-21
Online:2022-06-25
Published:2022-06-25
Contact:
··Taleb Enivar
CLC Number:
·Ablimiti Merika,·Abibule Aishan,Shen SHI,et al. Compound Casson Syrup Alleviates Acute Chemical Liver Injury in Mice Caused by D-galactosamine[J]. Laboratory Animal and Comparative Medicine, 2022, 42(3): 194-200. DOI: 10.12300/j.issn.1674-5817.2021.140.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2021.140
组别 Group | 剂量 Dose/(mL·kg-1·d-1) | 实验前体质量 Pre-test body mass/g | 解剖前体质量 Preanatomic body mass/g | 肝脏指数 Liver index/ (mg·g-1) | 脾脏指数 Spleen index/ (mg·g-1) |
|---|---|---|---|---|---|
正常对照 Normal control | - | 24.00±1.07 | 29.19±1.70 | 4.95±0.61 | 0.30±0.07 |
模型对照 Model control | - | 25.00±2.39 | 28.85±4.88 | 5.93±0.84* | 0.36±0.01 |
炎消迪娜儿糖浆对照 YS control | 12 | 20.13±3.31 | 27.16±2.98 | 4.42±0.75# | 0.32±0.05 |
低剂量复方卡森糖浆 Low-dose of CCS | 4 | 27.50±0.76 | 30.99±1.36 | 4.50±0.38 | 0.26±0.05# |
中剂量复方卡森糖浆 Medium-dose of CCS | 8 | 22.88±3.36 | 28.91±1.75 | 5.35±0.83 | 0.28±0.06# |
高剂量复方卡森糖浆 High-dose of CCS | 16 | 26.13±0.99 | 28.66±1.81 | 4.64±0.29 | 0.32±0.06 |
Table 1 Effect of compound Casson syrup (CCS) on liver and spleen indexes of mice induced by D-galactose
组别 Group | 剂量 Dose/(mL·kg-1·d-1) | 实验前体质量 Pre-test body mass/g | 解剖前体质量 Preanatomic body mass/g | 肝脏指数 Liver index/ (mg·g-1) | 脾脏指数 Spleen index/ (mg·g-1) |
|---|---|---|---|---|---|
正常对照 Normal control | - | 24.00±1.07 | 29.19±1.70 | 4.95±0.61 | 0.30±0.07 |
模型对照 Model control | - | 25.00±2.39 | 28.85±4.88 | 5.93±0.84* | 0.36±0.01 |
炎消迪娜儿糖浆对照 YS control | 12 | 20.13±3.31 | 27.16±2.98 | 4.42±0.75# | 0.32±0.05 |
低剂量复方卡森糖浆 Low-dose of CCS | 4 | 27.50±0.76 | 30.99±1.36 | 4.50±0.38 | 0.26±0.05# |
中剂量复方卡森糖浆 Medium-dose of CCS | 8 | 22.88±3.36 | 28.91±1.75 | 5.35±0.83 | 0.28±0.06# |
高剂量复方卡森糖浆 High-dose of CCS | 16 | 26.13±0.99 | 28.66±1.81 | 4.64±0.29 | 0.32±0.06 |
组别 Group | 剂量 Dose/(mL·kg-1·d-1) | ALT z/(U·L-1) | AST z/(U·L-1) | AST/ALT | ALP z/(U·L-1) |
|---|---|---|---|---|---|
| 正常对照 Normal control | - | 34.63±1.41 | 144.50±27.30 | 4.18±0.84# | 171.50±20.10# |
| 模型对照 Model control | - | 101.13±51.3* | 241.25±41.04* | 2.39±1.69 | 217.50±81.39* |
| 炎消迪娜儿糖浆对照 YS control | 12 | 53.60±10.43 | 216.20±50.45 | 4.19±1.47 | 157.80±17.30# |
| 低剂量复方卡森糖浆 Low-dose of CCS | 4 | 59.63±37.74 | 182.63±56.29 | 3.44±0.99 | 169.13±28.98# |
| 中剂量复方卡森糖浆 Medium-dose of CCS | 8 | 45.25±17.80 | 159.13±31.84 | 3.79±0.95 | 173.38±27.64# |
| 高剂量复方卡森糖浆 High-dose of CCS | 16 | 38.80±4.76 | 145.80±13.03 | 3.83±0.75 | 149.60±24.81# |
Table 3 Effects of compound Casson syrup on MDA, GSH, SOD in liver tissues of mice with chemical liver injury caused by
组别 Group | 剂量 Dose/(mL·kg-1·d-1) | ALT z/(U·L-1) | AST z/(U·L-1) | AST/ALT | ALP z/(U·L-1) |
|---|---|---|---|---|---|
| 正常对照 Normal control | - | 34.63±1.41 | 144.50±27.30 | 4.18±0.84# | 171.50±20.10# |
| 模型对照 Model control | - | 101.13±51.3* | 241.25±41.04* | 2.39±1.69 | 217.50±81.39* |
| 炎消迪娜儿糖浆对照 YS control | 12 | 53.60±10.43 | 216.20±50.45 | 4.19±1.47 | 157.80±17.30# |
| 低剂量复方卡森糖浆 Low-dose of CCS | 4 | 59.63±37.74 | 182.63±56.29 | 3.44±0.99 | 169.13±28.98# |
| 中剂量复方卡森糖浆 Medium-dose of CCS | 8 | 45.25±17.80 | 159.13±31.84 | 3.79±0.95 | 173.38±27.64# |
| 高剂量复方卡森糖浆 High-dose of CCS | 16 | 38.80±4.76 | 145.80±13.03 | 3.83±0.75 | 149.60±24.81# |
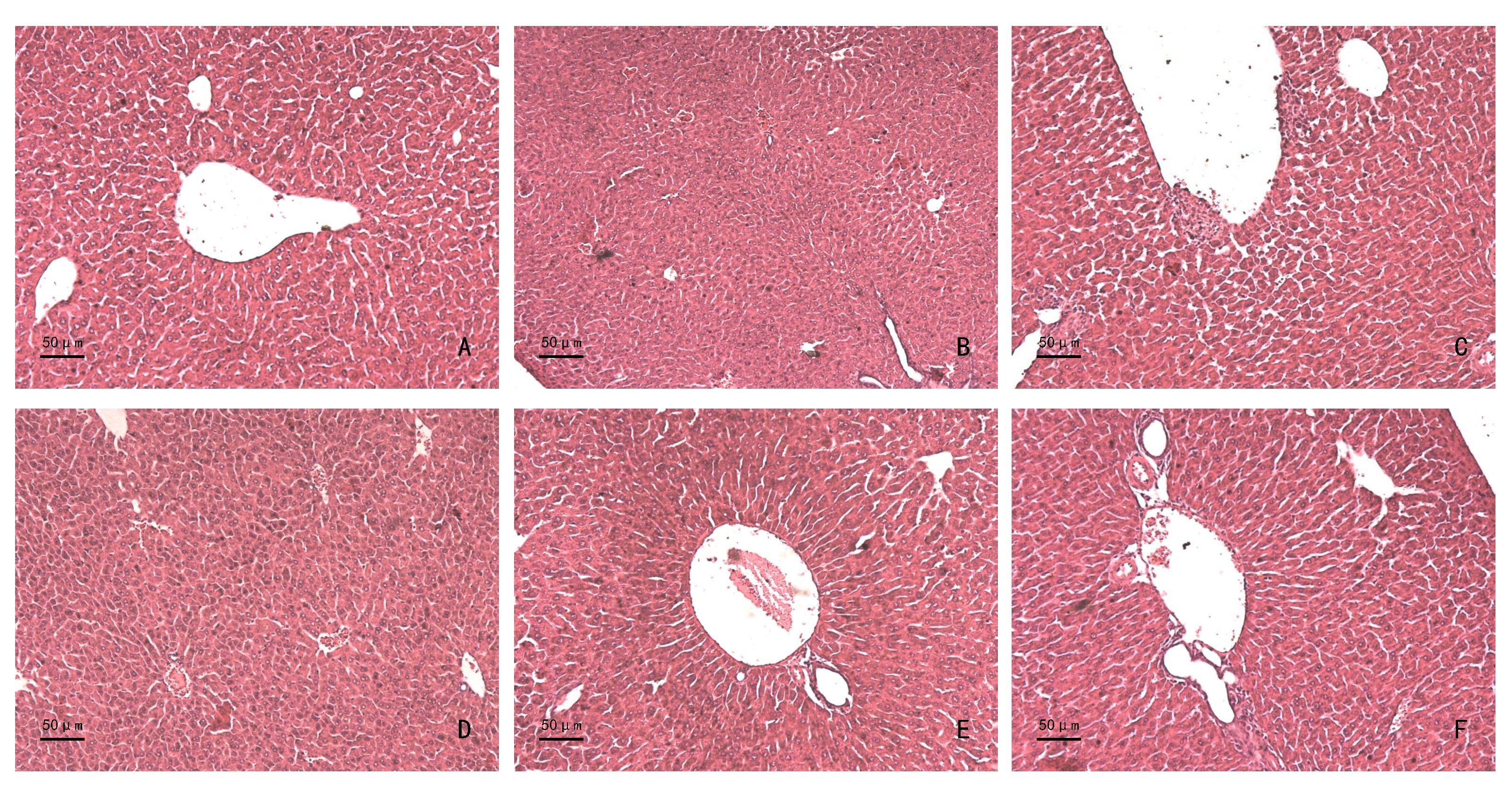
Figure 1 Effect of compound Casson syrup on liver tissue caseology of D-galactose induced chemical liver injury mice(HE staining, ×200)Note: A is normal control group; B is model control group; C is Yanxiaodinar syrup positive group; D, E and F are compound Casson syrup low-dose, medium-dose and high-dose groups, respectively.
| 1 | 代云云, 谢晓蓉, 王茂鹤, 等. 我国四大民族医药体系概述[J]. 中华中医药杂志, 2021, 36(3):1522-1525. |
| DAI Y Y, XIE X R, WANG M H, et al. Overview of four national medicine system[J]. China J Tradit Chin Med Pharm, 2021, 36(3):1522-1525. | |
| 2 | 刘燕, 张莉, 黄华. 具抗炎作用维药的研究进展[J]. 中国中药杂志, 2014, 39(9):1714-1720. DOI:10.4268/cjcmm20140933 . |
| LIU Y, ZHANG L, HUANG H. Advance in studies on anti-inflammatory effect of Uygur medicine[J]. China J Chin Mater Med, 2014, 39(9):1714-1720. DOI:10.4268/cjcmm20140933 . | |
| 3 | 张晓恒, 姚佳, 秦冬梅. 毛菊苣提取物对小鼠肝纤维化的保护作用[J]. 世界华人消化杂志, 2020, 28(3):86-91. |
| ZHANG X H, YAO J, QIN D M. Protective effects of Cichorium glandulosum Boiss extracts against liver fibrosis in mice[J]. World Chin J Dig, 2020, 28(3):86-91. | |
| 4 | 阿吉艾克拜尔·艾萨, 信学雷, 吴涛, 等. 毛菊苣功能成分及药理作用研究[Z]. 国家科技成果, 2017. |
| Aji-Ekbel·Isa, XIN X L, WU T, et al. Study on functional constituents and pharmacological effects of Chicory hairy [Z]. National Scientific and Technological Achievements, 2017. | |
| 5 | 吴晓琴, 宋晓琳, 彭瀛, 等. 花脸蘑多糖水提取物对D-氨基半乳糖致小鼠急性肝损伤的保护作用[J]. 延边大学医学学报, 2013, 36(1):22-24. DOI:10.16068/j.1000-1824.2013.01.005 . |
| WU X Q, SONG X L, PENG Y, et al. Protective effects of water extract of the Lepista sordida polysaccharides for D-galactosamine-induced acute liver injury in mice[J]. J Med Sci Yanbian Univ, 2013, 36(1):22-24. DOI:10.16068/j.1000-1824.2013.01.005 . | |
| 6 | 信学雷, 吴汉夔, 吕俏莹, 等. 维吾尔药毛菊苣提取物降糖活性的研究[J]. 天然产物研究与开发, 2012, 24(2):234-238. DOI:10.16333/j.1001-6880.2012.02.024 . |
| XIN X L, WU H K, LV Q Y, et al. Hypoglycemic effect of extracts from Cichorium glandulosum [J]. Nat Prod Res Dev, 2012, 24(2):234-238. DOI:10.16333/j.1001-6880.2012.02.024 . | |
| 7 | 古力米拉·买买提衣明, 古力那孜·阿不都热合满. 维药清热卡森颗粒治疗脂肪肝61例观察[J]. 中国民族医药杂志, 2014, 20(1):12-13. DOI:10.16041/j.cnki.cn15-1175.2014.01.011 . |
| Gulimila·Maimaitiyiming, Reheman Gulinaz·Abudu. Obser-vation on 61 cases of fatty liver treated with weiqingre Casson Granule[J]. J Med Pharm Chin Minor, 2014, 20(1):12-13. DOI:10.16041/j.cnki.cn15-1175.2014.01.011 . | |
| 8 | 王晓杰, 吾买尔江·牙合甫, 阿丽米热·买买提, 等. 菊苣多糖对小鼠药物性肝损伤保护作用的筛选[J]. 现代畜牧兽医, 2021(4):9-13. |
| WANG X J, Yakup·Omar, Mamat·Almira, et al. Screening of the protective effect of Cichorium intybus L.polysaccharide on drug-induced liver injury[J]. Mod J Animal Husb Vet Med, 2021(4):9-13. | |
| 9 | 秦冬梅, 胡利萍, 文志萍, 等. 维药毛菊苣提取物对小鼠四氮化碳急性肝损伤的保护作用[J]. 中国药理学通报, 2012, 28(8):1180-1181. DOI:10.3969/j.issn.1001-1978.2012.08.032 . |
| QIN D M, HU L P, WEN Z P, et al. Protective effects of Cichorium glandulosum Boiss extracts on experimental liver injury in mice[J]. Chin Pharmacol Bull, 2012, 28(8):1180-1181. DOI:10.3969/j.issn.1001-1978.2012.08.032 . | |
| 10 | 张权林, 罗玉琴, 杨伟俊, 等. 刺山柑胶囊对肝损伤模型小鼠的保护作用及对大鼠胆汁分泌的影响[J]. 中国药房, 2014, 25(19):1747-1750. DOI:10.6039/j.issn.1001-0408.2014.19.06 . |
| ZHANG Q L, LUO Y Q, YANG W J, et al. Protective effects of caper capsules on liver injury model mice and bile secretion of rats[J]. China Pharm, 2014, 25(19):1747-1750. DOI:10.6039/j.issn.1001-0408.2014.19.06 . | |
| 11 | 万晓莉, 谢笑龙, 刘杰, 等. 口服齐墩果酸对D-氨基半乳糖致小鼠急性肝损伤的保护作用[J]. 华西药学杂志, 2016, 31(1):26-29. DOI:10.13375/j.cnki.wcjps.2016.01.008 . |
| WAN X L, XIE X L, LIU J, et al. Hepatoprotective effect of oleanolic acid on D-galactosamine- induced acute liver injury in the mice[J]. West China J Pharm Sci, 2016, 31(1):26-29. DOI:10.13375/j.cnki.wcjps.2016.01.008 . | |
| 12 | 中华人民共和国卫生部药典委员会. 中华人民共和国卫生部药品标准: 维吾尔药分册 [S]. 乌鲁木齐: 新疆科技卫生出版社, 1998:157. |
| Pharmacopoeia Committee of the Ministry of Health of the People's Republic of China. Drug Standards of the Ministry of Health of the People's Republic of China: Uygur medicine volume [S]. Urumqi: Xinjiang People's Medical Publishing House, 1998:157. | |
| 13 | 代小佩. 我国病毒性肝炎发病率下降 其他类型肝炎有所上升[N]. 科技日报, 2021-07-29(2). |
| DAI X P. The incidence of viral hepatitis decreased and other types of hepatitis increased in China[N]. Science and Technology Daily, 2021-07-29(2). | |
| 14 | 熊亚茹, 张莹莹, 崔鲁炜, 等. 某复方口服液对小鼠酒精性肝损伤的辅助保护作用研究[J]. 预防医学情报杂志, 2021, 37(8):1164-1167. |
| XIONG Y R, ZHANG Y Y, CUI L W, et al. Auxiliary protection of A compound oral liquid on alcoholic liver injury in mice[J]. J Prev Med Inf, 2021, 37(8):1164-1167. | |
| 15 | 付双楠, 朱平生, 高达. 中医药防治化学性肝损伤的研究进展[J]. 中医学报, 2017, 32(3):449-454. DOI:10.16368/j.issn.1674-8999.2017.03.116 . |
| FU S N, ZHU P S, GAO D. Progress of TCM studies in TCM treatment of liver injury due to chemical factors[J]. Acta Chin Med, 2017, 32(3):449-454. DOI:10.16368/j.issn.1674-8999.2017.03.116 . | |
| 16 | CHEN M J, VIJAY V, SHI Q, et al. FDA-approved drug labeling for the study of drug-induced liver injury[J]. Drug Discov Today, 2011, 16(15-16):697-703. DOI:10.1016/j.drudis.2011.05.007 . |
| [1] | LIU Yueqin, XUE Weiguo, WANG Shuyou, SHEN Yaohua, JIA Shuyong, WANG Guangjun, SONG Xiaojing. Observation of Digestive Tract Tissue Morphology in Mice Using Probe-Based Confocal Laser Endomicroscopy [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 457-465. |
| [2] | KONG Zhihao, WEI Xiaofeng, YU Lingzhi, FENG Liping, ZHU Qi, SHI Guojun, WANG Chen. Isolation and Identification of Staphylococcus xylosus in Nude Mice with Squamous Skin Scurfs [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 368-375. |
| [3] | XU Qiuyu, YAN Guofeng, FU Li, FAN Wenhua, ZHOU Jing, ZHU Lian, QIU Shuwen, ZHANG Jie, WU Ling. A Mouse Model of Polycystic Ovary Syndrome Established Through Subcutaneous Administration of Letrozole Sustained-Release Pellets and Hepatic Transcriptome Analysis [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 119-129. |
| [4] | LIU Rongle, CHENG Hao, SHANG Fusheng, CHANG Shufu, XU Ping. Study on Cardiac Aging Phenotypes of SHJH hr Mice [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 13-20. |
| [5] | WU Zhihao, CAO Shuyang, ZHOU Zhengyu. Establishment of an Intestinal Fibrosis Model Associated with Inflammatory Bowel Disease in VDR-/- Mice Induced by Helicobacter hepaticus Infection and Mechanism Exploration [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 37-46. |
| [6] | ZHANG Nan, LI Huaiyin, LIAN Xiaodi, WEI Juanpeng, GAO Ming. Effects of Different Durations of Light Exposure on Body Weight and Learning and Memory Abilities of NIH Mice [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 73-78. |
| [7] | ZHAO Xiaona, WANG Peng, YE Maoqing, QU Xinkai. Establishment of a New Hyperglycemic Obesity Cardiac Dysfunction Mouse Model with Triacsin C [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 605-612. |
| [8] | TAN He, YANG Xiaohui, ZHANG Daxiu, WANG Guicheng. Optimal Adaptation Period for Metabolic Cage Experiments in Mice at Different Developmental Stages [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 502-510. |
| [9] | MENG Yu, LIANG Dongli, ZHENG Linlin, ZHOU Yuanyuan, WANG Zhaoxia. Optimization and Evaluation of Conditions for Orthotopic Nude Mouse Models of Human Liver Tumor Cells [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 511-522. |
| [10] | Jing QIN, Yong ZHAO, Caiqin ZHANG, Bing BAI, Changhong SHI. Construction and Evaluation of Theranostic Near-infrared Fluorescent Probe for Targeting Inflammatory Brain Edema [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 243-250. |
| [11] | Yisu ZHANG, Xinru LIU, Ruojie WU, Rui LIU, Hong OUYANG, Xiaohong LI. Establishment and Evaluation of Mouse Model of Pregnancy Pain-depression Comorbidity Induced by Chronic Unpredictable Stress, Complete Freund's Adjuvant and Formalin [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 259-269. |
| [12] | Dong WU, Rui SHI, Peishan LUO, Ling'en LI, Xijing SHENG, Mengyang WANG, Lu NI, Sujuan WANG, Huixin YANG, Jing ZHAO. Effects of Different Pellet Feed Hardness on Growth and Reproduction, Feed Utilization Rate, and Environmental Dust in Laboratory Mice [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 313-320. |
| [13] | Yun LIU, Tingting FENG, Wei TONG, Zhi GUO, Xia LI, Qi KONG, Zhiguang XIANG. Glycyrrhizic Acid Showed Therapeutic Effects on Severe Pulmonary Damages in Mice Induced by Pneumonia Virus of Mice Infection [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 251-258. |
| [14] | Jinhua HU, Jingjie HAN, Min JIN, Bin HU, Yuefen LOU. Effects of Puerarin on Bone Density in Rats and Mice: A Meta-analysis [J]. Laboratory Animal and Comparative Medicine, 2024, 44(2): 149-161. |
| [15] | Min LIANG, Yang GUO, Jinjin WANG, Mengyan ZHU, Jun CHI, Yanjuan CHEN, Chengji WANG, Zhilan YU, Ruling SHEN. Construction of Dmd Gene Mutant Mice and Phenotype Verification in Muscle and Immune Systems [J]. Laboratory Animal and Comparative Medicine, 2024, 44(1): 42-51. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||