
Laboratory Animal and Comparative Medicine ›› 2024, Vol. 44 ›› Issue (1): 42-51.DOI: 10.12300/j.issn.1674-5817.2023.089
• Animal Models of Human Diseases • Previous Articles Next Articles
Min LIANG1( ), Yang GUO1, Jinjin WANG2, Mengyan ZHU1, Jun CHI2, Yanjuan CHEN1, Chengji WANG1, Zhilan YU1, Ruling SHEN1(
), Yang GUO1, Jinjin WANG2, Mengyan ZHU1, Jun CHI2, Yanjuan CHEN1, Chengji WANG1, Zhilan YU1, Ruling SHEN1( )(
)( )
)
Received:2023-06-27
Revised:2023-11-28
Online:2024-02-25
Published:2024-02-25
Contact:
Ruling SHEN
CLC Number:
Min LIANG,Yang GUO,Jinjin WANG,et al. Construction of Dmd Gene Mutant Mice and Phenotype Verification in Muscle and Immune Systems[J]. Laboratory Animal and Comparative Medicine, 2024, 44(1): 42-51. DOI: 10.12300/j.issn.1674-5817.2023.089.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2023.089
序列名称 Sequence name | 序列(5'→3') Sequence (5'→3') |
|---|---|
小向导RNA sgRNA | TCTTTGAAAGAGCAACAAAATGG |
寡核苷酸供体DNA Oligo donor DNA | GCTCTGCAAAGTTCTTTGAAAGAGCAATAAAATGGCTTCAACTATCTGAGTGACACTGTGAAGGAGATGGCCAAGAAAGCACCTTCAGAAATATGCCAGAAATATCTGTC |
引物I Primer I | ATGCTTGATATTGAGTAGTTA |
引物 II Primer II | AATAGATTTGGCTTTTGAT |
测序引物 Sequencing primer | ATGCTTGATATTGAGTAGTTA |
Table 1 sgRNA and oligo donor DNA as well as PCR primer sequence information
序列名称 Sequence name | 序列(5'→3') Sequence (5'→3') |
|---|---|
小向导RNA sgRNA | TCTTTGAAAGAGCAACAAAATGG |
寡核苷酸供体DNA Oligo donor DNA | GCTCTGCAAAGTTCTTTGAAAGAGCAATAAAATGGCTTCAACTATCTGAGTGACACTGTGAAGGAGATGGCCAAGAAAGCACCTTCAGAAATATGCCAGAAATATCTGTC |
引物I Primer I | ATGCTTGATATTGAGTAGTTA |
引物 II Primer II | AATAGATTTGGCTTTTGAT |
测序引物 Sequencing primer | ATGCTTGATATTGAGTAGTTA |
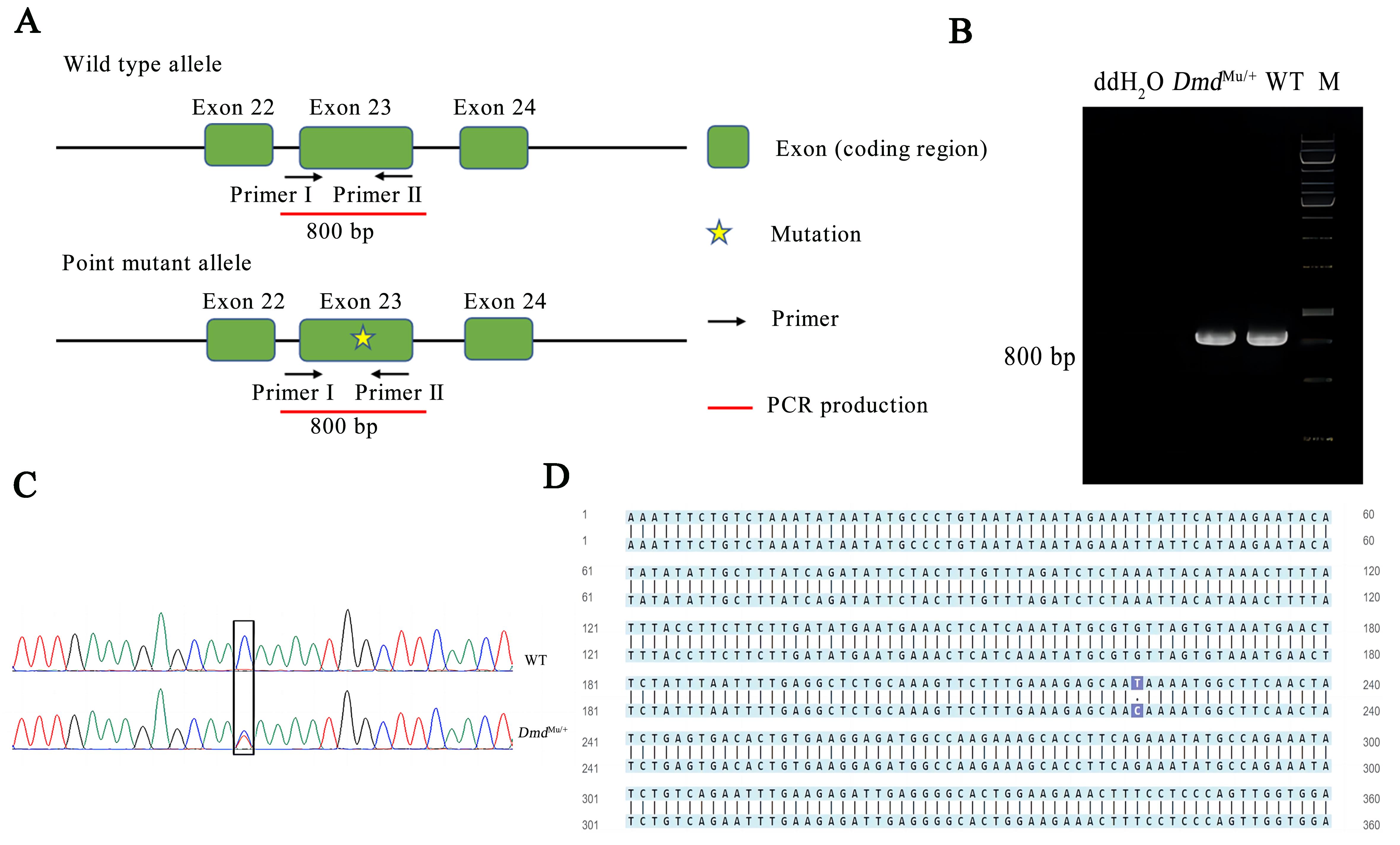
Figure2 Genotype results of Dmd point mutant miceNote:A, identification strategy diagram; B, PCR electrophoresis maps of DmdMu/+ mice; C-D, DNA sequencing results. DmdMu/+, heterozygous point mutant mice; WT, wild-type mice; M, DNA marker.
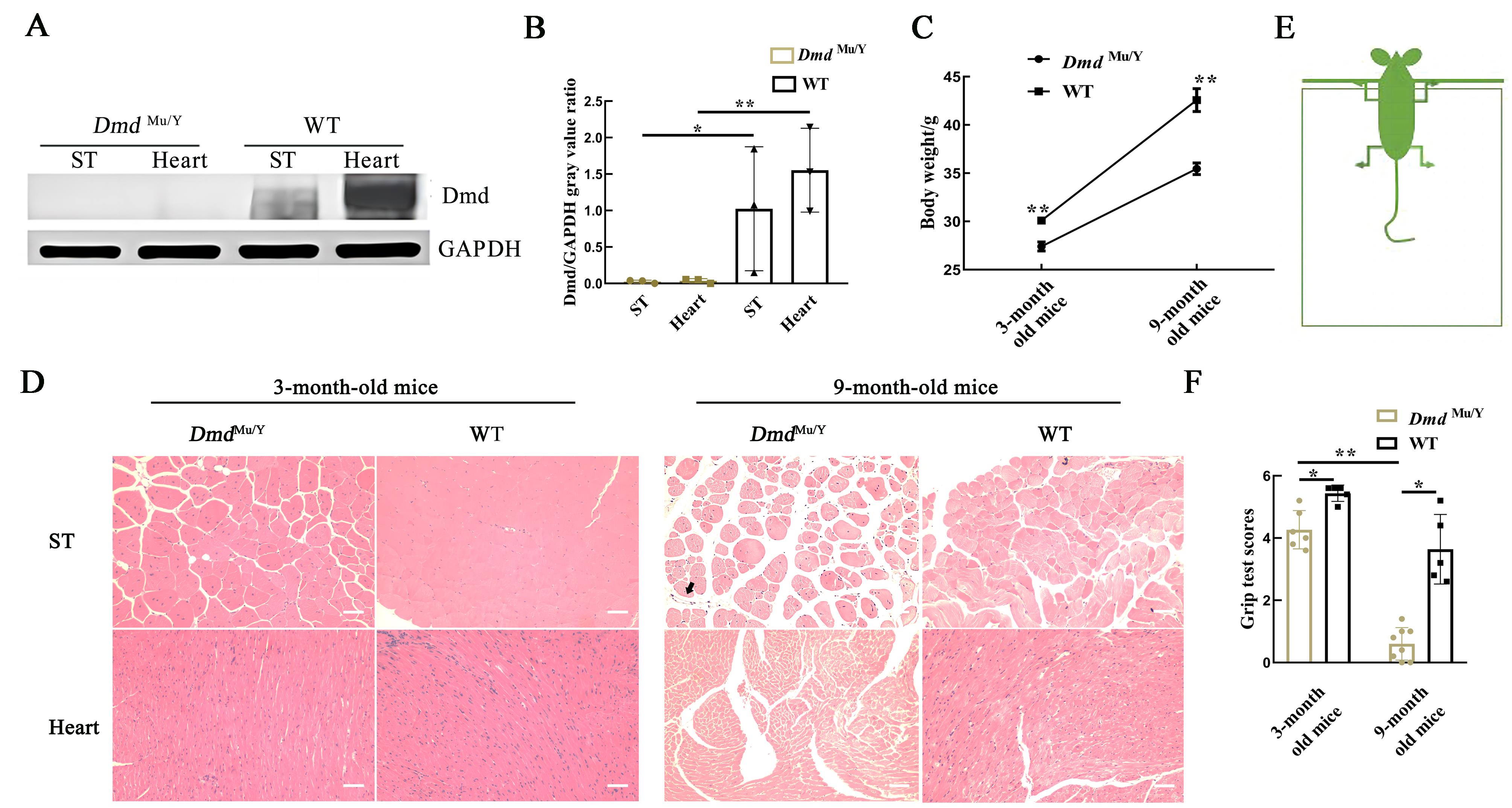
Figure 3 Phenotypic validation of DmdMu/Y miceNote:A-B, The expression levels of Dmd protein in semitendinosus (ST) and heart of DmdMu/Y mice were detected by Western blotting [wild-type C57BL/6J mice (WT) were used as the control group; GAPDH was internal control protein; n=3, *P<0.05, **P<0.01]; C, the body weight of 3-month and 9-month old DmdMu/Y mice [wild-type C57BL/6J mice (WT) were used as the control group; n=9, **P<0.01]; D, HE staining results of muscle tissue pathologic changes in 3-month and 9-month old DmdMu/Y mice [wild-type C57BL/6J mice (WT) were used as the control group; the gap between skeletal (ST) and myocardial (Heart) muscles of 9-month old DmdMu/Y mice became wider and the size of muscles varied; Infiltrating inflammatory cells were observed (sigh by arrow);n=3,bar=100 μm]. E, Schematic diagram of grid test; F, The statistical chart of the grid test [3-month old, DmdMu/Y mice n=6, WT mice n=5; 9-month old, DmdMu/Y mice n=8, WT mice n=5, *P<0.05, **P<0.01].
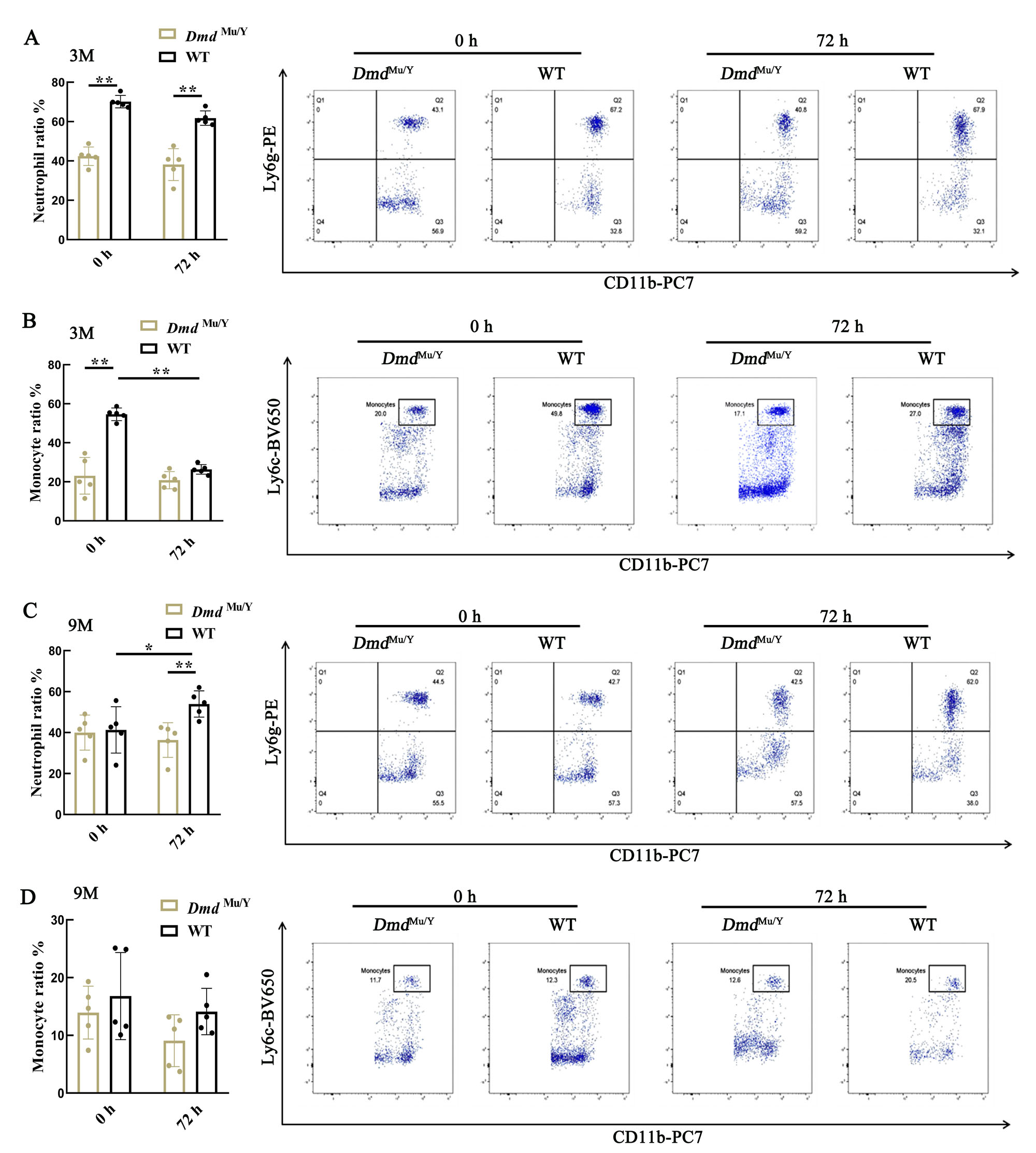
Figure 4 The proportion of neutrophils and monocytes in the blood of DmdMu/Y mice of LPS-induced acute inflammation model detected by flow cytometryNote: A-B, Changes in neutrophils ratio (A) and monocyte ratio (B) in 3-month old DmdMu/Y mice after injection of lipopolysaccharide (LPS) for 0 and 72 hours; C-D, Changes in neutrophils ratio (C) and monocyte ratio (D) in 9-month old DmdMu/Y mice after injection of LPS for 0 and 72 hours. The wild-type C57BL/6J mice (WT) were used as the control group; n=5, *P<0.05, **P<0.01.
| 1 | NALLAMILLI B R R, CHAUBEY A, VALENCIA C A, et al. A single NGS-based assay covering the entire genomic sequence of the DMD gene facilitates diagnostic and newborn screening confirmatory testing[J]. Hum Mutat, 2021, 42(5):626-638. DOI: 10.1002/humu.24191 . |
| 2 | FORTUNATO F, FARNÈ M, FERLINI A. The DMD gene and therapeutic approaches to restore dystrophin[J]. Neuromuscul Disord, 2021, 31(10):1013-1020. DOI: 10.1016/j.nmd.2021.08.004 . |
| 3 | WANG L A, XU M, LI H A, et al. Genotypes and phenotypes of DMD small mutations in Chinese patients with dystrophinopathies[J]. Front Genet, 2019, 10:114. DOI: 10.3389/fgene.2019.00114 . |
| 4 | BRANDSEMA J F, DARRAS B T. Dystrophinopathies[J]. Semin Neurol, 2015, 35(4):369-384. DOI: 10.1055/s-0035-1558982 . |
| 5 | LINDSAY A, RUSSELL A P. The unconditioned fear response in dystrophin-deficient mice is associated with adrenal and vascular function[J]. Sci Rep, 2023, 13(1):5513. DOI: 10.1038/s41598-023-32163-w . |
| 6 | 李少英, 孙筱放, 黎青, 等. 中国人群中抗肌萎缩蛋白基因突变类型和分布特点及其与临床症状的相关性[J]. 遗传, 2011, 33(3):251-254. DOI: 10.3724/SP.J.1005.2011.00251 . |
| LI S Y, SUN X F, LI Q, et al. Association of mutation types and distribution characteristics of dystrophin gene with clinical symptoms in Chinese population[J]. Hereditas, 2011, 33(3):251-254. DOI: 10.3724/SP.J.1005.2011.00251 . | |
| 7 | BHATTARAI S, LI Q A, DING J, et al. TLR4 is a regulator of trained immunity in a murine model of Duchenne muscular dystrophy[J]. Nat Commun, 2022, 13:879. DOI: 10.1038/s41467-022-28531-1 . |
| 8 | PETROF B J, PODOLSKY T, BHATTARAI S, et al. Trained immunity as a potential target for therapeutic immunomodulation in Duchenne muscular dystrophy[J]. Front Immunol, 2023, 14:1183066. DOI: 10.3389/fimmu.2023. 1183066 . |
| 9 | TULANGEKAR A, SZTAL T E. Inflammation in Duchenne muscular dystrophy-exploring the role of neutrophils in muscle damage and regeneration[J]. Biomedicines, 2021, 9(10):1366. DOI: 10.3390/biomedicines9101366 . |
| 10 | TRIPODI L, VILLA C, MOLINARO D, et al. The immune system in Duchenne muscular dystrophy pathogenesis[J]. Biomedicines, 2021, 9(10):1447. DOI: 10.3390/biomedicines 9101447 . |
| 11 | KURIBARA H, HIGUCHI Y, TADOKORO S. Effects of central depressants on rota-rod and traction performances in mice[J]. Jpn J Pharmacol, 1977, 27(1):117-126. DOI: 10.1254/jjp.27.117 . |
| 12 | 姚庆和, 张华, 高国栋. 帕金森病小鼠模型行为学检测方法的比较研究[J]. 中国实验动物学报, 2006, 14(4): 264-270. DOI: 10.3969/j.issn.1005-4847.2006.04.006 . |
| YAO Q H, ZHANG H, GAO G D. Comparison of behavioral tests in MPTP-induced Parkinson's disease in mice[J]. Acta Lab Animalis Sci Sin, 2006, 14(4): 264-270. DOI: 10.3969/j.issn.1005-4847.2006.04.006 . | |
| 13 | MCGREEVY J W, HAKIM C H, MCINTOSH M A, et al. Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy[J]. Dis Model Mech, 2015, 8(3):195-213. DOI: 10.1242/dmm.018424 . |
| 14 | GIBBS E M, HORSTICK E J, DOWLING J J. Swimming into prominence: the zebrafish as a valuable tool for studying human myopathies and muscular dystrophies[J]. FEBS J, 2013, 280(17):4187-4197. DOI: 10.1111/febs.12412 . |
| 15 | SICINSKI P, GENG Y, RYDER-COOK A S, et al. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation[J]. Science, 1989, 244(4912):1578-1580. DOI: 10.1126/science.2662404 . |
| 16 | BARTHÉLÉMY I, PINTO-MARIZ F, YADA E, et al. Predictive markers of clinical outcome in the GRMD dog model of Duchenne muscular dystrophy[J]. Dis Model Mech, 2014, 7(11):1253-1261. DOI: 10.1242/dmm.016014 . |
| 17 | STIRM M, FONTEYNE L M, SHASHIKADZE B, et al. Pig models for Duchenne muscular dystrophy - from disease mechanisms to validation of new diagnostic and therapeutic concepts[J]. Neuromuscul Disord, 2022, 32(7):543-556. DOI: 10.1016/j.nmd.2022.04.005 . |
| 18 | LI J, WANG K Y, ZHANG Y C, et al. Therapeutic exon skipping through a CRISPR-guided cytidine deaminase rescues dystrophic cardiomyopathy in vivo [J]. Circulation, 2021, 144(22):1760-1776. DOI: 10.1161/CIRCULATIONAHA.121.054628 . |
| 19 | ZHANG Y, LI H, NISHIYAMA T, et al. A humanized knockin mouse model of Duchenne muscular dystrophy and its correction by CRISPR-Cas9 therapeutic gene editing[J]. Mol Ther Nucleic Acids, 2022, 29:525-537. DOI: 10.1016/j.omtn. 2022.07.024 . |
| 20 | WILLMANN R, POSSEKEL S, DUBACH-POWELL J, et al. Mammalian animal models for Duchenne muscular dystrophy[J]. Neuromuscul Disord, 2009, 19(4):241-249. DOI: 10.1016/j.nmd.2008.11.015 . |
| 21 | 董奇超, 陈慧敏. Duchenne型肌营养不良基因治疗研究进展[J]. 中国当代儿科杂志, 2018, 20(8): 691-697. DOI: 10.7499/j.issn.1008-8830.2018.08.017 . |
| DONG Q C, CHEN H M. A review of gene therapy for Duchenne muscular dystrophy[J]. Chin J Contemp Pediatr, 2018, 20(8): 691-697. DOI: 10.7499/j.issn.1008-8830.2018.08.017 . | |
| 22 | BULFIELD G, SILLER W G, WIGHT P A, et al. X chromosome-linked muscular dystrophy (mdx) in the mouse[J]. Proc Natl Acad Sci USA, 1984, 81(4):1189-1192. DOI: 10.1073/pnas. 81. 4.1189 . |
| 23 | GRIFFIN J L, WILLIAMS H J, SANG E, et al. Metabolic profiling of genetic disorders: a multitissue 1H nuclear magnetic resonance spectroscopic and pattern recognition study into dystrophic tissue[J]. Anal Biochem, 2001, 293(1):16-21. DOI: 10.1006/abio.2001.5096 . |
| 24 | MORRONI J, SCHIRONE L, VECCHIO D, et al. Accelerating the Mdx heart histo-pathology through physical exercise[J]. Life, 2021, 11(7):706. DOI: 10.3390/life11070706 . |
| 25 | RAZZOLI M, LINDSAY A, LAW M L, et al. Social stress is lethal in the mdx model of Duchenne muscular dystrophy[J]. EBioMedicine, 2020, 55:102700. DOI: 10.1016/j.ebiom. 2020. 102700 . |
| 26 | CHANG L L, NIU F N, CHEN J, et al. Ghrelin improves muscle function in dystrophin-deficient mdx mice by inhibiting NLRP3 inflammasome activation[J]. Life Sci, 2019, 232:116654. DOI: 10.1016/j.lfs.2019.116654 . |
| 27 | 李想, 崔迪, 邱守涛. 白细胞介素-6在骨骼肌质量调节中的作用[J]. 中国生物化学与分子生物学报, 2023, 39(6):778-788. DOI: 10.13865/j.cnki.cjbmb.2022.09.1245 . |
| LI X, CUI D, QIU S T. Interleukin-6 in skeletal muscle mass regulation[J]. Chin J Biochem Mol Biol, 2023, 39(6):778-788. DOI: 10.13865/j.cnki.cjbmb.2022.09.1245 . | |
| 28 | JACKAMAN C, TOMAY F, DUONG L, et al. Aging and cancer: the role of macrophages and neutrophils[J]. Ageing Res Rev, 2017, 36:105-116. DOI: 10.1016/j.arr.2017.03.008 . |
| 29 | 王颖. 脂多糖信号通路中的蛋白质修饰[J]. 中国生物化学与分子生物学报, 2008, 24(6):505-511. DOI: 10.13865/j.cnki.cjbmb.2008.06.013 . |
| WANG Y. Post-translational modifications in regulation of lipopolysaccharide-activated signal transduction pathway[J]. Chin J Biochem Mol Biol, 2008, 24(6):505-511. DOI: 10.13865/j.cnki.cjbmb.2008.06.013 . |
| [1] | LIU Siyu, LAI Yuezhao, GUO Wenting, CHEN Xuejin. Advances in Nucleic Acid Drugs and Gene Therapies based on Animal Models of Duchenne Muscular Dystrophy [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 613-625. |
| [2] | Yisu ZHANG, Xinru LIU, Ruojie WU, Rui LIU, Hong OUYANG, Xiaohong LI. Establishment and Evaluation of Mouse Model of Pregnancy Pain-depression Comorbidity Induced by Chronic Unpredictable Stress, Complete Freund's Adjuvant and Formalin [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 259-269. |
| [3] | Han LI, Xiaorui ZHANG, Chengfang ZHANG. Mechanism of Intermittent Fasting in Improving Olanzapine-induced Metabolic Disorders in Mice [J]. Laboratory Animal and Comparative Medicine, 2023, 43(1): 3-10. |
| [4] | LAI Suomei, DING Yifu, LI Jinsong. A New Strategy for Constructing Mouse Models of Complex Diseases: Semi-cloning Technology Based on Sperm-like Haploid Embryonic Stem Cells [J]. Laboratory Animal and Comparative Medicine, 2021, 41(5): 369-383. |
| [5] | LI Zifa, ZHANG Hao, REN Meng, XU Kaiyong, HU Minghui, ZHOU Miaomiao, WANG Kezhou. Protective Effect of Quercetin on Lipid Metabolism Disorder in Mice Livers Caused by Cadmium [J]. Laboratory Animal and Comparative Medicine, 2021, 41(4): 305-312. |
| [6] | HONG Shenghui, ZHANG Xuliang, WANG Qianqian, LIU Ping, LIU Diwen. Construction of Inhibin Gene Knockout Mice and Preliminary Analysis of the Phenotype [J]. Laboratory Animal and Comparative Medicine, 2020, 40(4): 306-. |
| [7] | LEI Shan, LIU Qiang, HUANG Wei-jin, WANG You-chun. Influence of Strain, Gender and Hair Coat of Mice on Establishing Bioluminescent Imaging Pseudovirus Mouse Model [J]. Laboratory Animal and Comparative Medicine, 2019, 39(6): 423-428. |
| [8] | GAO Meng-qiao, AI Dong-xu, LI Yu, SUN Fei, WANG Jin, FAN Jun-wen, YUAN Zheng, LIU Yuan, SUN Zhao-zeng. The Construction of Ring Finger Protein 126 Gene Knockout Mouse by Using CRISPR/Cas9 Technique [J]. Laboratory Animal and Comparative Medicine, 2019, 39(1): 21-25. |
| [9] | ZHANG Qi, WANG Jian-fei. Progress and Perspective for Xenotransplantation [J]. Laboratory Animal and Comparative Medicine, 2018, 38(6): 407-411. |
| [10] | AI Dong-xu, ZHONG De-gang, SUN Fei, Li Yu, LI Chong, QIN Tong-tong, GAO Meng-qiao, DONG Shi-shi, SUN Zhao-zeng, LI Lian-rui. Construction of Lyst Gene Defective C57BL/6 Mice by CRISPR/Cas9 Technology [J]. Laboratory Animal and Comparative Medicine, 2018, 38(3): 202-206. |
| [11] | YANG Hua, ZHAO Ya-Juan, OU Qiang. Establishment of Nonalcoholic Fatty Liver Fibrosis Model and Expression of Inflammatory Factors in Mice [J]. Laboratory Animal and Comparative Medicine, 2017, 37(1): 20-24. |
| [12] | XIAO Nan1,ZHAO Wei2, BA Cai-feng2, SU Rong-jian1, SU Yu-hong1,3. Study on Gene Mutation and Muscles Subtypes’Expression in DMD Model Mice [J]. Laboratory Animal and Comparative Medicine, 2008, 28(6): 356-360. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||