
Laboratory Animal and Comparative Medicine ›› 2025, Vol. 45 ›› Issue (2): 229-238.DOI: 10.12300/j.issn.1674-5817.2024.178
• Development and Utilization of Laboratory Animal Resources • Previous Articles Next Articles
LIU Xin1, QI Mengdi1, WANG Wenguang1, HAN Yuanyuan1, LU Meili1,2, LI Na1, DAI Jiejie1( ), LU Caixia1(
), LU Caixia1( )(
)( )
)
Received:2024-12-03
Revised:2025-02-09
Online:2025-04-25
Published:2025-05-12
Contact:
DAI Jiejie, LU Caixia
CLC Number:
LIU Xin,QI Mengdi,WANG Wenguang,et al. Study on Susceptibility and Infection Characteristics of Dengue Virus in Cells Sourced from Different Tissues of Tree Shrews[J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 229-238. DOI: 10.12300/j.issn.1674-5817.2024.178.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2024.178
细胞 Cell | 接种后时间/h Time post-infection/h | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 | 24 | 36 | 48 | 60 | 72 | 84 | 96 | 108 | 120 | 132 | 144 | |
| TAECs | - | - | + | ++ | +++ | ++++ | ++++ | ++++ | ||||
| TASMCs | - | - | + | ++ | +++ | ++++ | ++++ | ++++ | ||||
| THs | - | - | + | + | ++ | +++ | ++++ | ++++ | ||||
| pTRECs | - | ± | + | ++ | +++ | ++++ | ++++ | ++++ | ||||
| TSFs | ± | + | ++ | +++ | +++ | ++++ | ++++ | ++++ | ||||
| TCSCs | - | - | ± | + | + | ++ | +++ | +++ | ++++ | ++++ | ||
| TBMECs | - | - | - | ± | + | ++ | +++ | +++ | ++++ | ++++ | ||
| TRMECs | - | - | - | ± | ± | + | + | + | + | + | ||
| Vero | - | - | - | ± | + | + | + | + | ++ | ++ | +++ | ++++ |
| BHK-21 | - | - | - | + | ++ | +++ | +++ | ++++ | ++++ | ++++ | ++++ | |
| A549 | - | - | ± | + | + | ++ | ++ | +++ | +++ | +++ | ++++ | ++++ |
| C6/36 | - | - | + | + | ++ | ++ | +++ | +++ | +++ | +++ | +++ | ++++ |
Table 1 Changes of cytopathic effect (CPE) in different cell types after dengue virus (DENV) infection
细胞 Cell | 接种后时间/h Time post-infection/h | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 | 24 | 36 | 48 | 60 | 72 | 84 | 96 | 108 | 120 | 132 | 144 | |
| TAECs | - | - | + | ++ | +++ | ++++ | ++++ | ++++ | ||||
| TASMCs | - | - | + | ++ | +++ | ++++ | ++++ | ++++ | ||||
| THs | - | - | + | + | ++ | +++ | ++++ | ++++ | ||||
| pTRECs | - | ± | + | ++ | +++ | ++++ | ++++ | ++++ | ||||
| TSFs | ± | + | ++ | +++ | +++ | ++++ | ++++ | ++++ | ||||
| TCSCs | - | - | ± | + | + | ++ | +++ | +++ | ++++ | ++++ | ||
| TBMECs | - | - | - | ± | + | ++ | +++ | +++ | ++++ | ++++ | ||
| TRMECs | - | - | - | ± | ± | + | + | + | + | + | ||
| Vero | - | - | - | ± | + | + | + | + | ++ | ++ | +++ | ++++ |
| BHK-21 | - | - | - | + | ++ | +++ | +++ | ++++ | ++++ | ++++ | ++++ | |
| A549 | - | - | ± | + | + | ++ | ++ | +++ | +++ | +++ | ++++ | ++++ |
| C6/36 | - | - | + | + | ++ | ++ | +++ | +++ | +++ | +++ | +++ | ++++ |
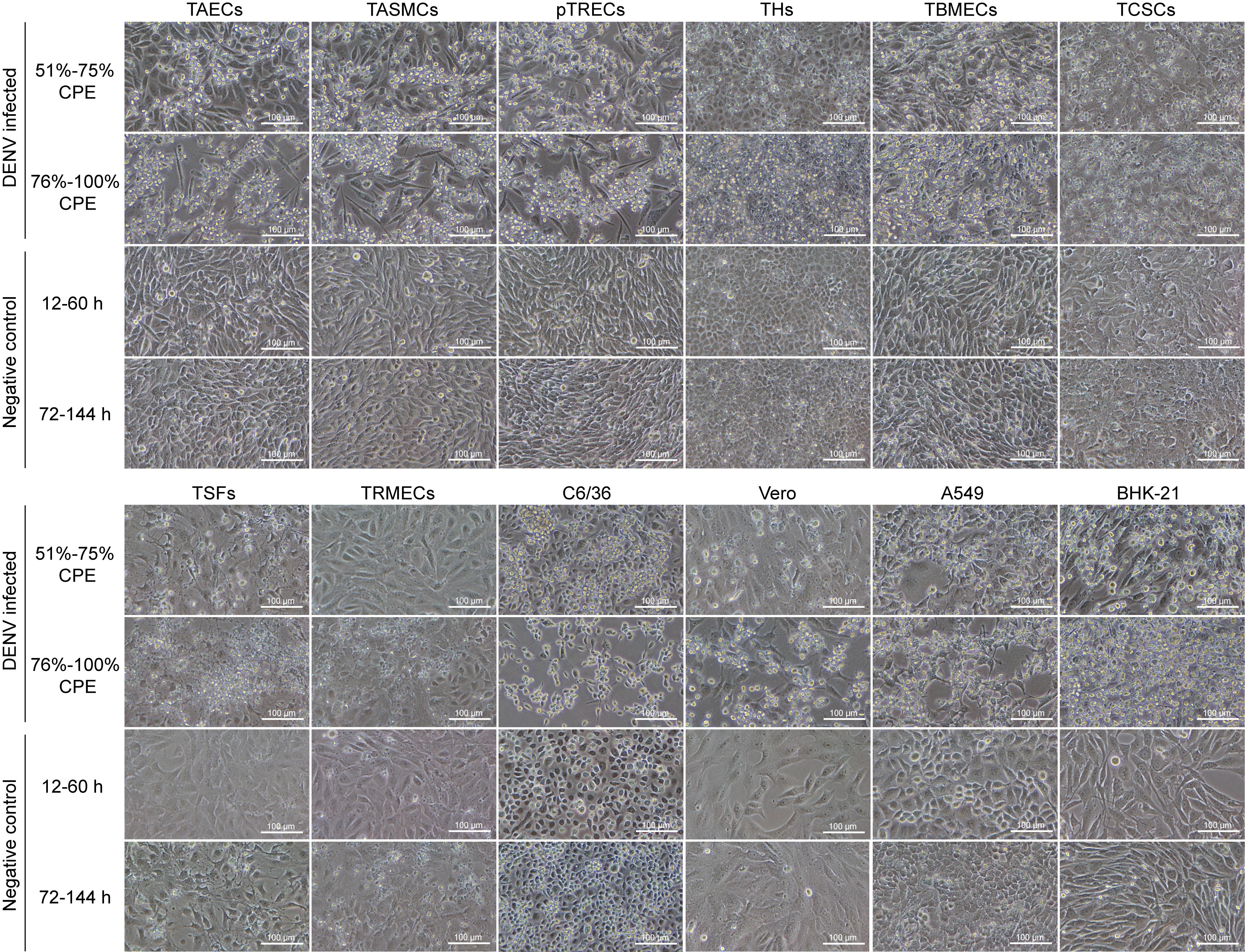
Figure 1 Morphological changes in tree shrew cells of different tissues and positive control cells before and after dengue virus (DENV) infection under inverted microscope (×200)Note: TAECs, tree shrew aortic endothelial cells; TASMCs, tree shrew aortic smooth muscle cells; pTRECs, primary tree shrew renal epithelial cells; THs, tree shrew hepatocytes; TBMECs, tree shrew brain microvascular endothelial cells; TCSCs, tree shrew corneal stromal cells; TSFs, tree shrew skin fibroblasts; TRMECs, tree shrew retinal microvascular endothelial cells; The C6/36, Vero, A549 and BHK-21 cells were employed as positive controls.
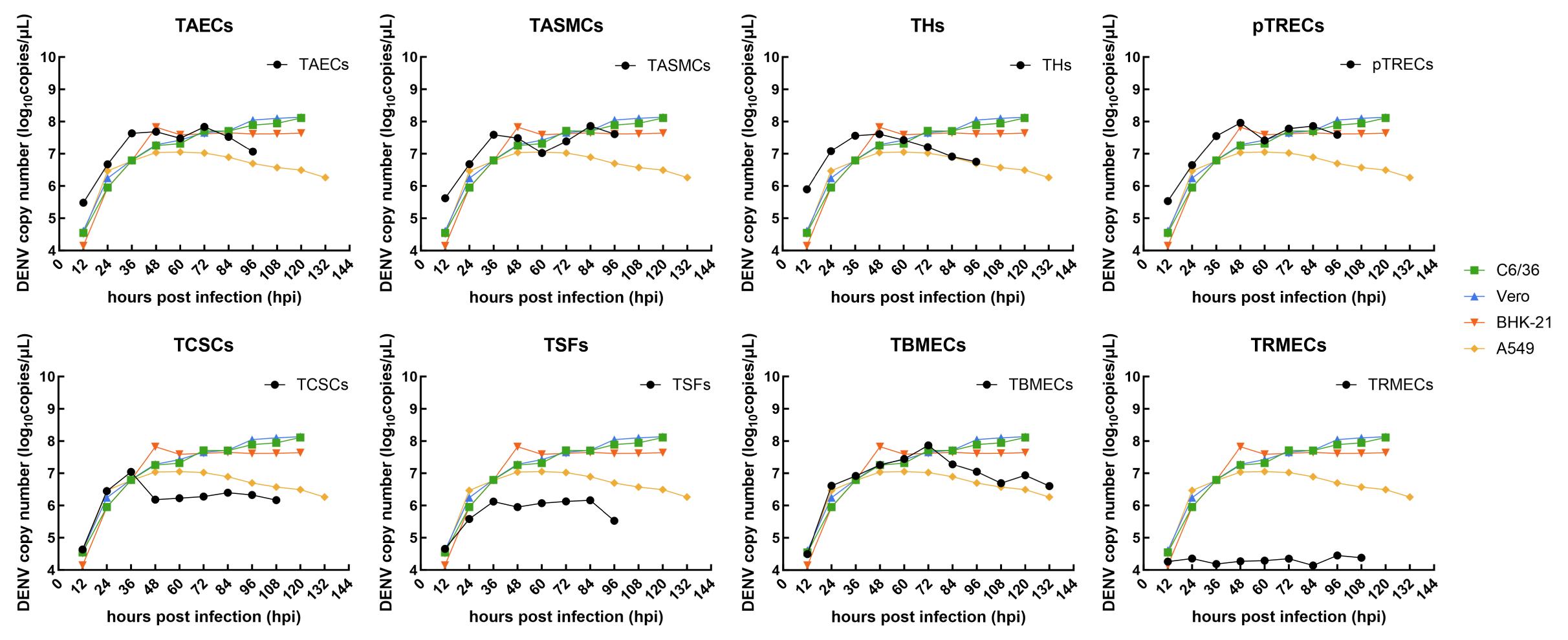
Figure 2 Viral proliferation curves of tree shrew cells of different tissues after dengue virus (DENV) infection via real-time fluorescent quatitative PCRNote: TAECs, tree shrew aortic endothelial cells; TASMCs, tree shrew aortic smooth muscle cells; THs, tree shrew hepatocytes; pTRECs, primary tree shrew renal epithelial cells; TSFs, tree shrew skin fibroblasts; TCSCs, tree shrew corneal stromal cells; TBMECs, tree shrew brain microvascular endothelial cells; TRMECs, tree shrew retinal microvascular endothelial cells; The C6/36, Vero, A549 and BHK-21 cells were employed as positive controls.
细胞 Cell | 病毒载量峰值/(拷贝·μL-1) Peak viral load/ (copies·μL-1) | 时间/h Time/ h |
|---|---|---|
| pTRECs | (9.18±0.21)×107 | 48 |
| TBMECs | (7.43±0.75)×107 | 72 |
| TASMCs | (7.27±0.43)×107 | 84 |
| TAECs | (6.87±0.30)×107 | 72 |
| THs | (4.08±0.02)×107 | 48 |
| TCSCs | (1.11±0.05)×107 | 36 |
| TSFs | (1.50±0.05)×106 | 84 |
| Vero | (1.39±0.22)×108 | 120 |
| C6/36 | (1.28±0.21)×108 | 120 |
| BHK-21 | (6.73±0.13)×107 | 48 |
| A549 | (1.13±0.05)×107 | 60 |
Table 2 Peak viral load and time-to-peak in different cell types after dengue virus (DENV) infection
细胞 Cell | 病毒载量峰值/(拷贝·μL-1) Peak viral load/ (copies·μL-1) | 时间/h Time/ h |
|---|---|---|
| pTRECs | (9.18±0.21)×107 | 48 |
| TBMECs | (7.43±0.75)×107 | 72 |
| TASMCs | (7.27±0.43)×107 | 84 |
| TAECs | (6.87±0.30)×107 | 72 |
| THs | (4.08±0.02)×107 | 48 |
| TCSCs | (1.11±0.05)×107 | 36 |
| TSFs | (1.50±0.05)×106 | 84 |
| Vero | (1.39±0.22)×108 | 120 |
| C6/36 | (1.28±0.21)×108 | 120 |
| BHK-21 | (6.73±0.13)×107 | 48 |
| A549 | (1.13±0.05)×107 | 60 |
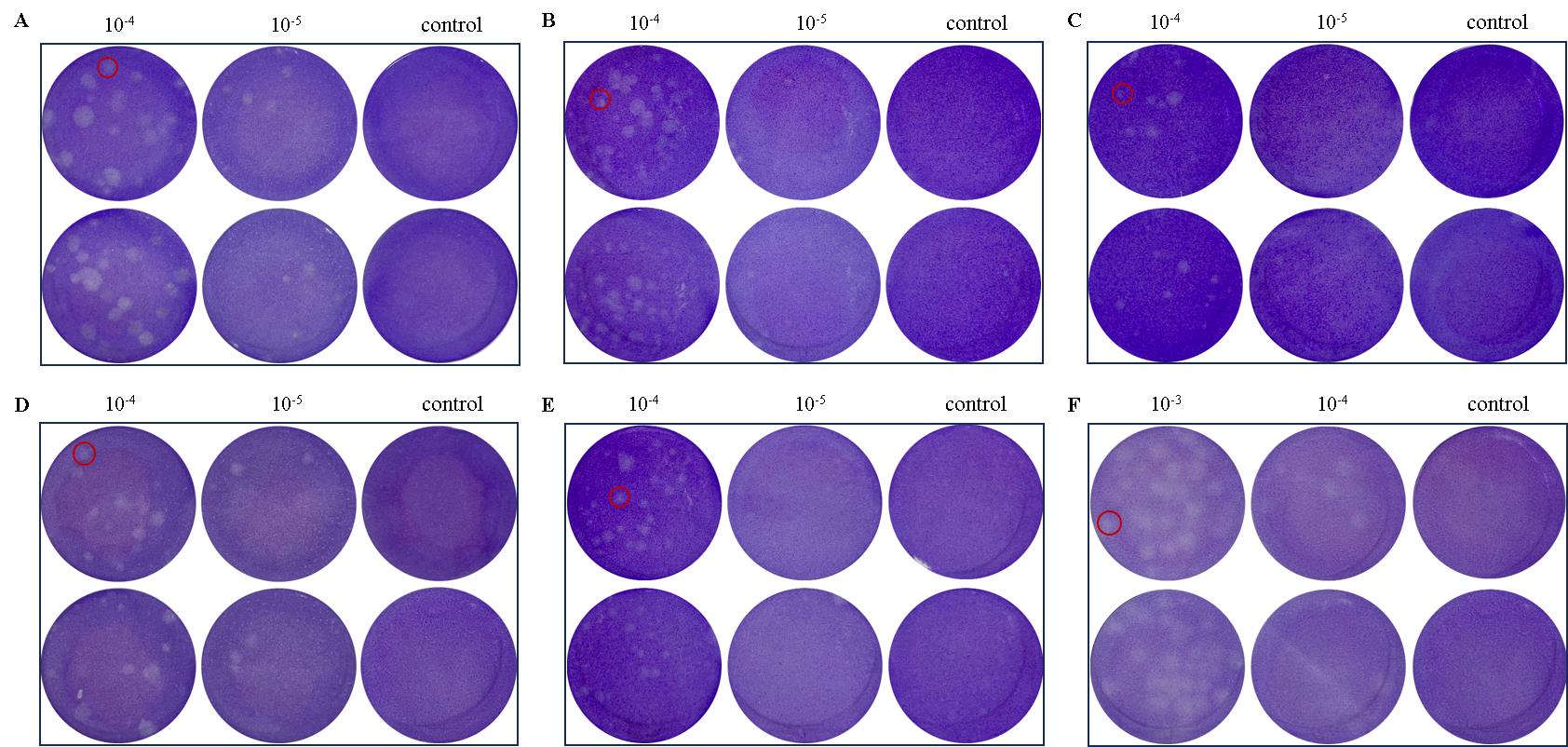
Figure 3 Quantification of dengue virus (DENV) titer in tree shrew cells of different tissues via plaque assayNote: A, Plaques formed by DENV proliferating in C6/36 (positive control); B, Plaques formed in tree shrew aortic endothelial cells (TAECs); C, Plaques formed tree shrew aortic smooth muscle cells (TASMCs); D, Plaques formed in tree shrew hepatocytes (THs); E, Plaques formed by DENV proliferating in primary tree shrew renal epithelial cells (pTRECs); F, Plaques in primary tree shrew brain microvascular endothelial cells (TBMECs). The C6/36 cells were employed as the positive control. Red circles demarcate individual plaques. Viral dilutions are indicated as 10-3, 10-4, and 10-5. The “control” denotes uninfected wells, serving as a negative reference.
| 1 | STABELL A C, MEYERSON N R, GULLBERG R C, et al. Dengue viruses cleave STING in humans but not in nonhuman primates, their presumed natural reservoir[J]. eLife, 2018, 7: e31919. DOI:10.7554/eLife.31919 . |
| 2 | World Health Organization. Dengue-Global situation [EB/OL]. (2024-05-30)[2024-09-27]. https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON518. |
| 3 | WILDER-SMITH A. Dengue vaccine development: challenges and prospects[J]. Curr Opin Infect Dis, 2022, 35(5):390-396. DOI:10.1097/QCO.0000000000000871 . |
| 4 | MALAVIGE G N, OGG G S. Molecular mechanisms in the pathogenesis of dengue infections[J]. Trends Mol Med, 2024, 30(5):484-498. DOI:10.1016/j.molmed.2024.03.006 . |
| 5 | KAYESH M E H, TSUKIYAMA-KOHARA K. Mammalian animal models for dengue virus infection: a recent overview[J]. Arch Virol, 2022, 167(1):31-44. DOI:10.1007/s00705-021-05298-2 . |
| 6 | CHIU H C, HANNEMANN H, HEESOM K J, et al. High-throughput quantitative proteomic analysis of dengue virus type 2 infected A549 cells[J]. PLoS One, 2014, 9(3): e93305. DOI:10.1371/journal.pone.0093305 . |
| 7 | RIVERA J A, RENGIFO A C, SARMIENTO L, et al. Nuclei ultrastructural changes of C6/36 cells infected with virus dengue type 2[J]. Biomedica, 2018, 38(0):135-143. DOI:10.7705/biomedica.v38i0.3997 . |
| 8 | 杨佳佳, 姜黎明, 罗佳, 等. 登革病毒感染C6/36、Vero、BHK-21和THP-1细胞CPE的比较及病毒检测[J]. 中国生物制品学杂志, 2017, 30(4):362-365, 371. DOI: 10.13200/j.cnki.cjb.001705 . |
| YANG J J, JIANG L M, LUO J, et al. CPE caused by dengue virus in C6/36, Vero, BHK-21 and THP 1 cells and virus test[J]. Chin J Biol, 2017, 30(4):362-365, 371. DOI: 10.13200/j.cnki.cjb.001705 . | |
| 9 | 林垚, 洪珊, 王晓丹, 等. Ⅲ型登革病毒在C6/36及Vero细胞中复制对其毒力的影响[J]. 中国生物制品学杂志, 2019, 32(10):1070-1073, 1079. DOI: 10.13200/j.cnki.cjb.002826 . |
| LIN Y, HONG S, WANG X D, et al. Effect of replication of dengue virus type Ⅲ in C6/36 and Vero cells on virulence[J]. Chin J Biol, 2019, 32(10):1070-1073, 1079. DOI: 10.13200/j.cnki.cjb.002826 . | |
| 10 | LEE H C, YEN Y T, CHEN W Y, et al. Dengue type 4 live-attenuated vaccine viruses passaged in vero cells affect genetic stability and dengue-induced hemorrhaging in mice[J]. PLoS One, 2011, 6(10): e25800. DOI:10.1371/journal.pone. 0025800 . |
| 11 | EMENY J M, MORGAN M J. Regulation of the interferon system: evidence that vero cells have a genetic defect in interferon production[J]. J Gen Virol, 1979, 43(1):247-252. DOI:10.1099/0022-1317-43-1-247 . |
| 12 | ZHAO X P, TANG Z Y, KLUMPP B, et al. Primary hepatocytes of Tupaia belangeri as a potential model for hepatitis C virus infection[J]. J Clin Invest, 2002, 109(2):221-232. DOI:10.1172/JCI13011 . |
| 13 | ZHANG L, SHEN Z L, FENG Y, et al. Infectivity of Zika virus on primary cells support tree shrew as animal model[J]. Emerg Microbes Infect, 2019, 8(1):232-241. DOI:10.1080/22221751. 2018.1559707 . |
| 14 | KAYESH M E H, KITAB B, SANADA T, et al. Susceptibility and initial immune response of Tupaia belangeri cells to dengue virus infection[J]. Infect Genet Evol, 2017, 51:203-210. DOI:10.1016/j.meegid.2017.04.003 . |
| 15 | 丁相荣, 陈柳, 霍姝汭, 等. 树鼩角膜基质永生化细胞系的构建及其在病毒感染性方面的研究[J]. 中国实验动物学报, 2024, 32(5):610-619. DOI: 10.3969/j.issn.1005-4847.2024.05.008 . |
| DING X R, CHEN L, HUO S R, et al. Construction of immortalized tree shrew corneal stromal cell line and investigation of viral infectivity[J]. Acta Lab Anim Sci Sin, 2024, 32(5):610-619. DOI: 10.3969/j.issn.1005-4847.2024.05.008 . | |
| 16 | GUZMAN M G, GUBLER D J, IZQUIERDO A, et al. Dengue infection[J]. Nat Rev Dis Primers, 2016, 2:16055. DOI:10.1038/nrdp.2016.55 . |
| 17 | HUITS R, ANGELO K M, AMATYA B, et al. Clinical characteristics and outcomes among travelers with severe dengue: a GeoSentinel analysis[J]. Ann Intern Med, 2023, 176(7):940-948. DOI:10.7326/M23-0721 . |
| 18 | 孙爱娟. 登革1型病毒和登革2型病毒在C6/36细胞及白纹伊蚊体内共感染的研究[D]. 合肥: 安徽医科大学, 2018. |
| SUN A J. Research on co-infection of DENV-1 and DENV-2 in C6/36 cells and Aedes albopictus[D]. Hefei: Anhui Medical University, 2018. | |
| 19 | HARAPAN H, MICHIE A, SASMONO R T, et al. Dengue: a minireview[J]. Viruses, 2020, 12(8): E829. DOI:10.3390/v12080829 . |
| 20 | 国家卫生健康委办公厅, 国家中医药局综合司. 关于印发麻疹等传染病诊疗方案(2024年版)的通知[EB/OL]. (2024-07-19)[2024-10-14]. https://www.gov.cn/zhengce/zhengceku/202407/content_6965110.htm. |
| General Office of the National Health Commission, Comprehensive Departmentof the National Administration of Traditional Chinese Medicine. Notice on issuing the diagnosis and treatment protocols for measles and other infectious diseases (2024 edition) [EB/OL]. (2024-07-19) [2024-10-14]. https://www.gov.cn/zhengce/zhengceku/202407/content_6965110.htm. | |
| 21 | PANG X J, ZHANG R D, CHENG G. Progress towards understanding the pathogenesis of dengue hemorrhagic fever[J]. Virol Sin, 2017, 32(1):16-22. DOI:10.1007/s12250-016-3855-9 . |
| 22 | SHARP T M, ANDERSON K B, KATZELNICK L C, et al. Knowledge gaps in the epidemiology of severe dengue impede vaccine evaluation[J]. Lancet Infect Dis, 2022, 22(2): e42-e51. DOI:10.1016/S1473-3099(20)30871-9 . |
| 23 | VIJAYAKRISHNAN S, JIU Y M, HARRIS J R. Virus infected cells (subcellular biochemistry book 106)[M]. Berlin: Springer, 2023:197-210. |
| 24 | KNEZEVIC I, STACEY G, PETRICCIANI J, et al. Evaluation of cell substrates for the production of biologicals: Revision of WHO recommendations. Report of the WHO Study Group on Cell Substrates for the Production of Biologicals, 22-23 April 2009, Bethesda, USA[J]. Biologicals, 2010, 38(1):162-169. DOI:10.1016/j.biologicals.2009.08.019 . |
| 25 | 程尧, 程小玲, 申瑷琳, 等. 人用疫苗生产用细胞基质使用现状及研究进展[J]. 国际生物制品学杂志, 2023, 46(5):235-239. DOI: 10.3760/cma.j.cn311962-20221108-00078 . |
| CHENG Y, CHENG X L, SHEN A L, et al. Current status and research progress of cell substrate for human vaccine production[J]. Int J Biol, 2023, 46(5):235-239. DOI: 10.3760/cma.j.cn311962-20221108-00078 . | |
| 26 | AUBRIT F, PERUGI F, LÉON A, et al. Cell substrates for the production of viral vaccines[J]. Vaccine, 2015, 33(44):5905-5912. DOI:10.1016/j.vaccine.2015.06.110 . |
| 27 | 李爱灵, 王红燕, 张月兰, 等. Vero细胞传代过程中致瘤性的检测[J]. 中华微生物学和免疫学杂志, 2011, 31(5):456-461. DOI: 10.3760/cma.j.issn.0254-5101.2011.05.017 . |
| LI A L, WANG H Y, ZHANG Y L, et al. Experimental investigation on tumorigenicity of Vero cell in the process of passage[J]. Chin J Microbiol Immunol, 2011, 31(5):456-461. DOI: 10.3760/cma.j.issn.0254-5101.2011.05.017 . | |
| 28 | BELOUKAS A. Dengue: Another viral infection with mucocutaneous manifestations[J]. J Eur Acad Dermatol Venereol, 2024, 38(1):15-16. DOI:10.1111/jdv.19589 . |
| 29 | WEI K C, WEI W J, LIU Y S, et al. Assessment of prolonged dengue virus infection in dermal fibroblasts and hair-follicle dermal papilla cells[J]. Viruses, 2020, 12(3):267. DOI:10.3390/v12030267 . |
| 30 | PAGLIARI C, QUARESMA J S, DOS-SANTOS W C, et al. Mechanisms of programmed cell death associated to severe dengue in human renal lesions[J]. Microb Pathog, 2024, 194:106794. DOI:10.1016/j.micpath.2024.106794 . |
| 31 | THONGTAN T, PANYIM S, SMITH D R. Apoptosis in dengue virus infected liver cell lines HepG2 and Hep3B[J]. J Med Virol, 2004, 72(3):436-444. DOI:10.1002/jmv.20004 . |
| 32 | DIELIS A W, SHADID S. A 66-year old woman with large vessel vasculitis after a dengue virus infection[J]. Brit J Diab Vasc Dis, 2013, 13(1):56-57. DOI:10.1177/1474651412475099 . |
| 33 | ARIAS-ARIAS J L, VEGA-AGUILAR F, CORRALES-AGUILAR E, et al. Dengue virus infection of primary human smooth muscle cells[J]. Am J Trop Med Hyg, 2018, 99(6):1451-1457. DOI:10.4269/ajtmh.18-0175 . |
| 34 | 周旋. 登革3型病毒感染人脑微血管内皮细胞的机制研究[D]. 广州: 南方医科大学, 2014. |
| ZHOU X. Mechanism study of dengue virus type 3 infecting the human brain microvascular endothelial cells[D]. Guangzhou: Southern Medical University, 2014. | |
| 35 | LUCENA-NETO F D, FALCÃO L F M, MORAES E C D S, et al. Dengue fever ophthalmic manifestations: a review and update[J]. Rev Med Virol, 2023, 33(2): e2422. DOI:10.1002/rmv.2422 . |
| 36 | NG A W, TEOH S C. Dengue eye disease[J]. Surv Ophthalmol, 2015, 60(2):106-114. DOI:10.1016/j.survophthal.2014.07.003 . |
| [1] | WANG Jiaoxiang, ZHANG Lu, CHEN Shuhan, JIAO Deling, ZHAO Heng, WEI Taiyun, GUO Jianxiong, XU Kaixiang, WEI HongJiang. Construction and Functional Validation of GTKO/hCD55 Gene-Edited Xenotransplant Donor Pigs [J]. Laboratory Animal and Comparative Medicine, 2025, (): 1-12. |
| [2] | WANG Tingjun, LUO Hao, CHEN Qi. Informationization Upgrade and Application of Laboratory Animal Center Based on Artificial Intelligence [J]. Laboratory Animal and Comparative Medicine, 2025, (): 1-10. |
| [3] | ZHENG Qingyong, YANG Donghua, MA Zhichao, ZHOU Ziyu, LU Yang, WANG Jingyu, XING Lina, KANG Yingying, DU Li, ZHAO Chunxiang, DI Baoshan, TIAN Jinhui. Precautions for Conducting Systematic Reviews and Meta-Analyses of Animal Experiments [J]. Laboratory Animal and Comparative Medicine, 2025, (): 1-11. |
| [4] | XIAO Linlin, YANG Yixuan, LI Shanshan, RUO Lanshiyu, YIN Siwei, SUN Juming, SHI Wei, OUYANG Yiqiang, LI Xiyi. A Rat Model of Alzheimer's Disease by Introducing Human Triple Mutant APP Gene into Hippocampus using Brain Stereotactic Technology [J]. Laboratory Animal and Comparative Medicine, 2025, (): 1-10. |
| [5] | LIU Wei, XU Zhongkan, HOU Fengtian, ZHANG Xinyan, QIAO Han, MA Liying. Evaluation of Proficiency Testing Results of Illuminance for animal in Laboratory Animal Facilities [J]. Laboratory Animal and Comparative Medicine, 2025, (): 1-8. |
| [6] | LIU Yueqin, XUE Weiguo, WANG Shuyou, SHEN Yaohua, JIA Shuyong, WANG Guangjun, SONG Xiaojing. Observation of Morphology of Digestive Tract in the Mice Appling Probe - based Confocal Laser Endomicroscopy [J]. Laboratory Animal and Comparative Medicine, 2025, (): 1-9. |
| [7] | OU Meizhen, LI Yongfeng, WEN Sha, LIAO Zhouxiang, HUANG Xuejing, YANG Lichao, HE Min. Preparation of Monoclonal Antibody to VASN in Tree Shrew and Exploration of Its Application [J]. Laboratory Animal and Comparative Medicine, 2025, (): 1-12. |
| [8] | JIANG Juan, SONGNing , LIAN Wenbo, SHAO Congcong, GU Wenwen, SHI Yan. The Comparison of Phenotypes in a Mouse Model of Intrauterine Adhesions Induced by Two Concentrations of Ethanol [J]. Laboratory Animal and Comparative Medicine, 2025, (): 1-9. |
| [9] | LIN Zhenhua, CHU Xiangyu, WEI Zhenxi, DONG Chuanjun, ZHAO Zenglin, SUN Xiaoxia, LI Qingyu, ZHANG Qi. Evaluation of the safety and efficacy of bone cement in experimental pigs using vertebroplasty [J]. Laboratory Animal and Comparative Medicine, 2025, (): 1-7. |
| [10] | GONG Leilei, WANG Xiaoxia, FENG Xuewei, LI Xinlei, ZHAO Han, ZHANG Xueyan, FENG Xin. Model and Mechanism of Action of Premature Ovarian Insufficiency Induced by Cyclophosphamide at Different Concentrations Based on SIRT5-FOXO3a Signaling Pathway [J]. Laboratory Animal and Comparative Medicine, 2025, (): 1-7. |
| [11] | . [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 247-250. |
| [12] | MIN Fangui, FU Hongkun, LIU Yonggang, LIU Xiangmei, LIU Zhonghua, LI Yao, TAO Yufeng. Special Welfare and Ethical Requirements for Infectious Animal Experiments [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 239-246. |
| [13] | PAN Qianjia, GE Junyi, HU Nan, HUA Fei, GU Min. Differential Analysis of Oral Microbiota in db/db Mouse Model of Type 2 Diabetes Utilizing 16S rRNA Sequencing [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 147-157. |
| [14] | XU Qiuyu, YAN Guofeng, FU Li, FAN Wenhua, ZHOU Jing, ZHU Lian, QIU Shuwen, ZHANG Jie, WU Ling. A Mouse Model of Polycystic Ovary Syndrome Established Through Subcutaneous Administration of Letrozole Sustained-Release Pellets and Hepatic Transcriptome Analysis [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 119-129. |
| [15] | LIAN Hui, JIANG Yanling, LIU Jia, ZHANG Yuli, XIE Wei, XUE Xiaoou, LI Jian. Construction and Evaluation of a Rat Model of Abnormal Uterine Bleeding [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 130-146. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||