
Laboratory Animal and Comparative Medicine ›› 2025, Vol. 45 ›› Issue (2): 119-129.DOI: 10.12300/j.issn.1674-5817.2024.186
• Animal Models of Human Diseases • Next Articles
XU Qiuyu1, YAN Guofeng2, FU Li2, FAN Wenhua2, ZHOU Jing2, ZHU Lian2, QIU Shuwen1, ZHANG Jie1, WU Ling1( )(
)( )
)
Received:2024-12-17
Revised:2025-02-06
Online:2025-04-25
Published:2025-05-12
Contact:
WU Ling
CLC Number:
XU Qiuyu,YAN Guofeng,FU Li,et al. A Mouse Model of Polycystic Ovary Syndrome Established Through Subcutaneous Administration of Letrozole Sustained-Release Pellets and Hepatic Transcriptome Analysis[J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 119-129. DOI: 10.12300/j.issn.1674-5817.2024.186.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2024.186
引物名称 Primer name | 引物序列 Sequence (5'→3') |
|---|---|
| 36B4-F | AGATTCGGGATATGCTGTTGGC |
| 36B4-R | TCGGGTCCTAGACCAGTGTTC |
| HSD3B2-F | GGTTTTTGGGGCAGAGGATCA |
| HSD3B2-R | GGTACTGGGTGTCAAGAATGTCT |
| HMGCR-F | CAAACATTGTCACCGCCATC |
| HMGCR-R | CCACCCACCGTTCCTATCTC |
| IL4-F | GGTCTCAACCCCCAGCTAGT |
| IL4-R | GCCGATGATCTCTCTCAAGTGAT |
| CCL2-F | TTAAAAACCTGGATCGGAACCAA |
| CCL2-R | GCATTAGCTTCAGATTTACGGGT |
| COL1A1-F | GCTCCTCTTAGGGGCCACT |
| COL1A1-R | CCACGTCTCACCATTGGGG |
Table 1 Sequences of primers
引物名称 Primer name | 引物序列 Sequence (5'→3') |
|---|---|
| 36B4-F | AGATTCGGGATATGCTGTTGGC |
| 36B4-R | TCGGGTCCTAGACCAGTGTTC |
| HSD3B2-F | GGTTTTTGGGGCAGAGGATCA |
| HSD3B2-R | GGTACTGGGTGTCAAGAATGTCT |
| HMGCR-F | CAAACATTGTCACCGCCATC |
| HMGCR-R | CCACCCACCGTTCCTATCTC |
| IL4-F | GGTCTCAACCCCCAGCTAGT |
| IL4-R | GCCGATGATCTCTCTCAAGTGAT |
| CCL2-F | TTAAAAACCTGGATCGGAACCAA |
| CCL2-R | GCATTAGCTTCAGATTTACGGGT |
| COL1A1-F | GCTCCTCTTAGGGGCCACT |
| COL1A1-R | CCACGTCTCACCATTGGGG |
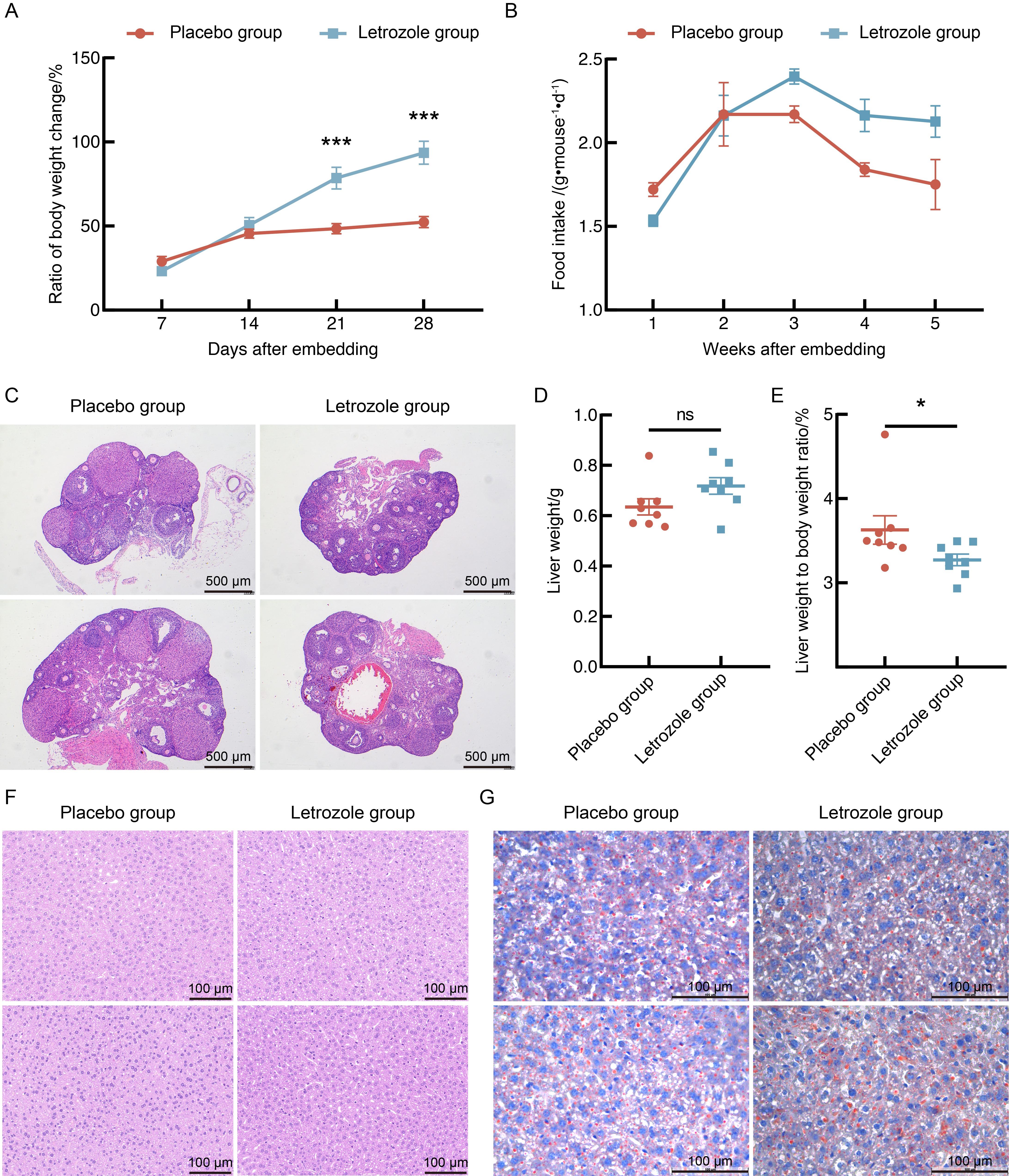
Figure 1 Effects of letrozole and high-fat diet modeling on body weight, food intake and liver in miceNote: A, Weekly rate of body weight change of mice during the first 4 weeks compared to their initial body weight; B, Weekly food intake of mice in 24 hours during the modeling period; C, Ovary HE staining of mice (×50); D, Liver weight of mice; E, Liver-to-body weight ratio of mice; F, Liver HE staining of mice (×400); G, Liver Oil Red O staining of mice (×400). Each group consisted of 8 mice. Compared with placebo group, *P<0.05, ***P<0.001, nsP>0.05. In fig. C, F and G, the upper and lower panels show two independent biological replicates.
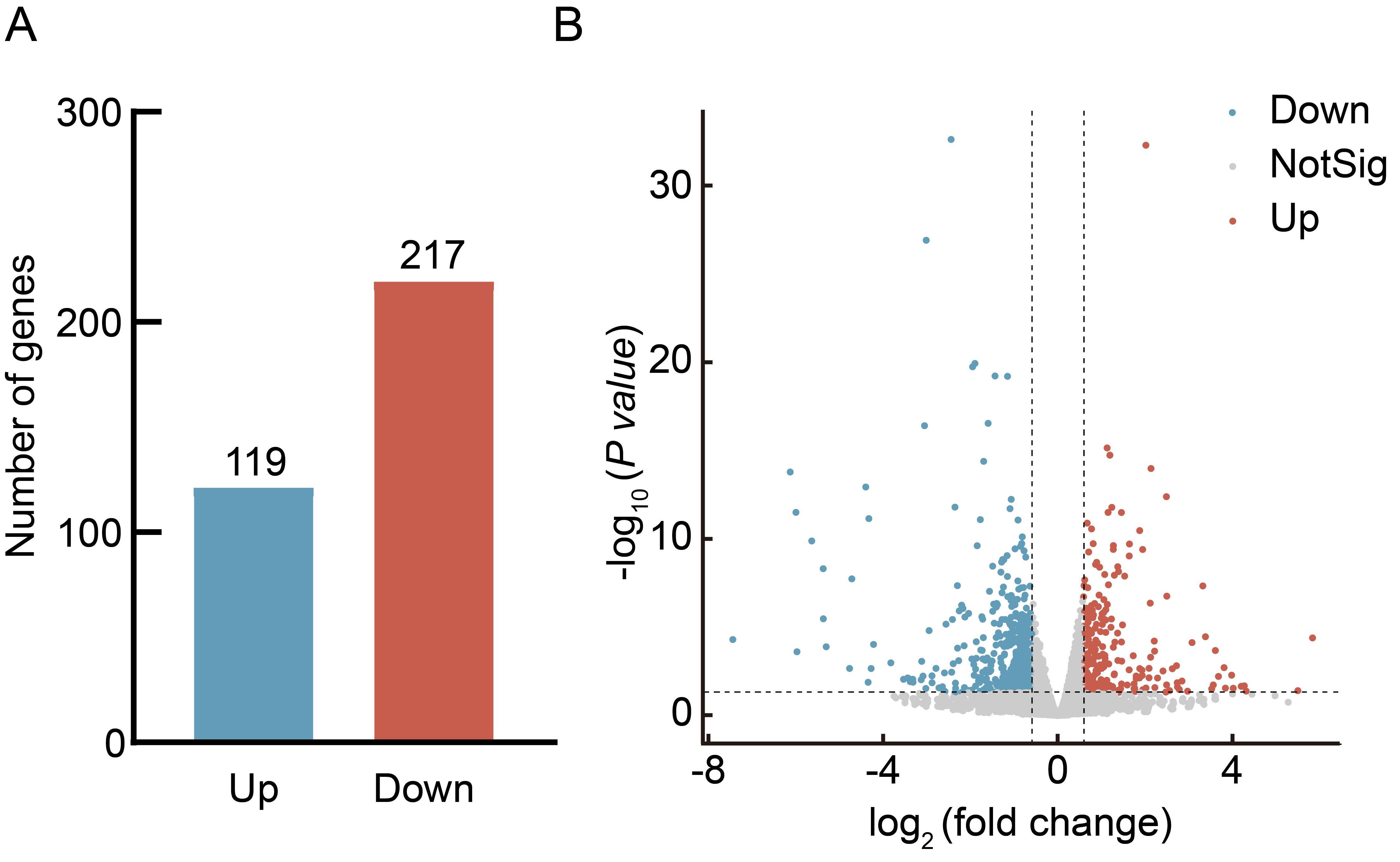
Figure 2 Analysis of differentially expressed genes in liver of mice after letrozole and high-fat diet modelingNote:A, Bar chart of the number of differentially expressed genes between the letrozole model group and the placebo control group; B, Volcano plot of differentially expressed genes between the letrozole model group and the placebo control group.
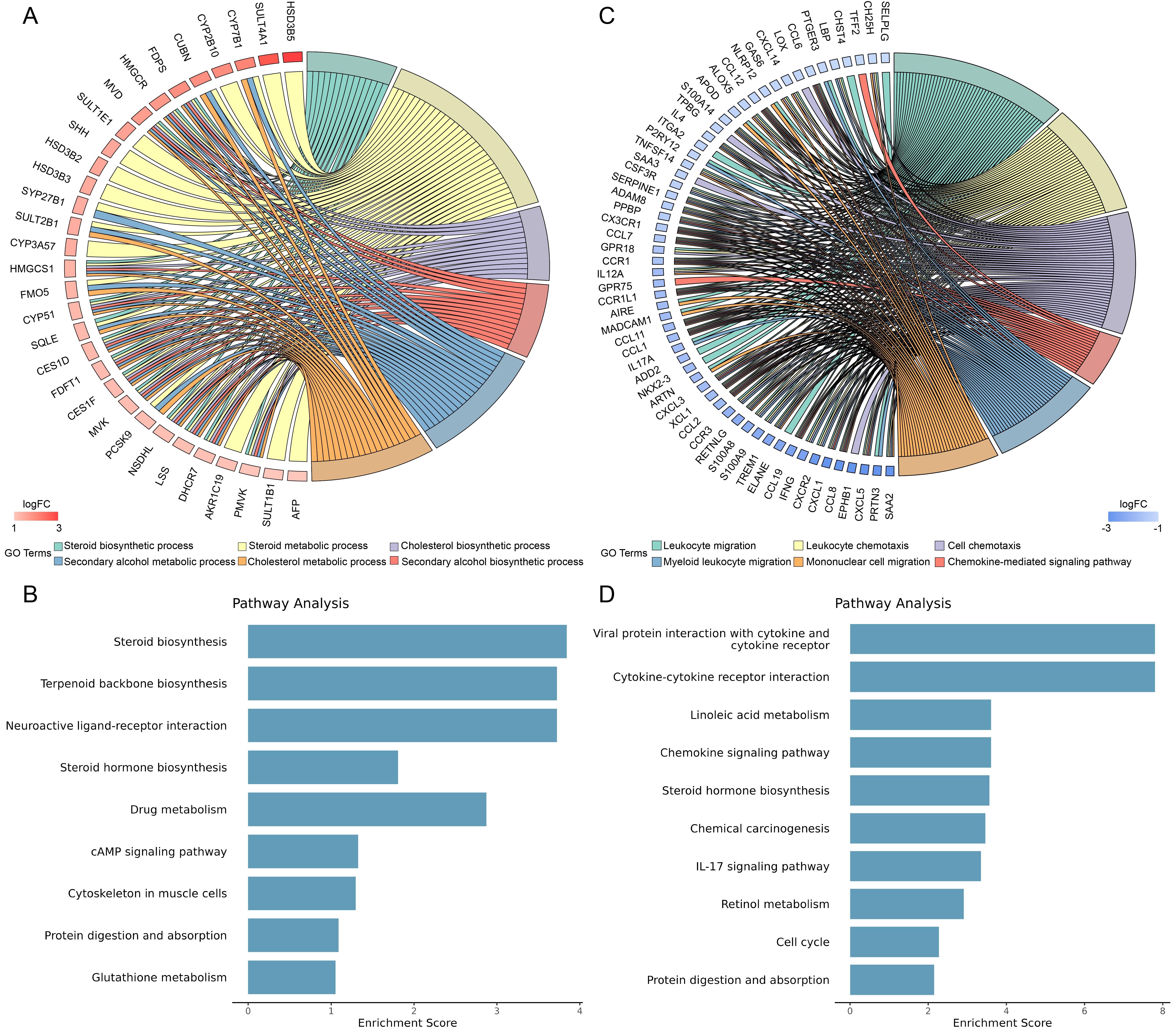
Figure 3 Functional annotation and enrichment analysis of differentially expressed genes in mice after letrozole and high-fat diet modelingNote: A, Representative GO functional annotations of upregulated differentially expressed genes in the letrozole model group compared to the placebo control group; B, Representative KEGG enrichment analysis of upregulated differentially expressed genes in the letrozole model group compared to the placebo control group; C, Representative GO functional annotations of downregulated differentially expressed genes in the letrozole model group compared to the placebo control group; D, Representative KEGG enrichment analysis of downregulated differentially expressed genes in the letrozole model group compared to the placebo control group.
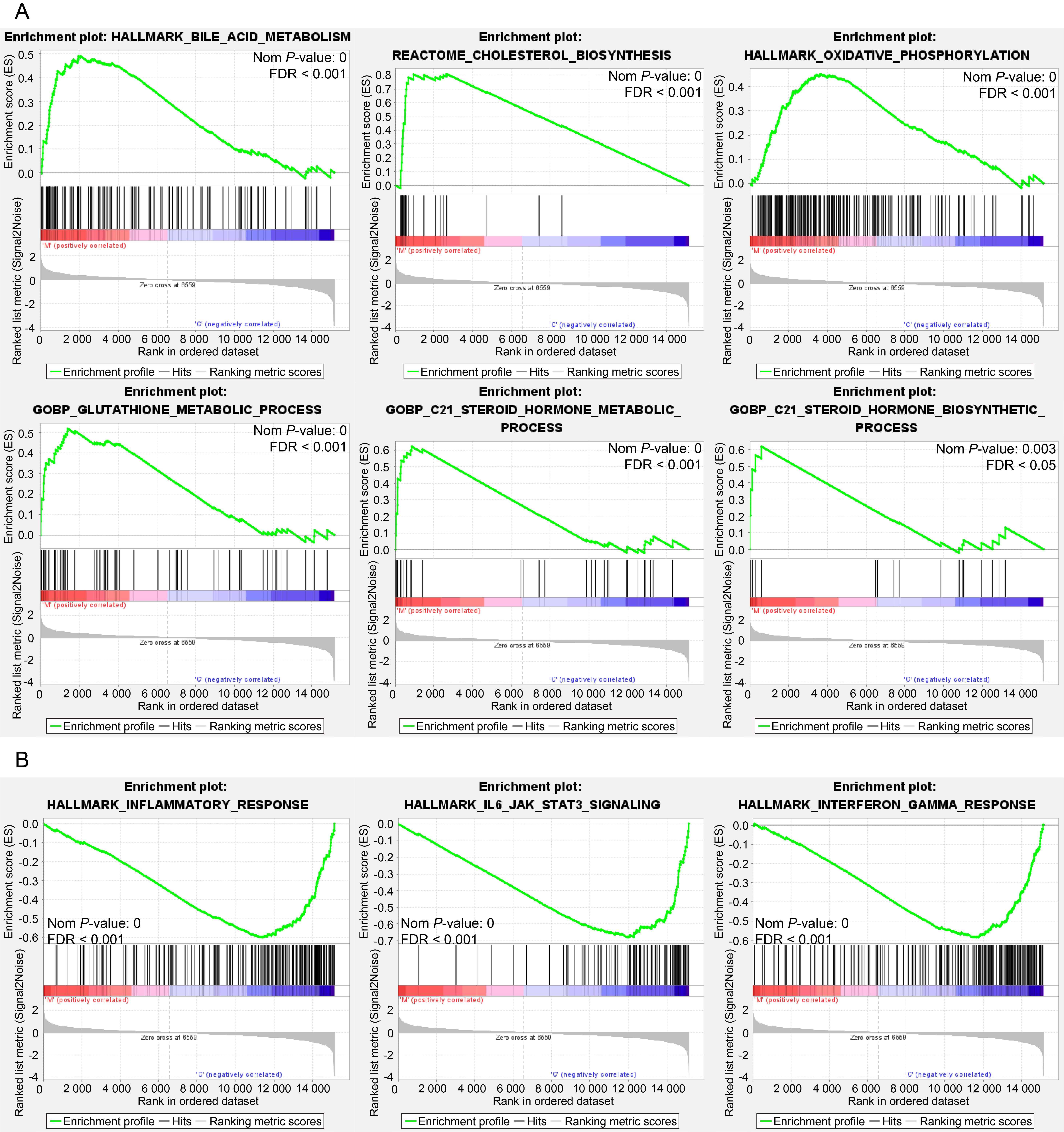
Figure 4 GSEA enrichment analysis of differentially expressed genes after letrozole and high-fat diet modelingNote: A-B, GSEA enrichment analysis of genes in the letrozole model group compared to the placebo control group (A,upregulated gene sets; B, downregulated gene sets). FDR, False discovery rate.
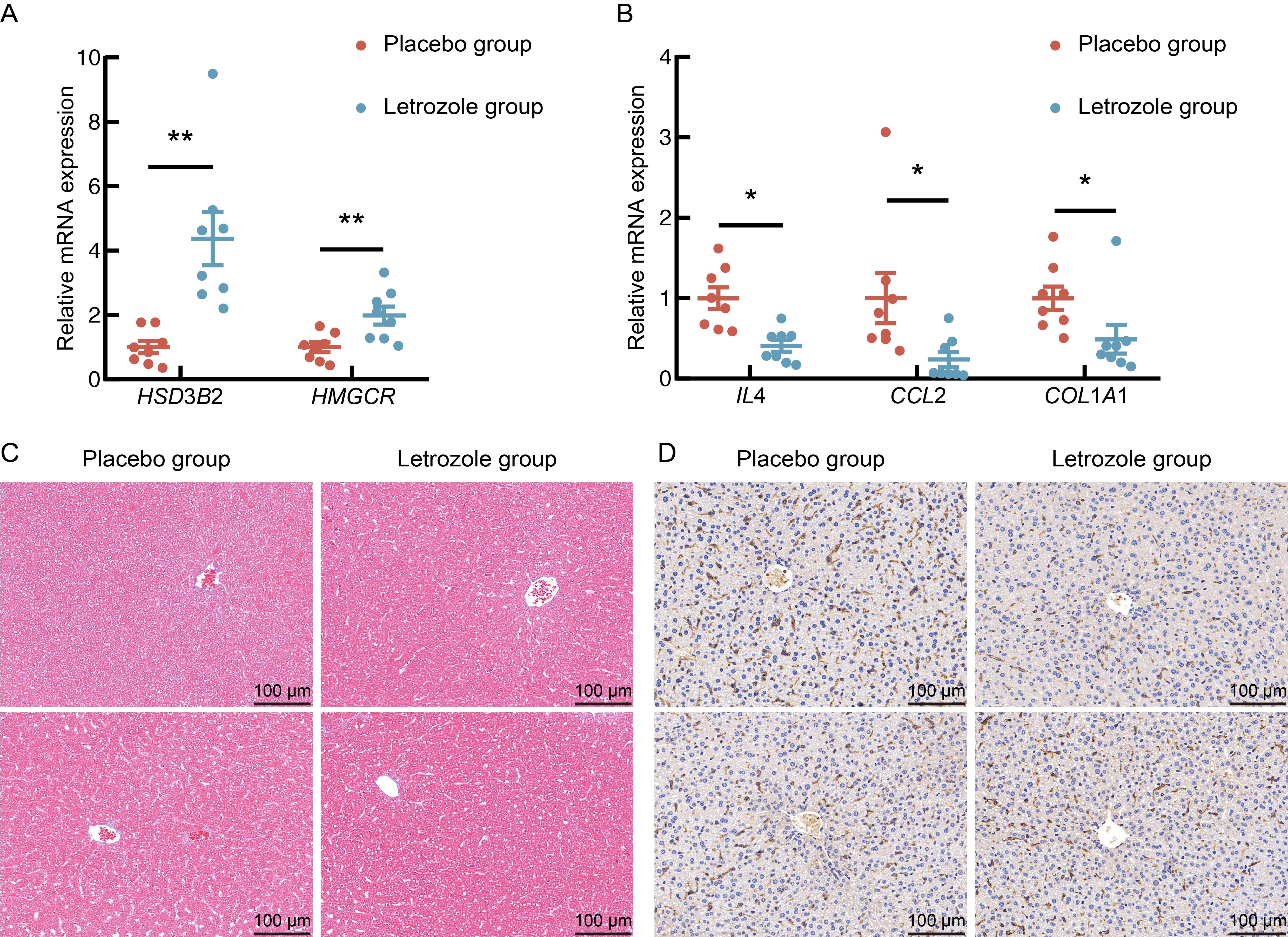
Figure 5 Real-time quantitative PCR verification of differentially expressed genes in mice after letrozole and high-fat diet modelingNote: A, Relative expression levels of 3 beta- and steroid delta-isomerase 2 (HSD3B2) and 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR); B, Relative expression levels of interleukin-4 (IL4),C-C motif chemokine ligand 2 (CCL2), and collagen, type I, alpha 1 (COL1A1); C,Liver Masson staining (×400); D, Liver F4/80 immunohistochemistry staining (×400). Each group consisted of 8 mice. Compared with placebo group, *P<0.05, **P<0.01. In fig. C-D, the upper and lower panels show two independent biological replicates.
| 1 | Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome[J]. Fertil Steril, 2008, 89(3):505-522. DOI:10.1016/j.fertnstert.2007.09.041 . |
| 2 | TEEDE H J, TAY C T, LAVEN J J E, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome[J]. J Clin Endocrinol Metab, 2023, 108(10):2447-2469. DOI:10.1210/clinem/dgad463 . |
| 3 | HOEGER K M, DOKRAS A, PILTONEN T. Update on PCOS: consequences, challenges, and guiding treatment[J]. J Clin Endocrinol Metab, 2021, 106(3): e1071-e1083. DOI:10.1210/clinem/dgaa839 . |
| 4 | KAUFFMAN A S, THACKRAY V G, RYAN G E, et al. A novel letrozole model recapitulates both the reproductive and metabolic phenotypes of polycystic ovary syndrome in female mice[J]. Biol Reprod, 2015, 93(3):69. DOI:10.1095/biolreprod.115.131631 . |
| 5 | BEGUM N, MANIPRIYA K, VEERESH B. Role of high-fat diet on letrozole-induced polycystic ovarian syndrome in rats[J]. Eur J Pharmacol, 2022, 917:174746. DOI:10.1016/j.ejphar. 2022. 174746 . |
| 6 | VASSILATOU E, VASSILIADI D A, SALAMBASIS K, et al. Increased prevalence of polycystic ovary syndrome in premenopausal women with nonalcoholic fatty liver disease[J]. Eur J Endocrinol, 2015, 173(6):739-747. DOI:10.1530/EJE-15-0567 . |
| 7 | PASCHOU S A, POLYZOS S A, ANAGNOSTIS P, et al. Nonalcoholic fatty liver disease in women with polycystic ovary syndrome[J]. Endocrine, 2020, 67(1):1-8. DOI:10.1007/s12020-019-02085-7 . |
| 8 | GIANNOULI A, STEFANAKI C, KOUSKOUTIS C, et al. Hepatokine profile in adolescents with polycystic ovary syndrome: a case-control study[J]. J Clin Med, 2023, 12(17):5744. DOI:10.3390/jcm12175744 . |
| 9 | LIU S, HU W J, HE Y R, et al. Serum Fetuin-a levels are increased and associated with insulin resistance in women with polycystic ovary syndrome[J]. BMC Endocr Disord, 2020, 20(1):67. DOI:10.1186/s12902-020-0538-1 . |
| 10 | BEDNARSKA S, FRYCZAK J, SIEJKA A. Serum β-Klotho concentrations are increased in women with polycystic ovary syndrome[J]. Cytokine, 2020, 134:155188. DOI:10.1016/j.cyto. 2020.155188 . |
| 11 | SHAH M Z UL HAQ, SHRIVASTAVA V K, MIR M A, et al. Role of diacerein on steroidogenesis and folliculogenesis related genes in ovary of letrozole-induced PCOS mice[J]. Chem Biol Interact, 2023, 377:110468. DOI:10.1016/j.cbi.2023.110468 . |
| 12 | VIRTANEN N, SAARELA U, KARPALE M, et al. Roxadustat alleviates metabolic traits in letrozole-induced PCOS mice[J]. Biochem Pharmacol, 2024, 229:116522. DOI:10.1016/j.bcp. 2024.116522 . |
| 13 | TREFTS E, GANNON M, WASSERMAN D H. The liver[J]. Curr Biol, 2017, 27(21): R1147-R1151. DOI:10.1016/j.cub.2017.09.019 . |
| 14 | LIU D, GAO X, PAN X F, et al. The hepato-ovarian axis: genetic evidence for a causal association between non-alcoholic fatty liver disease and polycystic ovary syndrome[J]. BMC Med, 2023, 21(1):62. DOI:10.1186/s12916-023-02775-0 . |
| 15 | WU Y X, YANG X Y, HAN B S, et al. Naringenin regulates gut microbiota and SIRT1/PGC-1ɑ signaling pathway in rats with letrozole-induced polycystic ovary syndrome[J]. Biomed Pharmacother, 2022, 153:113286. DOI:10.1016/j.biopha.2022. 113286 . |
| 16 | PIECZYŃSKA J M, PRUSZYŃSKA-OSZMAŁEK E, KOŁODZIEJSKI P A, et al. The role of a high-fat, high-fructose diet on letrozole-induced polycystic ovarian syndrome in prepubertal mice[J]. Nutrients, 2022, 14(12):2478. DOI:10.3390/nu14122478 . |
| 17 | RYU K J, PARK H, HAN Y I, et al. Effects of time-restricted feeding on letrozole-induced mouse model of polycystic ovary syndrome[J]. Sci Rep, 2023, 13(1):1943. DOI:10.1038/s41598-023-28260-5 . |
| 18 | LI Z, LIU Y, WANG Y, et al. Sodium oligomannate's amelioration of reproductive and metabolic phenotypes in a letrozole-induced PCOS-like mouse model depends on the gut microbiome[J]. Biol Reprod, 2024, 111(2):361-375. DOI:10.1093/biolre/ioae058 . |
| 19 | BANASZEWSKA B, DULEBA A J, SPACZYNSKI R Z, et al. Lipids in polycystic ovary syndrome: role of hyperinsulinemia and effects of metformin[J]. Am J Obstet Gynecol, 2006, 194(5):1266-1272. DOI:10.1016/j.ajog.2005.11.009 . |
| 20 | SALILEW-WONDIM D, WANG Q, TESFAYE D, et al. Polycystic ovarian syndrome is accompanied by repression of gene signatures associated with biosynthesis and metabolism of steroids, cholesterol and lipids[J]. J Ovarian Res, 2015, 8:24. DOI:10.1186/s13048-015-0151-5 . |
| 21 | LI H, YU X H, OU X, et al. Hepatic cholesterol transport and its role in non-alcoholic fatty liver disease and atherosclerosis[J]. Prog Lipid Res, 2021, 83:101109. DOI:10.1016/j.plipres. 2021.101109 . |
| 22 | TAKEI A, NAGASHIMA S, TAKEI S, et al. Myeloid HMG-CoA reductase determines adipose tissue inflammation, insulin resistance, and hepatic steatosis in diet-induced obese mice[J]. Diabetes, 2020, 69(2):158-164. DOI:10.2337/db19-0076 . |
| 23 | SIDDIQUI S, MATEEN S, AHMAD R, et al. A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS)[J]. J Assist Reprod Genet, 2022, 39(11):2439-2473. DOI:10.1007/s10815-022-02625-7 . |
| 24 | JOHNSON D E, O'KEEFE R A, GRANDIS J R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer[J]. Nat Rev Clin Oncol, 2018, 15(4):234-248. DOI:10.1038/nrclinonc.2018.8 . |
| 25 | ASHOUR D, REBS S, ARAMPATZI P, et al. An interferon gamma response signature links myocardial aging and immunosenescence[J]. Cardiovasc Res, 2023, 119(14):2458-2468. DOI:10.1093/cvr/cvad068 . |
| 26 | ESCOBAR-MORREALE H F, LUQUE-RAMÍREZ M, GONZÁLEZ F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis[J]. Fertil Steril, 2011, 95(3):1048-1058.e1-2. DOI:10.1016/j.fertnstert. 2010.11.036 . |
| 27 | PHELAN N, O'CONNOR A, KYAW TUN T, et al. Leucocytosis in women with polycystic ovary syndrome (PCOS) is incompletely explained by obesity and insulin resistance[J]. Clin Endocrinol, 2013, 78(1):107-113. DOI:10.1111/j.1365-2265. 2012.04454.x . |
| 28 | EHSES J A, BÖNI-SCHNETZLER M, FAULENBACH M, et al. Macrophages, cytokines and beta-cell death in Type 2 diabetes[J]. Biochem Soc Trans, 2008, 36(Pt 3):340-342. DOI:10.1042/BST0360340 . |
| 29 | KRISHNAN A, MUTHUSAMI S, PERIYASAMY L, et al. Effect of DHT-induced hyperandrogenism on the pro-inflammatory cytokines in a rat model of polycystic ovary morphology[J]. Medicina, 2020, 56(3):100. DOI:10.3390/medicina56030100 . |
| [1] | LIU Rongle, CHENG Hao, SHANG Fusheng, CHANG Shufu, XU Ping. Study on Cardiac Aging Phenotypes of SHJH hr Mice [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 13-20. |
| [2] | WU Zhihao, CAO Shuyang, ZHOU Zhengyu. Establishment of an Intestinal Fibrosis Model Associated with Inflammatory Bowel Disease in VDR-/- Mice Induced by Helicobacter hepaticus Infection and Mechanism Exploration [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 37-46. |
| [3] | ZHANG Nan, LI Huaiyin, LIAN Xiaodi, WEI Juanpeng, GAO Ming. Effects of Different Durations of Light Exposure on Body Weight and Learning and Memory Abilities of NIH Mice [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 73-78. |
| [4] | ZHAO Xiaona, WANG Peng, YE Maoqing, QU Xinkai. Establishment of a New Hyperglycemic Obesity Cardiac Dysfunction Mouse Model with Triacsin C [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 605-612. |
| [5] | TAN He, YANG Xiaohui, ZHANG Daxiu, WANG Guicheng. Optimal Adaptation Period for Metabolic Cage Experiments in Mice at Different Developmental Stages [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 502-510. |
| [6] | MENG Yu, LIANG Dongli, ZHENG Linlin, ZHOU Yuanyuan, WANG Zhaoxia. Optimization and Evaluation of Conditions for Orthotopic Nude Mouse Models of Human Liver Tumor Cells [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 511-522. |
| [7] | QI Longju, CHEN Shiyuan, LIAO Zehua, SHI Yuanhu, SUN Yuyu, WANG Qinghua. Transcriptomic Analysis of Menstrual Blood-Derived Stem Cells Transplantation Combined with Exercise Training in Promoting Spinal Cord Injury Recovery in Rats [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 531-542. |
| [8] | Jing QIN, Yong ZHAO, Caiqin ZHANG, Bing BAI, Changhong SHI. Construction and Evaluation of Theranostic Near-infrared Fluorescent Probe for Targeting Inflammatory Brain Edema [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 243-250. |
| [9] | Yisu ZHANG, Xinru LIU, Ruojie WU, Rui LIU, Hong OUYANG, Xiaohong LI. Establishment and Evaluation of Mouse Model of Pregnancy Pain-depression Comorbidity Induced by Chronic Unpredictable Stress, Complete Freund's Adjuvant and Formalin [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 259-269. |
| [10] | Dong WU, Rui SHI, Peishan LUO, Ling'en LI, Xijing SHENG, Mengyang WANG, Lu NI, Sujuan WANG, Huixin YANG, Jing ZHAO. Effects of Different Pellet Feed Hardness on Growth and Reproduction, Feed Utilization Rate, and Environmental Dust in Laboratory Mice [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 313-320. |
| [11] | Yun LIU, Tingting FENG, Wei TONG, Zhi GUO, Xia LI, Qi KONG, Zhiguang XIANG. Glycyrrhizic Acid Showed Therapeutic Effects on Severe Pulmonary Damages in Mice Induced by Pneumonia Virus of Mice Infection [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 251-258. |
| [12] | Jinhua HU, Jingjie HAN, Min JIN, Bin HU, Yuefen LOU. Effects of Puerarin on Bone Density in Rats and Mice: A Meta-analysis [J]. Laboratory Animal and Comparative Medicine, 2024, 44(2): 149-161. |
| [13] | Min LIANG, Yang GUO, Jinjin WANG, Mengyan ZHU, Jun CHI, Yanjuan CHEN, Chengji WANG, Zhilan YU, Ruling SHEN. Construction of Dmd Gene Mutant Mice and Phenotype Verification in Muscle and Immune Systems [J]. Laboratory Animal and Comparative Medicine, 2024, 44(1): 42-51. |
| [14] | Jianhua ZHENG, Yunzhi FA, Qiaoyan DONG, Yefeng QIU, Jingqing CHEN. Construction and Evaluation of a Mouse Model with Intestinal Injury by Acute Hypoxic Stress in Plateau [J]. Laboratory Animal and Comparative Medicine, 2024, 44(1): 31-41. |
| [15] | Qianqian TANG, Xiuli ZHANG, Zai CHANG. Statistical Analysis of the Leakage Situation in the Automated Watering System for Mice in Tsinghua University Laboratory Animal Resources Center [J]. Laboratory Animal and Comparative Medicine, 2024, 44(1): 85-91. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||