
Laboratory Animal and Comparative Medicine
• XXXX XXXX •
ZHENG Qingyong1,2,3( )(
)( ), YANG Donghua1,2,3,4, MA Zhichao5, ZHOU Ziyu5, LU Yang6, WANG Jingyu1,7, XING Lina1,2,3, KANG Yingying1,2,3, DU Li8, ZHAO Chunxiang9, DI Baoshan10,11, TIAN Jinhui1,2,3(
), YANG Donghua1,2,3,4, MA Zhichao5, ZHOU Ziyu5, LU Yang6, WANG Jingyu1,7, XING Lina1,2,3, KANG Yingying1,2,3, DU Li8, ZHAO Chunxiang9, DI Baoshan10,11, TIAN Jinhui1,2,3( )(
)( )
)
Online:2025-05-16
Contact:
TIAN Jinhui
CLC Number:
ZHENG Qingyong,YANG Donghua,MA Zhichao,et al. Precautions for Conducting Systematic Reviews and Meta-Analyses of Animal Experiments[J]. Laboratory Animal and Comparative Medicine. DOI: 10.12300/j.issn.1674-5817.2025.017.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2025.017
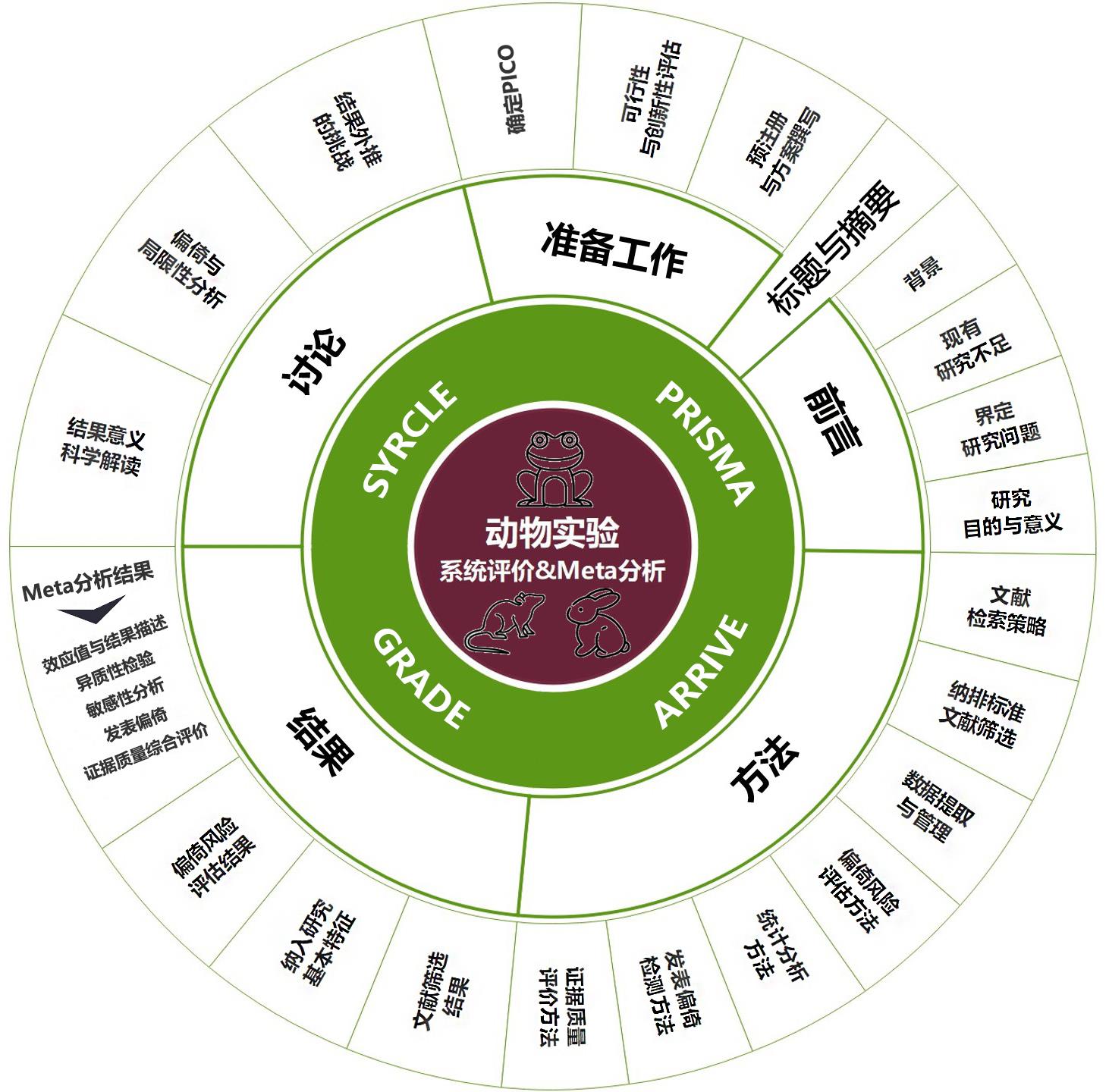
Figure 1 Implementation framework for systematic review and meta-analysis of animal experimentsNote:PICO, population、intervention、comparison、outcome; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; ARRIVE, Animal Research: Reporting of In Vivo Experiments; GRADE, Grades of Recommendations Assessment, Development and Evaluation; SYRCLE, SYRCLE’s risk of bias tool for animal studies.
| 序号No. | 模块 Module | 具体报告内容 Report details |
|---|---|---|
| 1 | 标题与背景信息 | ·明确标注“临床前研究”、“动物实验”或具体物种·研究目的与问题的陈述 ·简要说明研究背景及意义 ·体现研究的转化医学关联与潜在应用价值 |
| 2 | 研究问题 (PICO要素) | ·P:动物种属、品系、周龄、性别等·I:干预措施(药物、手术等)、给药途径、剂量标准化 ·C:对照条件(安慰剂、不同剂量或干预方式等)·O:主要和次要结局指标 |
| 3 | 文献检索策略 | ·检索数据库(如PubMed、Embase等,含动物实验特殊数据库)·检索时间范围·检索词及逻辑关系(结合MeSH词汇与自由词)·灰色文献的检索与记录 |
| 4 | 纳排标准 | 明确纳入标准(如动物物种、实验设计、干预措施、结局指标等)与排除标准(如数据不完整、无对照组等) |
| 5 | 偏倚风险评估 | 使用的评估工具(如SYRCLE工具),简述偏倚风险评估的具体维度(如随机化、分组隐藏、检测盲法实施等) |
| 6 | 数据提取与管理 | ·提取的变量(实验条件、干预措施、主要和次要结局指标等) ·数据处理方法(如单位换算、分组标准化、重复数据的处理方式) |
| 7 | 统计分析方法 | ·异质性分析方法(如I²统计量、Q检验) ·敏感性分析及亚组分析的设计、处理跨物种实验结果的整合方法、规范化跨实验室环境差异的策略等 |
| 8 | 伦理与利益冲突 | 声明研究的伦理原则、披露研究者的利益冲突 |
| 9 | 其他信息 | 预期开始与完成时间、当前研究状态、研究工作基础、已完成或正在进行的类似研究等 |
Table 1 Framework Content of the Main Report of the Research Protocol
| 序号No. | 模块 Module | 具体报告内容 Report details |
|---|---|---|
| 1 | 标题与背景信息 | ·明确标注“临床前研究”、“动物实验”或具体物种·研究目的与问题的陈述 ·简要说明研究背景及意义 ·体现研究的转化医学关联与潜在应用价值 |
| 2 | 研究问题 (PICO要素) | ·P:动物种属、品系、周龄、性别等·I:干预措施(药物、手术等)、给药途径、剂量标准化 ·C:对照条件(安慰剂、不同剂量或干预方式等)·O:主要和次要结局指标 |
| 3 | 文献检索策略 | ·检索数据库(如PubMed、Embase等,含动物实验特殊数据库)·检索时间范围·检索词及逻辑关系(结合MeSH词汇与自由词)·灰色文献的检索与记录 |
| 4 | 纳排标准 | 明确纳入标准(如动物物种、实验设计、干预措施、结局指标等)与排除标准(如数据不完整、无对照组等) |
| 5 | 偏倚风险评估 | 使用的评估工具(如SYRCLE工具),简述偏倚风险评估的具体维度(如随机化、分组隐藏、检测盲法实施等) |
| 6 | 数据提取与管理 | ·提取的变量(实验条件、干预措施、主要和次要结局指标等) ·数据处理方法(如单位换算、分组标准化、重复数据的处理方式) |
| 7 | 统计分析方法 | ·异质性分析方法(如I²统计量、Q检验) ·敏感性分析及亚组分析的设计、处理跨物种实验结果的整合方法、规范化跨实验室环境差异的策略等 |
| 8 | 伦理与利益冲突 | 声明研究的伦理原则、披露研究者的利益冲突 |
| 9 | 其他信息 | 预期开始与完成时间、当前研究状态、研究工作基础、已完成或正在进行的类似研究等 |
检索维度 Search facets | 数据库/平台名称 Database/Platform | 内容/特点 Content & features |
|---|---|---|
| 通用数据库 | PubMed、Embase、Web of Science、Scopus、OVID、LILACS等 | 全球主流生物医学文献 |
| CBM、CNKI、万方等 | 涵盖中文动物实验研究 | |
| 专业数据库 | MGI小鼠基因组数据库 | 小鼠基因型-表型数据、标准化疾病模型 |
| 实验动物资源库 | 中国实验动物模型与实验设计规范 | |
| Animal Study Registry | 动物实验前瞻性注册库,追踪未发表或进行中研究 | |
| 灰色文献 | OpenGrey | 欧洲技术报告、会议论文 |
| ProQuest Dissertations & Theses、EThOS等 | 未公开学位论文中的实验数据 | |
| CAB Abstracts | 农业与兽医科学数据(疾病模型、毒理学) | |
| 预印本平台 | bioRxiv、medRxiv等 | 涵盖最新动物实验预印本研究 |
Table 2 Retrieval References for Databases in Systematic Reviews of Animal Experiments
检索维度 Search facets | 数据库/平台名称 Database/Platform | 内容/特点 Content & features |
|---|---|---|
| 通用数据库 | PubMed、Embase、Web of Science、Scopus、OVID、LILACS等 | 全球主流生物医学文献 |
| CBM、CNKI、万方等 | 涵盖中文动物实验研究 | |
| 专业数据库 | MGI小鼠基因组数据库 | 小鼠基因型-表型数据、标准化疾病模型 |
| 实验动物资源库 | 中国实验动物模型与实验设计规范 | |
| Animal Study Registry | 动物实验前瞻性注册库,追踪未发表或进行中研究 | |
| 灰色文献 | OpenGrey | 欧洲技术报告、会议论文 |
| ProQuest Dissertations & Theses、EThOS等 | 未公开学位论文中的实验数据 | |
| CAB Abstracts | 农业与兽医科学数据(疾病模型、毒理学) | |
| 预印本平台 | bioRxiv、medRxiv等 | 涵盖最新动物实验预印本研究 |
| 1 | SANDERCOCK P, ROBERTS I. Systematic reviews of animal experiments[J]. Lancet, 2002, 360(9333): 586. |
| 2 | 郑卿勇, 李腾飞, 许建国, 等. 动物实验证据整合方法研究的进展与挑战[J]. 实验动物与比较医学, 2024, 44(5): 567-576. |
| ZHENG Q Y, LI T F, XU J G, et al. Advances and challenges in the research of integration methods of animal experimental evidence[J]. Lab Anim Comp Med, 2024, 44(5): 567-576. | |
| 3 | HOOIJMANS CR, RITSKES-HOITINGA M. Progress in using systematic reviews of animal studies to improve translational research[J]. PLoS Med, 2013, 10(7): e1001482. |
| 4 | ANGIUS D, WANG H, SPINNER R J, et al. A systematic review of animal models used to study nerve regeneration in tissue-engineered scaffolds[J]. Biomaterials, 2012, 33(32): 8034-8039. |
| 5 | 赵霏, 唐晓宇, 寇城坤, 等. 动物实验系统评价/Meta分析的质量和报告特征[J]. 中国循证医学杂志, 2018, 18(8): 871-877. |
| ZHAO F, TANG X Y, KOU C K, et al. Methodological and reporting quality of systematic review/meta-analysis of animal studies[J]. Chin J Evid-Based Med, 2018, 18(8): 871-877. | |
| 6 | 胡凯燕, 邢丽娜, 姜彦彪, 等. 促进临床前动物实验系统评价发展,提高其成果的转化和利用[J]. 中国循证心血管医学杂志, 2019, 11(12): 1423-1425. |
| HU K Y, XING L N, JIANG Y B, et al. Promoting the development of preclinical animal experimental systematic reviews, and improving the transformation and utilization of its results[J]. Chin J Evid-Based Cardiovasc Med, 2019, 11(12): 1423-1425. | |
| 7 | HOOIJMANS C R, ROVERS M M, DE VRIES R B M, et al. SYRCLE's risk of bias tool for animal studies[J]. BMC Med Res Methodol, 2014, 14: 43. |
| 8 | KOREVAAR D A, HOOFT L, RIET G TER. Systematic reviews and meta-analyses of preclinical studies: publication bias in laboratory animal experiments[J]. Lab Anim, 2011, 45(4): 225-230. |
| 9 | DU SERT NP, HURST V, AHLUWALIA A, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research[J]. J Cereb Blood Flow Metab, 2020, 40(9): 1769-1777. |
| 10 | KILKENNY C, BROWNE W J, CUTHILL IC, et al. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research[J]. PLoS Biol, 2010, 8(6): e1000412. |
| 11 | PAGE M J, MCKENZIE J E, BOSSUYT P M, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews[J]. BMJ, 2021, 372: n71. |
| 12 | MCGRATH J C, DRUMMOND G B, MCLACHLAN E M, et al. Guidelines for reporting experiments involving animals: the ARRIVE guidelines[J]. Br J Pharmacol, 2010, 160(7): 1573-1576. |
| 13 | 王剑, 卢今, 马政文, 等. «动物研究:体内实验报告»即ARRIVE 2.0指南的解释和阐述(一)[J]. 实验动物与比较医学, 2023, 43(2): 213-224. |
| WANG J, LU J, MA Z W, et al. Explanation and elaboration for the ARRIVE guidelines 2.0-reporting animal research and in vivo experiments (I)[J]. Lab Anim Comp Med, 2023, 43(2): 213-224. | |
| 14 | 陈国元, 卢晓, 白玉, 等. «动物研究:体内实验报告»即ARRIVE 2.0指南的解释和阐述(二)[J]. 实验动物与比较医学, 2023, 43(3): 323-331. |
| CHEN G Y, LU X, BAI Y, et al. Explanation and elaboration for the ARRIVE guidelines 2.0-reporting animal research and in vivo experiments (II)[J]. Lab Anim Comp Med, 2023, 43(3): 323-331. | |
| 15 | 刘晓宇, 卢选成, 师晓萌, 等. «动物研究:体内实验报告»即ARRIVE 2.0指南的解释与阐述(三)[J]. 实验动物与比较医学, 2023, 43(4): 446-456. |
| LIU X Y, LU X C, SHI X M, et al. Explanation and elaboration for the ARRIVE guidelines 2.0-reporting animal research and in vivo experiments (III)[J]. Lab Anim Comp Med, 2023, 43(4): 446-456. | |
| 16 | 李夏莹, 田永路, 刘晓宇, 等. «动物研究:体内实验报告»即ARRIVE 2.0指南的解释和阐述(四)[J]. 实验动物与比较医学, 2023, 43(6): 659-668. |
| LI X Y, TIAN Y L, LIU X Y, et al. Explanation and elaboration for the ARRIVE guidelines 2.0-reporting animal research and in vivo experiments (IV)[J]. Lab Anim Comp Med, 2023, 43(6): 659-668. | |
| 17 | 马政文, 李夏莹, 刘晓宇, 等. «动物研究:体内实验报告»即ARRIVE 2.0指南的解释和阐述(五)[J]. 实验动物与比较医学, 2024, 44(1): 105-114. |
| MA Z W, LI X Y, LIU X Y, et al. Explanation and elaboration for the ARRIVE guidelines 2.0-reporting animal research and in vivo experiments (V)[J]. Lab Anim Comp Med, 2024, 44(1): 105-114. | |
| 18 | 张晶芳, 沈晓旭, 沈星辰, 等. 麝香保心丸干预动脉粥样硬化模型动物实验的系统评价[J]. 中国循证心血管医学杂志, 2024, 16(7): 778-781+788. |
| ZHANG J F, SHEN X X, SHEN X C, et al. Shexiang baoxin pills for treatment of atherosclerosisin modeled animal experiment: a systematic review[J]. Chin J Evid-Based Cardiovasc Med, 2024, 16(7): 778-781+788. | |
| 19 | WANG X, ZHOU Y, XIE D, et al. Melatonin intervention to prevent nanomaterial exposure-induced damages: a systematic review and meta-analysis of in vitro and in vivo studies[J]. J Appl Toxicol, 2025, 45(2): 179-199. |
| 20 | 刘津池, 刘畅, 华成舸. 随机对照试验偏倚风险评价工具RoB2(2019修订版)解读[J]. 中国循证医学杂志, 2021, 21(6): 737-744. |
| LIU J C, LIU C, HUA C G. Risk bias assessment tool RoB2 (revised version 2019) for randomized controlled trial: an interpretation[J]. Chin J Evid-Based Med, 2021, 21(6): 737-744. | |
| 21 | 郑卿勇, 周泳佳, 许建国, 等. 诊断试验准确性网状Meta分析方法进展与挑战[J]. 中国循证医学杂志, 2025, 25(3): 349-357. |
| ZHENG Q Y, ZHOU Y J, XU J G, et al. Advancements and challenges in network meta-analysis of diagnostic test accuracy[J]. Chin J Evid-Based Med, 2025, 25(3): 349-357. | |
| 22 | ROSENBERG MS. The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis[J]. Evolution, 2005, 59(2): 464-468. |
| 23 | 李腾飞, 郑卿勇, 许建国, 等. 提高动物实验系统评价/Meta分析的证据确定性:GRADE方法的实证研究[J]. 实验动物与比较医学, 2025, 45(1): 101-111. |
| LI T F, ZHENG Q Y, XU J G, et al. Improving the certainty of evidence in animal experiment systematic review/meta-analysis: an empirical study of the GRADE method[J]. Lab Anim Comp Med, 2025, 45(1): 101-111. | |
| 24 | GUYATT G H, OXMAN A D, VIST G E, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations[J]. BMJ, 2008, 336(7650): 924-926. |
| 25 | CORDELLI E, ARDOINO L, BENASSI B, et al. Effects of radiofrequency electromagnetic field (RF-EMF) exposure on pregnancy and birth outcomes: A systematic review of experimental studies on non-human mammals[J]. Environ Int, 2023, 180: 108178. |
| 26 | LI J, FAN S, LI H, et al. Evaluation of efficacy, safety and underlying mechanism on Traditional Chinese medicine as synergistic agents for cancer immunotherapy: A preclinical systematic review and meta-analysis[J]. J Ethnopharmacol, 2025, 338(Pt 1): 119035. |
| 27 | ZHU K, XU A, XIA W, et al. Association between NAT2 polymorphism and lung cancer risk: a systematic review and meta-analysis[J]. Front Oncol, 2021, 11: 567762. |
| 28 | HAJIMIRZAEI P, TABATABAEI F S A, NASIBI-SIS H, et al. Schwann cell transplantation for remyelination, regeneration, tissue sparing, and functional recovery in spinal cord injury: a systematic review and meta-analysis of animal studies[J]. Exp Neurol, 2025, 384: 115062. |
| 29 | VAN RHIJN-BROUWER F C C, WEVER K E, KIFFEN R, et al. Systematic review and meta-analysis of the effect of bone marrow-derived cell therapies on hind limb perfusion[J]. Dis Model Mech, 2024, 17(5):dmm050632. |
| 30 | CICONE F, VIERTL D, DENOËL T, et al. Comparison of absorbed dose extrapolation methods for mouse-to-human translation of radiolabelled macromolecules[J]. EJNMMI Res, 2022, 12(1): 21. |
| 31 | WU T, FENG H, HE M, et al. Efficacy of artemisinin and its derivatives in animal models of type 2 diabetes mellitus: a systematic review and meta-analysis[J]. Pharmacol Res, 2022, 175: 105994. |
| [1] | MIN Fangui, FU Hongkun, LIU Yonggang, LIU Xiangmei, LIU Zhonghua, LI Yao, TAO Yufeng. Special Welfare and Ethical Requirements for Infectious Animal Experiments [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 239-246. |
| [2] | LI Tengfei, ZHENG Qingyong, XU Jianguo, LI Yiyi, ZHOU Yongjia, XU Caihua, ZHANG Mingyue, TIAN Jiexiang, WANG Gang, TIAN Jinhui. Improving the Certainty of Evidence in Animal Experiment Systematic Review/Meta-Analysis: An Empirical Study of the GRADE Method [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 101-111. |
| [3] | ZHENG Qingyong, LI Tengfei, XU Jianguo, ZHOU Yongjia, MA Zhichao, WANG Na, LI Molan, YANG Wenjing, WU Peirun, WANG Haidong, TIAN Jinhui. Advances and Challenges in the Research of Integration Methods of Animal Experimental Evidence [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 567-576. |
| [4] | Editorial Board of Laboratory Animal and Comparative Medicine . Guideline Checklist for Publishing Research Papers on Animal Experimentation and Comparative Medicine in China (2024 Edition) [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 577-582. |
| [5] | Jinhua HU, Jingjie HAN, Min JIN, Bin HU, Yuefen LOU. Effects of Puerarin on Bone Density in Rats and Mice: A Meta-analysis [J]. Laboratory Animal and Comparative Medicine, 2024, 44(2): 149-161. |
| [6] | Zhengwen MA, Xiaying LI, Xiaoyu LIU, Yao LI, Jian WANG, Jin LU, Guoyuan CHEN, Xiao LU, Yu BAI, Xuancheng LU, Yonggang LIU, Wanyong PANG, Yufeng TAO. Interpretation and Elaboration for the ARRIVE Guidelines 2.0—Animal Research: Reporting In Vivo Experiments (V) [J]. Laboratory Animal and Comparative Medicine, 2024, 44(1): 105-114. |
| [7] | Jinhuan MIAO, Xia XU, Lu ZHOU, Haiyan CHENG, Yan HE. Visual Analysis of Animal Experiments on Traditional Chinese Medicine (TCM) Nursing Technology Based on VOSviewer [J]. Laboratory Animal and Comparative Medicine, 2023, 43(6): 626-635. |
| [8] | Xiaying LI, Yonglu TIAN, Xiaoyu LIU, Xuancheng LU, Guoyuan CHEN, Xiao LU, Yu BAI, Jing GAO, Yao LI, Yusheng WEI, Wanyong PANG, Yufeng TAO. Explanation and Elaboration for the ARRIVE Guidelines 2.0—Reporting Animal Research and In Vivo Experiments (Ⅳ) [J]. Laboratory Animal and Comparative Medicine, 2023, 43(6): 659-668. |
| [9] | Shuo WANG, Yunhui LÜ, Xiaokang WANG, Zhenhao ZHANG, Yongchun CUI. Construction and Verification of Quality Evaluation Indicator System for Extracorporeal Membrane Oxygenation Animal Experimental Platform [J]. Laboratory Animal and Comparative Medicine, 2023, 43(6): 604-611. |
| [10] | Xiaoyu LIU, Xuancheng LU, Xiaomeng SHI, Yuzhou ZHANG, Chao LÜ, Guoyuan CHEN, Xiao LU, Yu BAI, Jing GAO, Yao LI, Yonggang LIU, Yufeng TAO, Wanyong PANG. Explanation and Elaboration for the ARRIVE Guidelines 2.0—Reporting Animal Research and In Vivo Experiments (Ⅲ) [J]. Laboratory Animal and Comparative Medicine, 2023, 43(4): 446-456. |
| [11] | Guoyuan CHEN, Xiao LU, Yu BAI, Lingzhi YU, Ying QIAO, Jian WANG, Jin LU, Xiaoyu LIU, Xuancheng LU, Jing GAO, Yao LI, Wanyong PANG. Explanation and Elaboration of the ARRIVE Guidelines 2.0—Reporting Animal Research and In Vivo Experiments (Ⅱ) [J]. Laboratory Animal and Comparative Medicine, 2023, 43(3): 323-331. |
| [12] | Jian WANG, Jin LU, Zhengwen MA, Guoyuan CHEN, Xiao LU, Yu BAI, Xiaoyu LIU, Xuancheng LU, Jing GAO, Yao LI, Wanyong Pang. Explanation and Elaboration for the ARRIVE Guidelines 2.0—Reporting Animal Research and In Vivo Experiments (Ⅰ) [J]. Laboratory Animal and Comparative Medicine, 2023, 43(2): 213-224. |
| [13] | Xiangrong DING, Shurui HUO, Jiejie DAI. Research Progress on Influenza A Virus and Nervous System Disease of Human and Experimental Animals [J]. Laboratory Animal and Comparative Medicine, 2023, 43(2): 180-185. |
| [14] | Junyan ZHANG, Xiaoyu LIU, Yao LI, Guoyuan CHEN, Xiao LU, Yu BAI, Xuancheng LU, Wanyong PANG, Baojin WU. Introduction to the International Guide for Animal Research Reporting ARRIVE 2.0, and Its Implementation Plan in the Journal [J]. Laboratory Animal and Comparative Medicine, 2023, 43(1): 86-94. |
| [15] | Xiangmei LIU, Zhongchun MA, Hongkun FU, Feng GAO, Yufeng TAO. Discussion on Expression of Laboratory Accreditation Scope in the Field of Toxicology Testing [J]. Laboratory Animal and Comparative Medicine, 2022, 42(6): 526-530. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||