
Laboratory Animal and Comparative Medicine ›› 2025, Vol. 45 ›› Issue (4): 393-402.DOI: 10.12300/j.issn.1674-5817.2024.183
• Animal Models of Human Diseases • Previous Articles Next Articles
JIANG Juan1, SONG Ning2, LIAN Wenbo1, SHAO Congcong1, GU Wenwen1, SHI Yan1( )(
)( )
)
Received:2024-12-06
Revised:2025-03-04
Online:2025-08-25
Published:2025-09-01
Contact:
SHI Yan
CLC Number:
JIANG Juan,SONG Ning,LIAN Wenbo,et al. Comparison of Histopathological and Molecular Pathological Phenotypes in Mouse Models of Intrauterine Adhesions Induced by Two Concentrations of Ethanol Perfusion[J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 393-402. DOI: 10.12300/j.issn.1674-5817.2024.183.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2024.183
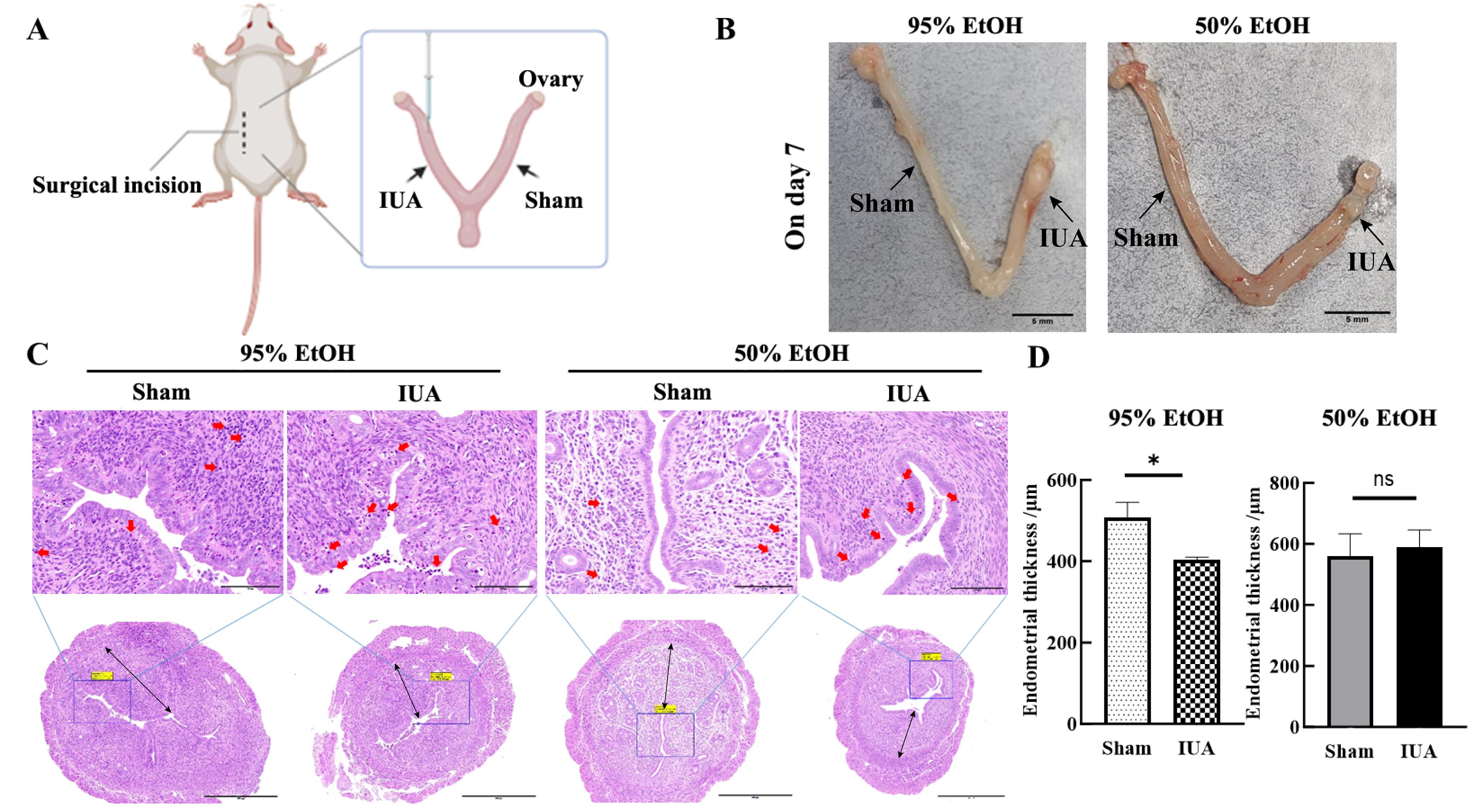
Figure 1 Construction of mouse intrauterine adhesion models induced by two concentrations of ethanol injury and HE staining results of uterine tissues collected one day 7 after injuryNote: A, Schematic diagram of the surgical process for establishing the ethanol perfusion model, Sham represents the sham operation group, and IUA represents intrauterine adhesions (IUA)model group(the ethanol injury group). B, On day 7 after modeling, images of uterine tissues of mice with two types of ethanol injury. In each image, the left side of the uterine tissue is the sham operation group, and the right side of uterine tissue is the injury group. The scale bar indicates 5 mm. C, HE staining results of uterine tissues on day 7 after modeling. In the upper images, the scale bar indicates 100 μm (×400); in the lower images, the scale bar indicates 500 μm (×100); the red arrow indicates inflammatory cell infiltration;the black line indicates the direction for measuring the thickness of endometrium. D, Statistical results of endometrial thickness in sham operation group and IUA model group, *P<0.05, nsP>0.05.
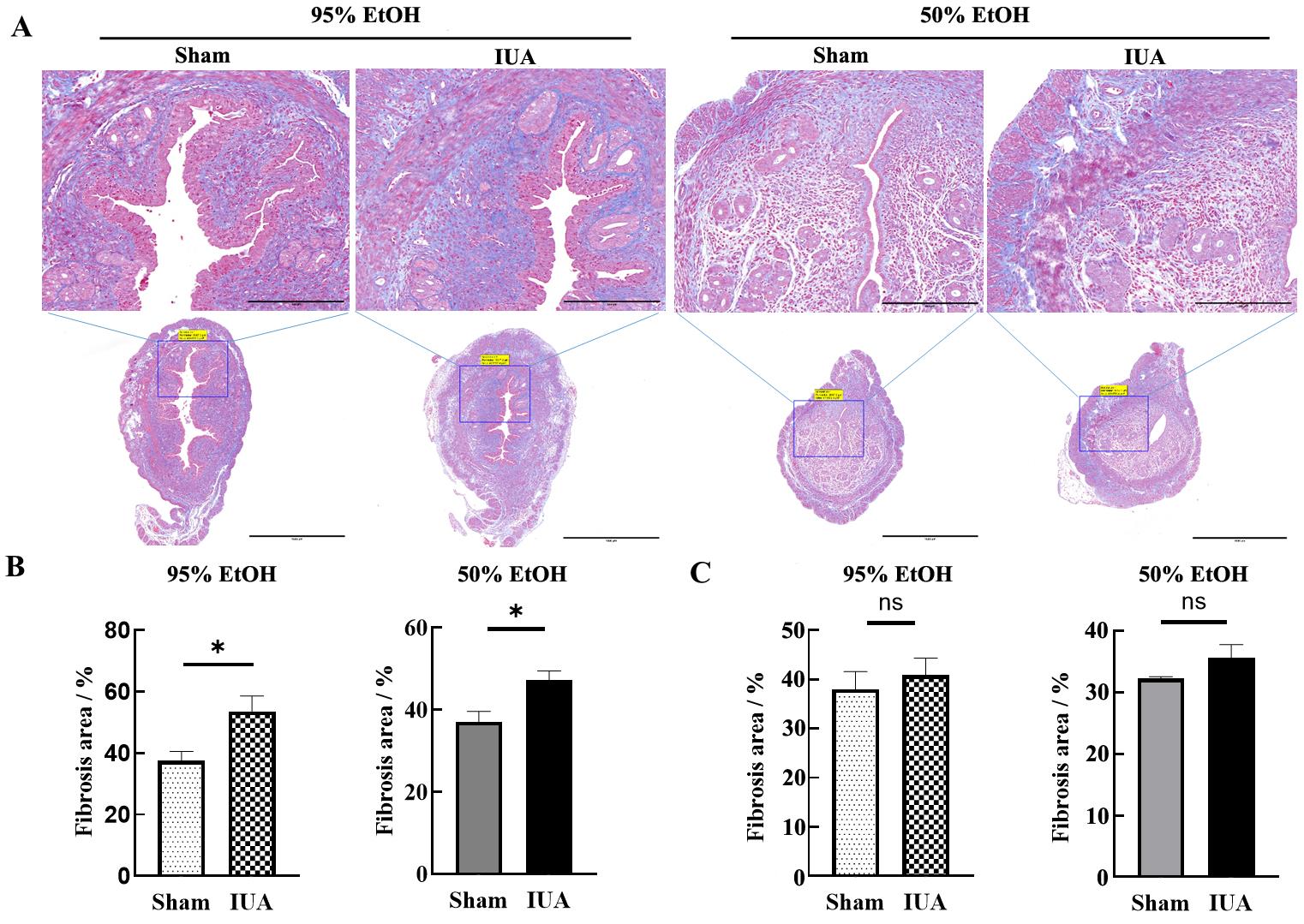
Figure 2 Construction of mouse intrauterine adhesion models induced by two concentrations of ethanol injury and Masson staining results of uterine tissues collected one day 7 after injuryNote: A, On day 7 after modeling, Masson staining was used to assess the content of collagen fibers. In the upper images, the scale bar indicates 250 μm (×400); in the lower images, the scale bar indicates 1 000 μm (×100). B, On day 7 after modeling, statistical results of the percentage of fibrotic area in the bilateral uterine tissues of mice, *P<0.05. C, On day 15 after modeling, statistical results of the percentage of fibrotic area in the bilateral uterine tissues of mice, nsP>0.05.
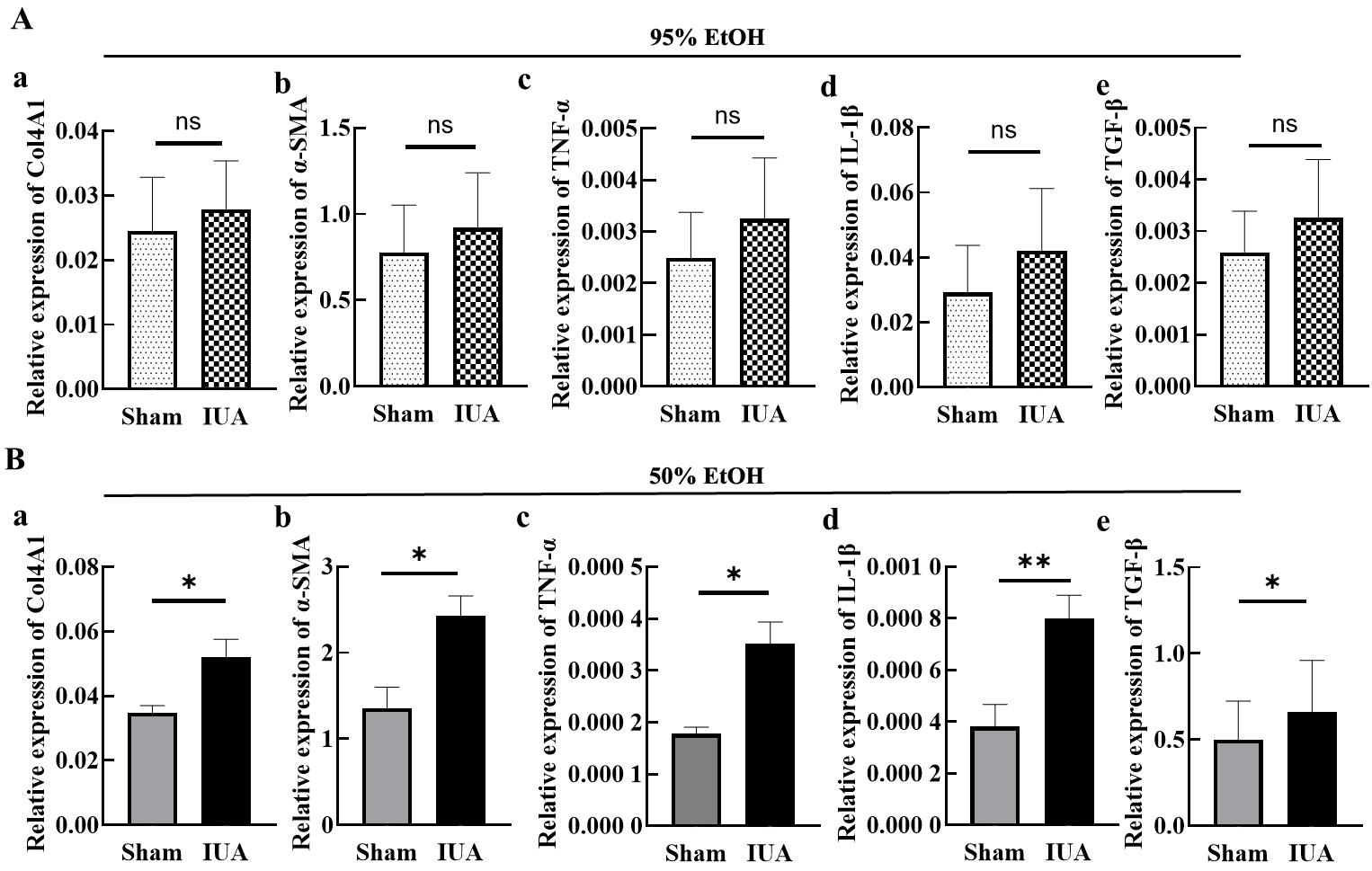
Figure 3 qPCR results of expression levels of fibrosis and inflammation markers in uterine tissues of IUA model mice on day 7 after modelingNote: A, The mRNA expression levels of Col4A1, α-SMA, TNF-α, IL-1β and TGF-β in uterine tissues one day 7 after modeling with 95% ethanol injury. B, The mRNA expression levels of Col4A1, α-SMA, TNF-α, IL-1β and TGF-β in uterine tissues one day 7 after modeling with 50% ethanol injury. nsP>0.05, *P<0.05, **P<0.01.
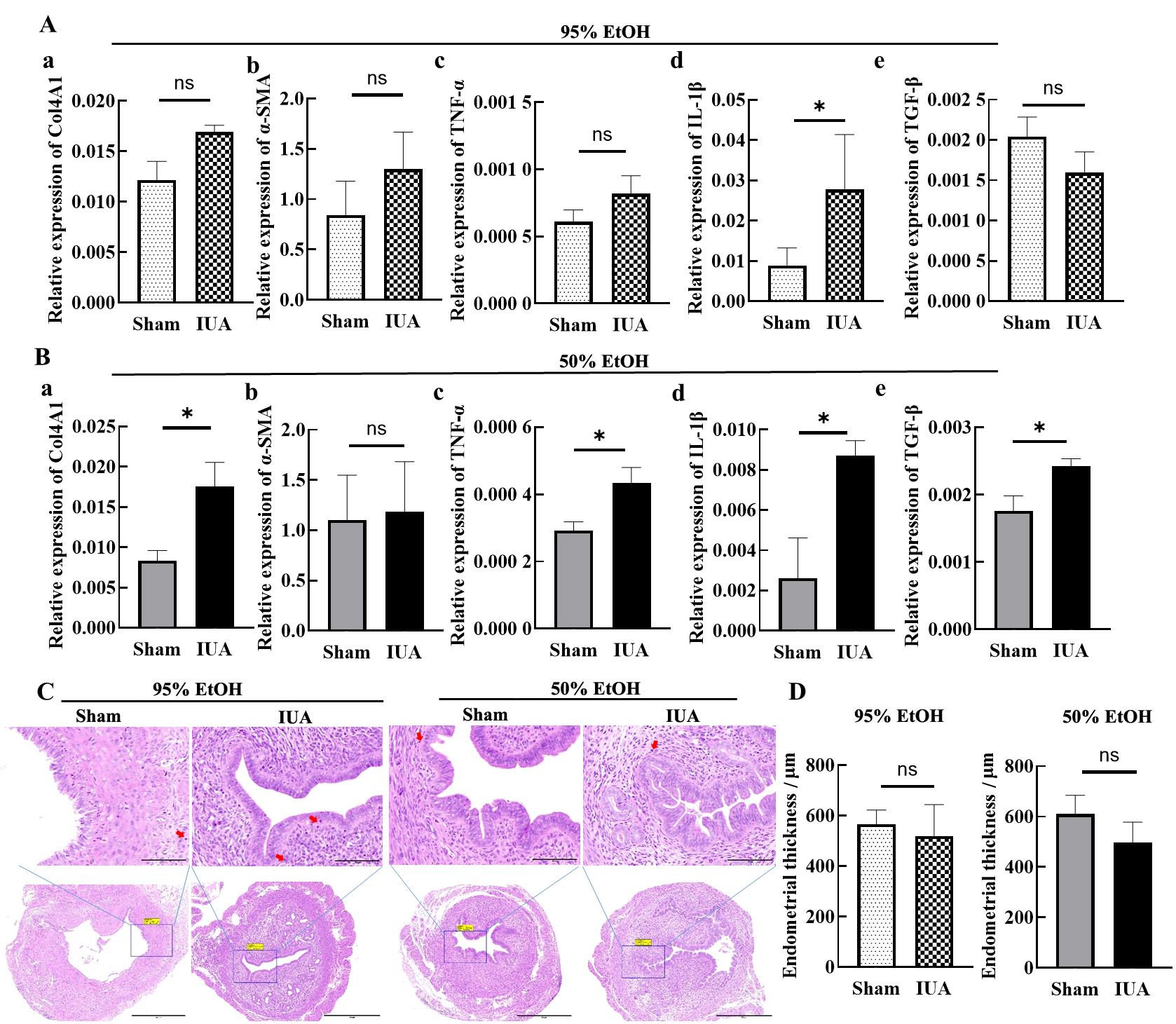
Figure 4 Detection results of fibrosis and inflammatory response in mouse uterine tissues on day 15 after IUA modelingNote: A, The mRNA expression levels of Col4A1, α-SMA, TNF-α, IL-1β and TGF-β in uterine tissues one day 15 after modeling with 95% ethanol injury. B, The mRNA expression levels of Col4A1, α-SMA, TNF-α, IL-1β and TGF-β in uterine tissues one day 15 after modeling with 50% ethanol injury. C, On day 15 after modeling, HE staining results of uterine tissues. In the upper images, the scale bar indicates 100 μm (×400); in the lower images, the scale bar indicates 500 μm (×100). The red arrow indicates inflammatory cell infiltration. D, On day 15 after modeling, statistical results of endometrial thickness in sham operation group and IUA model group. nsP>0.05, *P<0.05.
| [1] | 帕提曼·米吉提, 范余娟. 宫腔粘连的病因学研究及预防措施探讨[J]. 中国计划生育和妇产科, 2024, 16(7):32-35. DOI:10.3969/j.issn.1674-4020.2024.07.08 . |
| PATIMAN M J T, FAN Y J. Etiological research and preventive measures of intrauterine adhesions[J]. Chin J Fam Plan Gynecotokology, 2024, 16(7):32-35. DOI:10.3969/j.issn.1674-4020.2024.07.08 . | |
| [2] | HAN Q X, DU Y Z. Advances in the application of biomimetic endometrium interfaces for uterine bioengineering in female infertility[J]. Front Bioeng Biotechnol, 2020, 8:153. DOI:10.3389/fbioe.2020.00153 . |
| [3] | SANTAMARIA X, ISAACSON K, SIMÓN C. Asherman's syndrome: it may not be all our fault[J]. Hum Reprod, 2018, 33(8):1374-1380. DOI:10.1093/humrep/dey232 . |
| [4] | ERSOY G S, ZOLBIN M M, COSAR E, et al. CXCL12 promotes stem cell recruitment and uterine repair after injury in Asherman's syndrome[J]. Mol Ther Methods Clin Dev, 2017, 4:169-177. DOI:10.1016/j.omtm.2017.01.001 . |
| [5] | REIN D T, SCHMIDT T, HESS A P, et al. Hysteroscopic management of residual trophoblastic tissue is superior to ultrasound-guided curettage[J]. J Minim Invasive Gynecol, 2011, 18(6):774-778. DOI:10.1016/j.jmig.2011.08.003 . |
| [6] | CAI Y L, WU F Y, YU Y R, et al. Porous scaffolds from droplet microfluidics for prevention of intrauterine adhesion[J]. Acta Biomater, 2019, 84:222-230. DOI:10.1016/j.actbio.2018.11.016 . |
| [7] | KOU L F, JIANG X, XIAO S Y, et al. Therapeutic options and drug delivery strategies for the prevention of intrauterine adhesions[J]. J Control Release, 2020, 318:25-37. DOI:10.1016/j.jconrel.2019.12.007 . |
| [8] | 黄少凤, 林忠, 朱雪红, 等. 宫腔粘连大鼠模型研究进展[J]. 实验动物与比较医学, 2022, 42(6):560-565. DOI:10.12300/j.issn.1674-5817.2022.025 . |
| HUANG S F, LIN Z, ZHU X H, et al. Research progress in rat models of intrauterine adhesion[J]. Lab Anim Comp Med, 2022, 42(6):560-565. DOI:10.12300/j.issn.1674-5817.2022.025 . | |
| [9] | 梁姗姗, 植枝福, 黄滟岚. 宫腔粘连动物模型建立的研究进展[J]. 中华生殖与避孕杂志, 2022(8):874-877. DOI: 10.3760/cma.j.cn101441-20210320-00137 . |
| LIANG S S, ZHI Z F, HUANG Y L. Research progress on the establishment of animal model of intrauterine adhesion[J]. Chin J Reprod Contracept, 2022(8):874-877. DOI: 10.3760/cma.j.cn101441-20210320-00137 . | |
| [10] | CAO Y M, QI J, WANG J, et al. Injectable "homing-like" bioactive short-fibers for endometrial repair and efficient live births[J]. Adv Sci, 2024, 11(20): e2306507. DOI:10.1002/advs.202306507 . |
| [11] | QI J, LI X X, CAO Y M, et al. Locationally activated PRP via an injectable dual-network hydrogel for endometrial regeneration[J]. Biomaterials, 2024, 309:122615. DOI:10.1016/j.biomaterials.2024.122615 . |
| [12] | HUA Q, ZHANG Y, LI H J, et al. Human umbilical cord blood-derived MSCs trans-differentiate into endometrial cells and regulate Th17/Treg balance through NF-κB signaling in rabbit intrauterine adhesions endometrium[J]. Stem Cell Res Ther, 2022, 13(1):301. DOI:10.1186/s13287-022-02990-1 . |
| [13] | LIANG Y X, SHUAI Q Z, ZHANG X, et al. Incorporation of decidual stromal cells derived exosomes in sodium alginate hydrogel as an innovative therapeutic strategy for advancing endometrial regeneration and reinstating fertility[J]. Adv Healthc Mater, 2024, 13(13): e2303674. DOI:10.1002/adhm. 202303674 . |
| [14] | ZHANG S H, SUN Y L, JIANG D L, et al. Construction and optimization of an endometrial injury model in mice by transcervical ethanol perfusion[J]. Reprod Sci, 2021, 28(3):693-702. DOI:10.1007/s43032-020-00296-2 . |
| [15] | LIANG S S, HUANG Y L, XIA Y F, et al. Animal models in intrauterine adhesion research[J]. J Obstet Gynaecol, 2022, 42(8):3409-3415. DOI: 10.1080/01443615.2022.2124854 . |
| [16] | LI B N, ZHANG Q W, SUN J Y, et al. Human amniotic epithelial cells improve fertility in an intrauterine adhesion mouse model[J]. Stem Cell Res Ther, 2019, 10(1):257. DOI:10.1186/s13287-019-1368-9 . |
| [17] | PARK M, HONG S H, PARK S H, et al. Perivascular stem cell-derived cyclophilin A improves uterine environment with Asherman's syndrome via HIF1α-dependent angiogenesis[J]. Mol Ther, 2020, 28(8):1818-1832. DOI:10.1016/j.ymthe.2020. 05.015 . |
| [18] | 陈醒, 毛乐乐, 刁翯, 等. 大鼠子宫腔粘连模型的构建与改进[J]. 解剖学报, 2019, 50(1):123-127. DOI: 10.16098/j.issn.0529-1356.2019.01.021 . |
| CHEN X, MAO L L, DIAO H, et al. A rat model of intrauterine adhesion established by endometrial scraping[J]. Acta Anat Sin, 2019, 50(1):123-127. DOI: 10.16098/j.issn.0529-1356.2019.01.021 . | |
| [19] | LEE W L, LIU C H, CHENG M, et al. Focus on the primary prevention of intrauterine adhesions: current concept and vision[J]. Int J Mol Sci, 2021, 22(10):5175. DOI:10.3390/ijms22105175 . |
| [20] | DAI Y Y, XIN L B, HU S T, et al. A construct of adipose-derived mesenchymal stem cells-laden collagen scaffold for fertility restoration by inhibiting fibrosis in a rat model of endometrial injury[J]. Regen Biomater, 2023, 10: rbad080. DOI:10.1093/rb/rbad080 . |
| [21] | TANG P M, NIKOLIC-PATERSON D J, LAN H Y. Macrophages: versatile players in renal inflammation and fibrosis[J]. Nat Rev Nephrol, 2019, 15(3):144-158. DOI:10.1038/s41581-019-0110-2 . |
| [22] | LIU F, ZHU Z J, LI P, et al. Creation of a female rabbit model for intrauterine adhesions using mechanical and infectious injury[J]. J Surg Res, 2013, 183(1):296-303. DOI:10.1016/j.jss.2012.11.009 . |
| [23] | XU X X, ZHANG S S, LIN H L, et al. Metformin promotes regeneration of the injured endometrium via inhibition of endoplasmic reticulum stress-induced apoptosis[J]. Reprod Sci, 2019, 26(4):560-568. DOI:10.1177/1933719118804424 . |
| [24] | WEN J M, HOU B, LIN W G, et al. 3D-printed hydrogel scaffold-loaded granulocyte colony-stimulating factor sustained-release microspheres and their effect on endometrial regeneration[J]. Biomater Sci, 2022, 10(12):3346-3358. DOI:10.1039/d2bm00109h . |
| [25] | JANG H Y, MYOUNG S M, CHOE J M, et al. Effects of autologous platelet-rich plasma on regeneration of damaged endometrium in female rats[J]. Yonsei Med J, 2017, 58(6):1195-1203. DOI:10.3349/ymj.2017.58.6.1195 . |
| [1] | GONG Leilei, WANG Xiaoxia, FENG Xuewei, LI Xinlei, ZHAO Han, ZHANG Xueyan, FENG Xin. A Mouse Model and Mechanism Study of Premature Ovarian Insufficiency Induced by Different Concentrations of Cyclophosphamide [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 403-410. |
| [2] | LUO Lianlian, YUAN Yanchun, WANG Junling, SHI Guangsen. Advances in Mouse Models of Amyotrophic Lateral Sclerosis [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 290-299. |
| [3] | WU Zhihao, CAO Shuyang, ZHOU Zhengyu. Establishment of an Intestinal Fibrosis Model Associated with Inflammatory Bowel Disease in VDR-/- Mice Induced by Helicobacter hepaticus Infection and Mechanism Exploration [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 37-46. |
| [4] | Yisu ZHANG, Xinru LIU, Ruojie WU, Rui LIU, Hong OUYANG, Xiaohong LI. Establishment and Evaluation of Mouse Model of Pregnancy Pain-depression Comorbidity Induced by Chronic Unpredictable Stress, Complete Freund's Adjuvant and Formalin [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 259-269. |
| [5] | Can LAI, Lele LI, Tala HU, Yan MENG. Recent Advances of Animal Models of Renal Interstitial Fibrosis [J]. Laboratory Animal and Comparative Medicine, 2023, 43(2): 163-172. |
| [6] | Yiru WANG, Xiaoying JIANG, Ruoxi DONG, Yibin PAN, Xianghui HAN, Yongqing CAO. Modified Method for Inducing Acute Intestinal Fibrosis in Rats Using 2,4,6-Trinitrobenzene Sulfonic Acid [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 284-293. |
| [7] | Huijie GONG, Guangming ZHANG. Effects of Inagliptin on the Expression of Periosteal and TGF-β1 in Renal Fibrosis of Type 2 Diabetic Rats [J]. Laboratory Animal and Comparative Medicine, 2022, 42(2): 135-140. |
| [8] | Shiyan YU. Advances in the Application of Mouse Models to Study Digestive Mucosal Immunity and Infectious Diseases [J]. Laboratory Animal and Comparative Medicine, 2022, 42(1): 3-10. |
| [9] | SHEN Chen, WU Zhihao, YIN Jun, ZHU Liqi, ZHANG Quan. Helicobacter hepaticus Infection Promotes High Fat Diet-induced Non-alcoholic Fatty Liver Disease in Mice [J]. Laboratory Animal and Comparative Medicine, 2021, 41(2): 122-130. |
| [10] | SUN Chengcheng, LIU Jiangang, LIU Meixia, LI Hao, LUO Zenggang. Neurological Dysfunction and Pathological Changes of Rats with [J]. Laboratory Animal and Comparative Medicine, 2020, 40(6): 470-476. |
| [11] | CHEN Gaofeng, GAO Zhiling, LOU Weiwei, HUANG Lingying, JIN Shugen. Mice with Liver Fibrosis Based on Acoustic Radiation Force Impulse Imaging [J]. Laboratory Animal and Comparative Medicine, 2020, 40(5): 361-. |
| [12] | YAO Ding, ZHOU Jing, YAN Guofeng, WANG Huiyang, WANG Yadi, MA Zhengwen. Establishment of Salt-sensitive Hypertension Model in C57BL/6J Mice [J]. Laboratory Animal and Comparative Medicine, 2020, 40(4): 314-. |
| [13] | CHAI Wenjun, SUN Lei, LIU Xiaoli, PAN Hongyu, GUO Tianan, XU Ye, YAN Mingxia. Establishment of Bone Metastasis Mouse Models through Injecting Human Lung Cancer Cells into Left Ventricle#br# under Ultrasound Guidance#br# [J]. Laboratory Animal and Comparative Medicine, 2020, 40(3): 183-. |
| [14] | LI Lei, LI Xiangli, HU Huimin. Effect of Prostacyclin E1 on Hepatic Fiber in Rabbits after Embolization with Lipiodol #br# [J]. Laboratory Animal and Comparative Medicine, 2020, 40(3): 212-. |
| [15] | JIANG Hongli, MA Hongye, XUE Jinhong, SUN Lingshuang, CHEN Lei. The Role of Villin-1 in Model of Habu Nephritis Mice with Unilateral Nephrectomy [J]. Laboratory Animal and Comparative Medicine, 2020, 40(1): 1-8. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||