
Laboratory Animal and Comparative Medicine ›› 2025, Vol. 45 ›› Issue (4): 379-392.DOI: 10.12300/j.issn.1674-5817.2025.024
• Animal Models of Human Diseases • Next Articles
WANG Jiaoxiang1,2,3, ZHANG Lu1,2,4, CHEN Shuhan1,2,4, JIAO Deling1,2,4, ZHAO Heng1,2,4, WEI Taiyun1,2,4, GUO Jianxiong1,2,4, XU Kaixiang1,2,4, WEI Hongjiang1,2,3,4( )(
)( )
)
Received:2025-02-20
Revised:2025-04-27
Online:2025-08-25
Published:2025-09-01
Contact:
WEI Hongjiang
CLC Number:
WANG Jiaoxiang,ZHANG Lu,CHEN Shuhan,et al. Construction and Functional Validation of GTKO/hCD55 Gene-Edited Xenotransplant Donor Pigs[J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 379-392. DOI: 10.12300/j.issn.1674-5817.2025.024.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2025.024
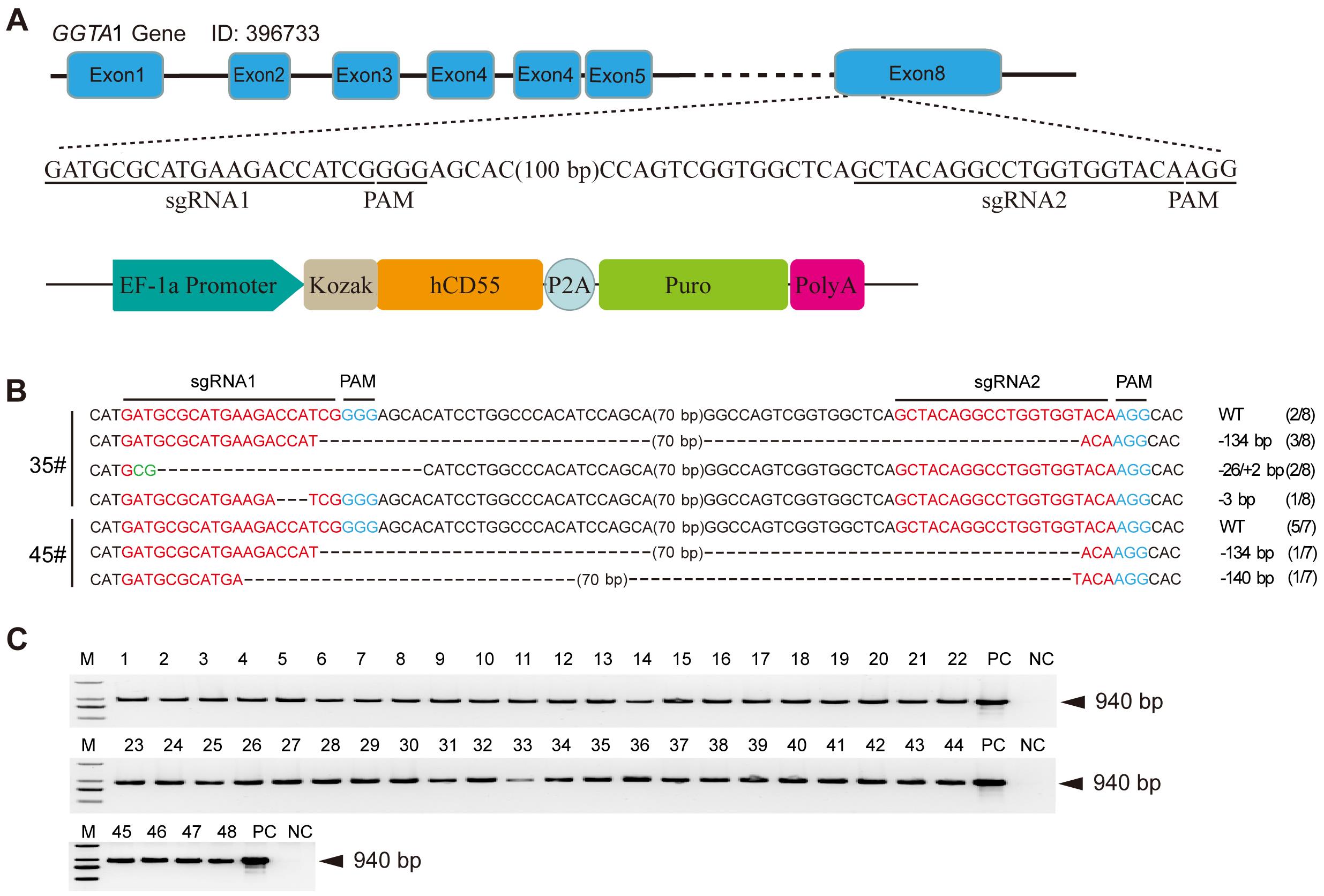
Figure 1 Screening of single-cell clones positive for GTKO/hCD55 gene editingNote: A, Design of sgRNA for GGTA1 gene knockout and schematic diagram of the vector for hCD55 gene overexpression; B, Genotype of the sgRNA targeted region of GGTA1 in positive single-cell clones 35# and 45#; C, PCR identification of the hCD55 gene from 48 single-cell clones; PC, Positive controls; NC, Negative controls; -, Indicates the knockout bases; +, Indicates the bases insertion; M, DNA ladder; WT, Wild type; WT (2/8) indicates among 8 single colonies sequencing after TA cloning, four genotypes were identified: two WT, three -134 bp, two -26/+2 bp and one -3 bp.
序号 No. | 仔猪编号 Piglet ID | 仔猪出生体重/g Body weight/g | 存活时间/d Survival time/d | 代孕母猪编号 Recipients sow ID | |
|---|---|---|---|---|---|
| 1 | DN-GTKO-hCD55F02P01 | 500 | 3 | P516 | |
| 2 | DN-GTKO-hCD55F02P02 | 400 | 1 | ||
| 3 | DN-GTKO-hCD55F02P03 | 720 | 死胎 | / | P452 |
| 4 | DN-GTKO-hCD55F02P04 | 380 | 死胎 | / | |
| 5 | DN-GTKO-hCD55F02P05 | 620 | 4 | ||
| 6 | DN-GTKO-hCD55F02P06 | 200 | / | P511 | |
| 7 | DN-GTKO-hCD55F02P07 | 560 | 死胎 | / | |
| 8 | DN-GTKO-hCD55F02P08 | 300 | / | ||
| 9 | DN-GTKO-hCD55F02P09 | 400 | / | ||
| 10 | DN-GTKO-hCD55F02P10 | 480 | 死胎 | / | P443 |
| 11 | DN-GTKO-hCD55F02P11 | 560 | 2 | ||
| 12 | DN-GTKO-hCD55F02P12 | 580 | 3 | ||
| 13 | DN-GTKO-hCD55F02P13 | 520 | 1 | ||
| 14 | DN-GTKO-hCD55F02P14 | 260 | 虚弱 | 2 | |
| 15 | DN-GTKO-hCD55F02P15 | 320 | 死胎 | / | |
| 16 | DN-GTKO-hCD55F02P16 | 620 | 2 | ||
| 17 | DN-GTKO-hCD55F02P17 | 740 | 1 | ||
| 18 | DN-GTKO-hCD55F02P18 | 560 | 死胎 | / | P525 |
| 19 | DN-GTKO-hCD55F02P19 | 540 | 21 | ||
| 20 | DN-GTKO-hCD55F02P20 | 660 | 206 |
Table 1 Embryo transfer and production of cloned piglets
序号 No. | 仔猪编号 Piglet ID | 仔猪出生体重/g Body weight/g | 存活时间/d Survival time/d | 代孕母猪编号 Recipients sow ID | |
|---|---|---|---|---|---|
| 1 | DN-GTKO-hCD55F02P01 | 500 | 3 | P516 | |
| 2 | DN-GTKO-hCD55F02P02 | 400 | 1 | ||
| 3 | DN-GTKO-hCD55F02P03 | 720 | 死胎 | / | P452 |
| 4 | DN-GTKO-hCD55F02P04 | 380 | 死胎 | / | |
| 5 | DN-GTKO-hCD55F02P05 | 620 | 4 | ||
| 6 | DN-GTKO-hCD55F02P06 | 200 | / | P511 | |
| 7 | DN-GTKO-hCD55F02P07 | 560 | 死胎 | / | |
| 8 | DN-GTKO-hCD55F02P08 | 300 | / | ||
| 9 | DN-GTKO-hCD55F02P09 | 400 | / | ||
| 10 | DN-GTKO-hCD55F02P10 | 480 | 死胎 | / | P443 |
| 11 | DN-GTKO-hCD55F02P11 | 560 | 2 | ||
| 12 | DN-GTKO-hCD55F02P12 | 580 | 3 | ||
| 13 | DN-GTKO-hCD55F02P13 | 520 | 1 | ||
| 14 | DN-GTKO-hCD55F02P14 | 260 | 虚弱 | 2 | |
| 15 | DN-GTKO-hCD55F02P15 | 320 | 死胎 | / | |
| 16 | DN-GTKO-hCD55F02P16 | 620 | 2 | ||
| 17 | DN-GTKO-hCD55F02P17 | 740 | 1 | ||
| 18 | DN-GTKO-hCD55F02P18 | 560 | 死胎 | / | P525 |
| 19 | DN-GTKO-hCD55F02P19 | 540 | 21 | ||
| 20 | DN-GTKO-hCD55F02P20 | 660 | 206 |
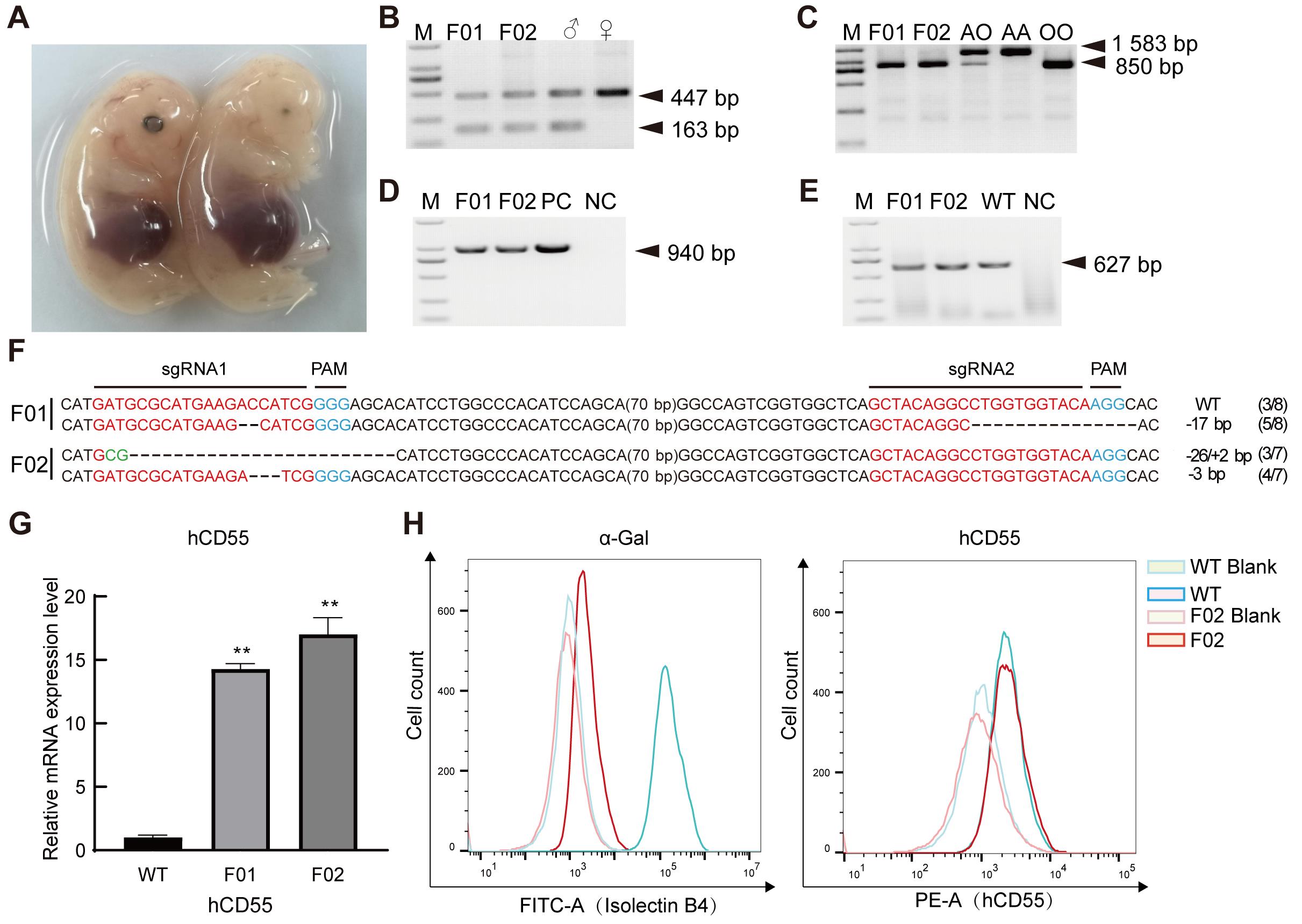
Figure 2 Identification of GTKO/hCD55 gene-edited cloned fetal pigsNote: A, Two cloned fetal pigs at 33 days of gestation; B,PCR results of sex identification of fetal pig heart tissue; C, PCR results of blood group gene identification of fetal pig heart tissue; D, PCR identification of hCD55 gene in fetal pig heart tissue; E, PCR results of the target region of GGTA1 gene sgRNA in fetal pig heart tissue; F, Genotype results of the target region of GGTA1 gene sgRNA in fetal pig heart tissue; G, hCD55 mRNA expression levels in fetal pig F01 and F02 heart tissues; H, Flow cytometric analysis results of α-Gal and hCD55 expression in fetal porcine F02 fibroblasts. M, DNA ladder; WT, Wild type; PC, Positive controls; NC, Negative controls; ♂, Male; ♀, Female; AO, AO blood type; AA, AA blood type; OO, OO blood type; Blank, Control without antibody; F02, Fetal number; **P<0.01.
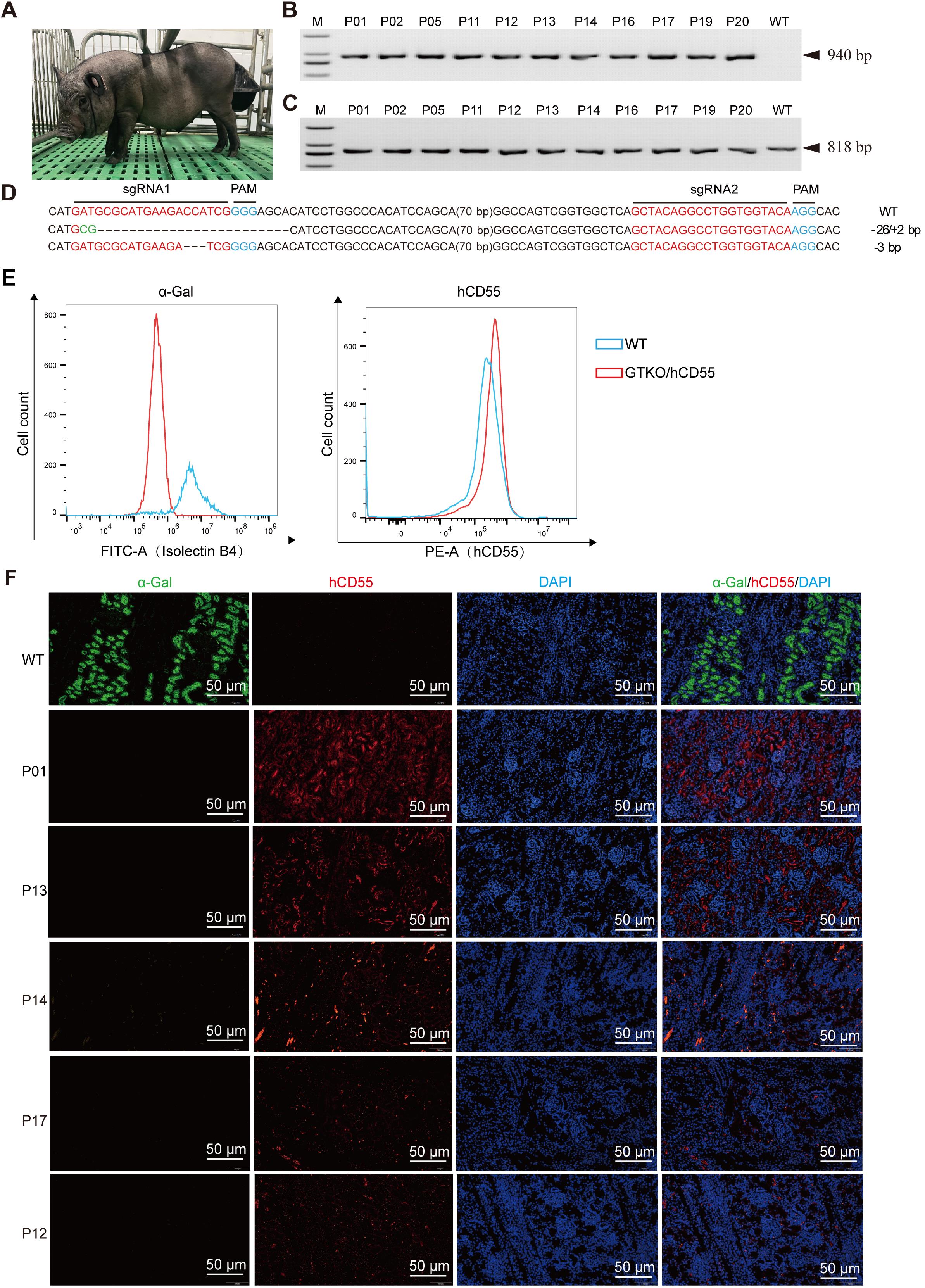
Figure 3 Identification of GTKO/hCD55 gene-edited cloned pigletsNote: A, Photo of cloned piglets with GTKO/hCD55 gene editing; B, PCR identification results of hCD55 gene in 11 piglets; C, PCR results of the sgRNA target region of the piglet GGTA1 gene; D, GGTA1 genotype of the sgRNA target region in piglet P20; E, Flow cytometry analysis results of α-Gal and hCD55 expression in P20 piglet-derived fibroblasts; F, Immunofluorescence results of piglet kidney tissues,the scale bar indicates 50 μm. M, DNA ladder; WT, wild type.
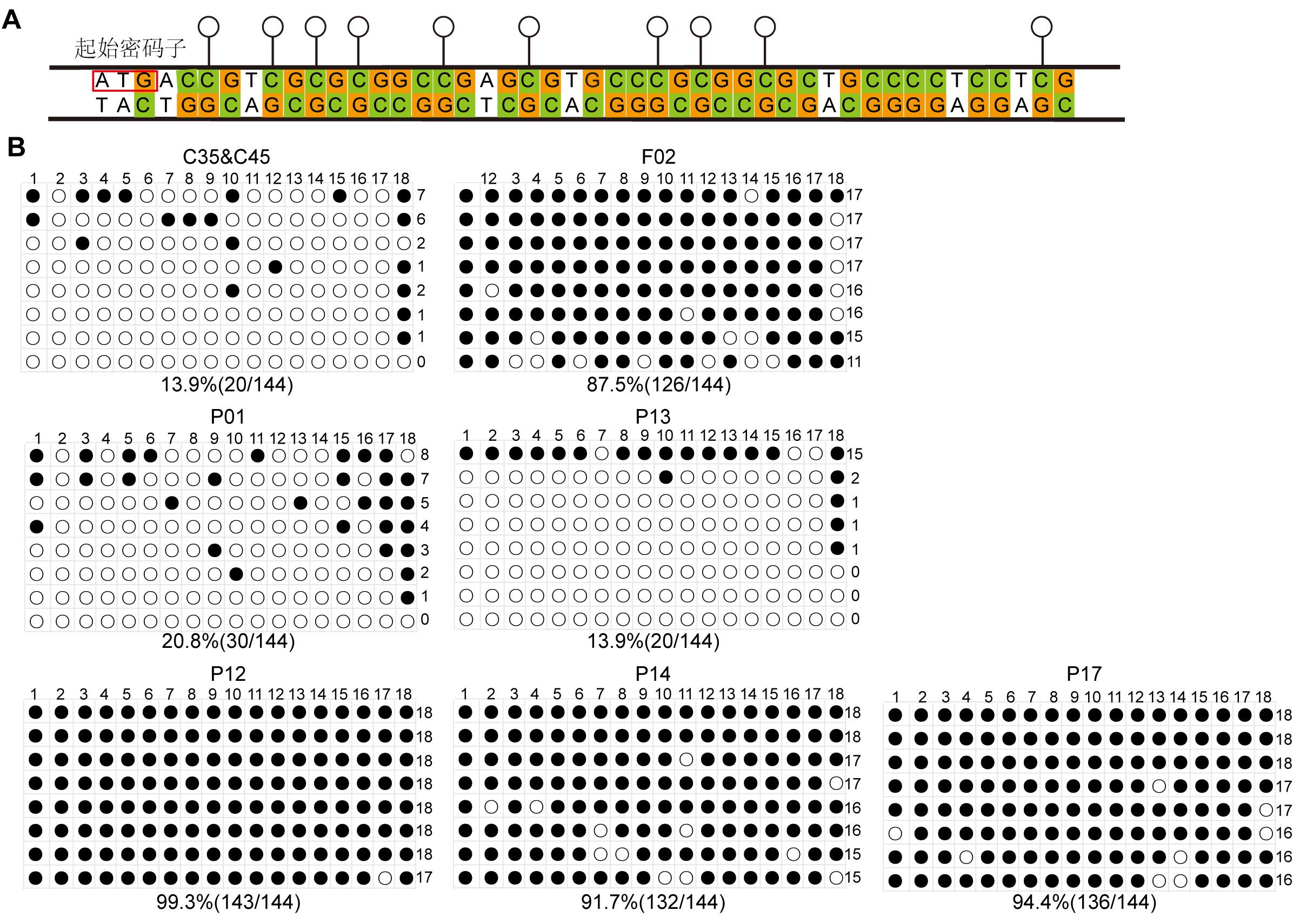
Figure 4 Methylation levels of hCD55 gene in mixed single-cell clones, fetal pigs, and cloned pigletsNote: A, Schematic representation of CpG island in the hCD55 gene; B, Methylation levels of hCD55 gene in mixed single-cell clones of C35&C45, cloned fetal pig F02 fibroblasts and cloned piglets (P01, P13, P12, P14, P17) kidney tissues. Solid black circles indicate methylation sites, and hollow circles indicate unmethylated sites.
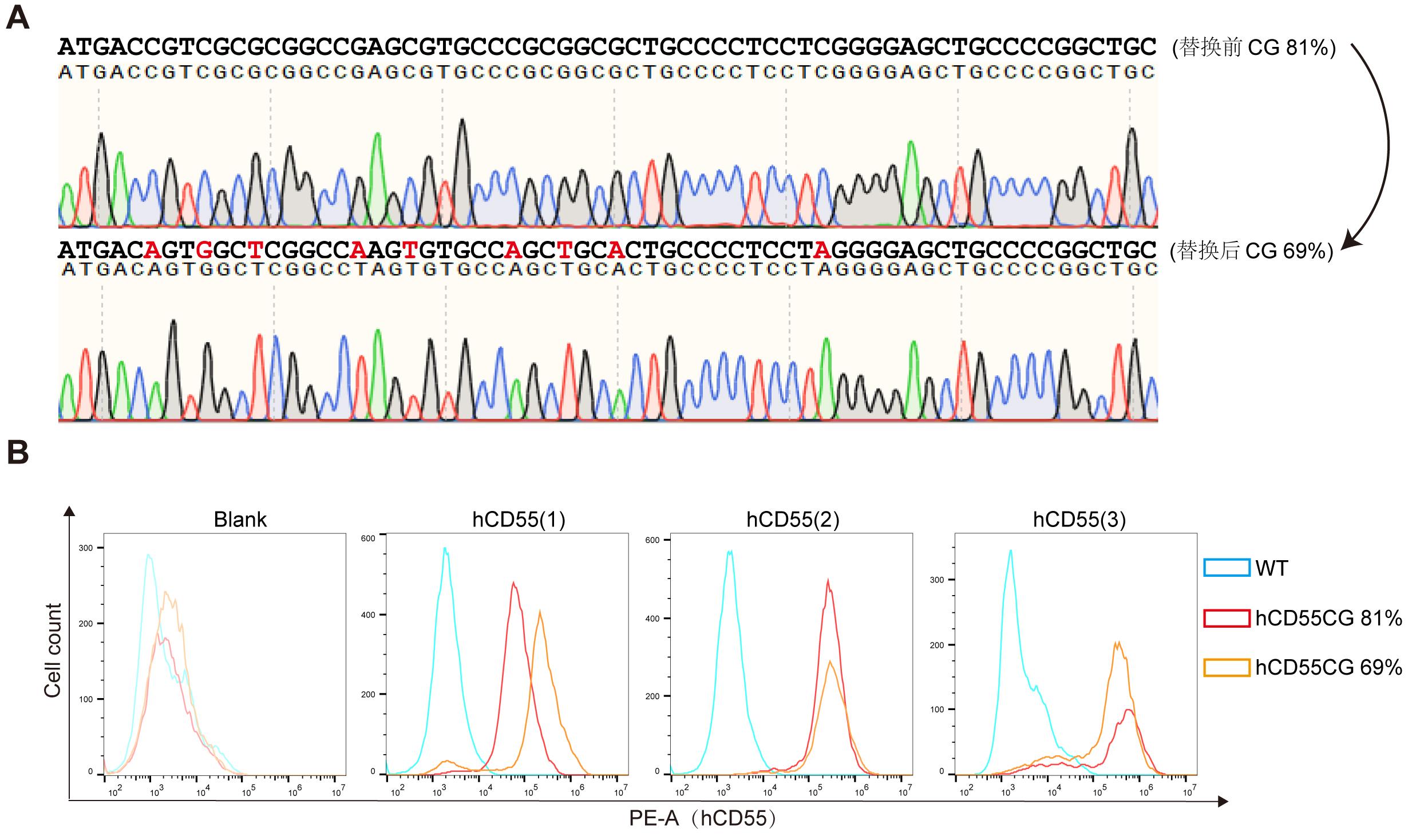
Figure 5 Expression levels of hCD55 in fibroblasts before and after optimization of CpG island region codonNote: A, Sanger sequencing results of CpG island region in hCD55 gene before and after codon optimization; B, Porcine fibroblasts were transfected with the codon unoptimized and codon optimized vectors (represents by hCD55CG 81% and hCD55CG 69% respectively), and the expression level of hCD55 in the cells was detected by flow cytometry (triplicate experimental results). Blank, control without antibody; WT, wild type.
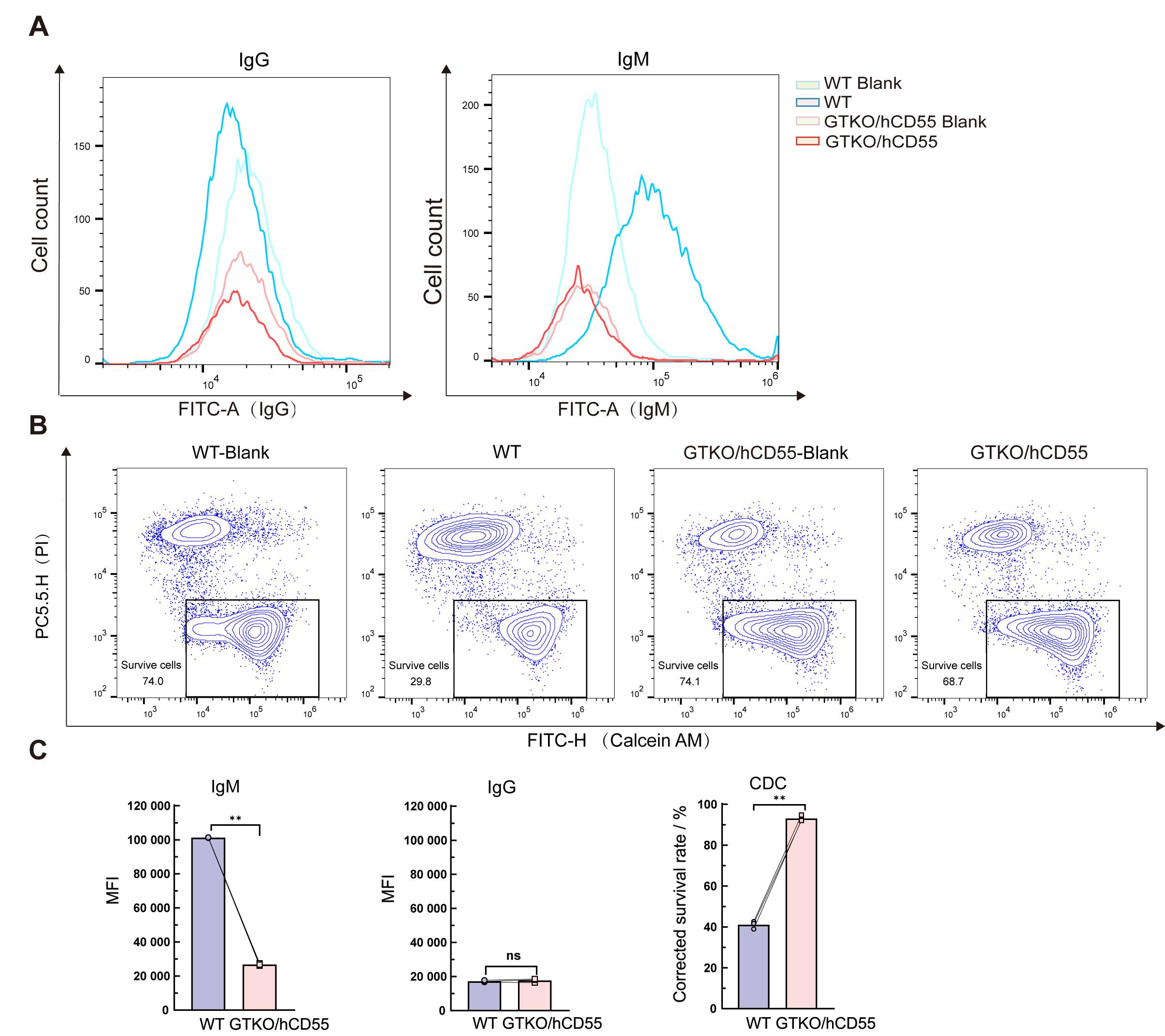
Figure 6 Evaluation of anti-immune rejection efficacy of GTKO/hCD55 gene-edited pigsNote: A, Binding extent analysis of human IgG and IgM antibodies to porcine fibroblasts after incubation with mixed human serum; B, Porcine cell death analysis induced by complement after incubation of porcine fibroblasts with mixed human serum; C, The statistical analysis results of figures A and B. WT, wild-type; GTKO/hCD55, experimental group; Blank, control without antibody; MFI, mean fluorescence intensity; CDC, complement-dependent cytotoxicity; **P<0.01; nsP>0.05.
| [1] | ALI A, KEMTER E, WOLF E. Advances in organ and tissue xenotransplantation[J]. Annu Rev Anim Biosci, 2024, 12:369-390. DOI:10.1146/annurev-animal-021122-102606 . |
| [2] | SINGH A K, GOERLICH C E, ZHANG T S, et al. Genetically engineered pig heart transplantation in non-human primates[J]. Commun Med, 2025, 5(1):6. DOI:10.1038/s43856-025-00731-y . |
| [3] | EISENSON D, HISADOME Y, SANTILLAN M, et al. Consistent survival in consecutive cases of life-supporting porcine kidney xenotransplantation using 10GE source pigs[J]. Nat Commun, 2024, 15(1):3361. DOI:10.1038/s41467-024-47679-6 . |
| [4] | ANAND R P, LAYER J V, HEJA D, et al. Design and testing of a humanized porcine donor for xenotransplantation[J]. Nature, 2023, 622(7982):393-401. DOI:10.1038/s41586-023-06594-4 . |
| [5] | MOHIUDDIN M M, SINGH A K, SCOBIE L, et al. Graft dysfunction in compassionate use of genetically engineered pig-to-human cardiac xenotransplantation: a case report[J]. Lancet, 2023, 402(10399):397-410. DOI:10.1016/S0140-6736(23)00775-4 . |
| [6] | GRIFFITH B P, GOERLICH C E, SINGH A K, et al. Genetically modified porcine-to-human cardiac xenotransplantation[J]. N Engl J Med, 2022, 387(1):35-44. DOI:10.1056/NEJMoa2201422 . |
| [7] | PAN W Q, ZHANG W M, ZHENG B H, et al. Cellular dynamics in pig-to-human kidney xenotransplantation[J]. Med, 2024, 5(8):1016-1029.e4. DOI:10.1016/j.medj.2024.05.003 . |
| [8] | KAWAI T, WILLIAMS W W, ELIAS N, et al. Xenotransplantation of a porcine kidney for end-stage kidney disease[J]. N Engl J Med, 2025, 392(19):1933-1940. DOI:10.1056/NEJMoa2412747 . |
| [9] | MALLAPATY S. First pig-to-human liver transplant recipient 'doing very well'[J]. Nature, 2024, 630(8015):18. DOI:10.1038/d41586-024-01613-4 . |
| [10] | KUWAKI K, TSENG Y L, DOR F J M F, et al. Heart transplantation in baboons using alpha1,3-galactosyl- transferase gene-knockout pigs as donors: initial experience[J]. Nat Med, 2005, 11(1):29-31. DOI:10.1038/nm1171 . |
| [11] | AZIMZADEH A M, KELISHADI S S, EZZELARAB M B, et al. Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement pathway-regulatory protein[J]. Xenotransplantation, 2015, 22(4):310-316. DOI:10.1111/xen.12176 . |
| [12] | MOHIUDDIN M M, GOERLICH C E, SINGH A K, et al. Progressive genetic modifications of porcine cardiac xeno- grafts extend survival to 9 months[J]. Xenotransplantation, 2022, 29(3): e12744. DOI:10.1111/xen.12744 . |
| [13] | CHABAN R, MCGRATH G, HABIBABADY Z, et al. Increased human complement pathway regulatory protein gene dose is associated with increased endothelial expression and prolonged survival during ex-vivo perfusion of GTKO pig lungs with human blood[J]. Xenotransplantation, 2023, 30(4): e12812. DOI:10.1111/xen.12812 . |
| [14] | WARD T, PIPKIN P A, CLARKSON N A, et al. Decay-accelerating factor CD55 is identified as the receptor for echovirus 7 using CELICS, a rapid immuno-focal cloning method[J]. EMBO J, 1994, 13(21):5070-5074. DOI:10.1002/j.1460-2075.1994.tb06836.x . |
| [15] | LUKACIK P, ROVERSI P, WHITE J, et al. Complement regulation at the molecular level: the structure of decay-accelerating factor[J]. Proc Natl Acad Sci USA, 2004, 101(5):1279-1284. DOI:10.1073/pnas.0307200101 . |
| [16] | MURAKAMI H, NAGASHIMA H, TAKAHAGI Y, et al. Transgenic pigs expressing human decay-accelerating factor regulated by porcine MCP gene promoter[J]. Mol Reprod Dev, 2002, 61(3):302-311. DOI:10.1002/mrd.10043 . |
| [17] | KIM S C, MATHEWS D V, BREEDEN C P, et al. Long-term survival of pig-to-rhesus macaque renal xenografts is dependent on CD4 T cell depletion[J]. Am J Transplant, 2019, 19(8):2174-2185. DOI:10.1111/ajt.15329 . |
| [18] | 李欣, 潘登科, 周佳, 等. 表达人源补体调节蛋白hCD55对异种胰岛移植的保护作用[J]. 器官移植, 2022, 13(4):475-482. DOI: 10.3969/j.issn.1674-7445.2022.04.010 . |
| LI X, PAN D K, ZHOU J, et al. Protective role of expression of human complement regulatory protein hCD55 in islet xenotransplantation[J]. Organ Transplant, 2022, 13(4):475-482. DOI: 10.3969/j.issn.1674-7445.2022.04.010 . | |
| [19] | 夏强兵, 冯豪, 王璐, 等. GTKO/hCD55基因工程猪到人异种移植CDC技术的探讨[J]. 中华器官移植杂志, 2022, 43(6): 364–369. DOI:10.3760/cma.j.cn421203-20220505-00096 . |
| XIA Q B, FENG H, WANG L, et al. Exploration of complement-dependent cytotoxicity technology for GTKO/hCD55 genetically engineered pig-to-human xenotransplantation[J]. Chin J Organ Transplant, 2022, 43(6):364-369. DOI:10.3760/cma.j.cn421203-20220505-00096 . | |
| [20] | YUE Y N, XU W H, KAN Y N, et al. Extensive germline genome engineering in pigs[J]. Nat Biomed Eng, 2021, 5(2):134-143. DOI:10.1038/s41551-020-00613-9 . |
| [21] | 王娇祥, 王艳, 胡珂, 等. 猪血型PCR鉴定方法的建立[J]. 实验动物与比较医学, 2023, 43(6):585-594. DOI: 10.12300/j.issn.1674-5817.2023.065 . |
| WANG J X, WANG Y, HU K, et al. Establishment of PCR identification method for pig blood type[J]. Lab Anim Comp Med, 2023, 43(6): 585-594. DOI: 10.12300/j.issn.1674-5817.2023.065 . | |
| [22] | LETI F, LLACI L, MALENICA I, et al. Methods for CpG methylation array profiling via bisulfite conversion[J]. Methods Mol Biol, 2018, 1706:233-254. DOI:10.1007/978-1-4939-7471-9_13 . |
| [23] | MOAZAMI N, STERN J M, KHALIL K, et al. Pig-to-human heart xenotransplantation in two recently deceased human recipients[J]. Nat Med, 2023, 29(8):1989-1997. DOI:10.1038/s41591-023-02471-9 . |
| [24] | LOCKE J E, KUMAR V, ANDERSON D, et al. Normal graft function after pig-to-human kidney xenotransplant[J]. JAMA Surg, 2023, 158(10): 1106-1108. DOI:10.1001/jamasurg.2023.2774 . |
| [25] | WANG J X, XU K X, LIU T, et al. Production and functional verification of 8-gene (GGTA1, CMAH, β4GalNT2, hCD46, hCD55, hCD59, hTBM, hCD39)-edited donor pigs for xenotransplantation[J]. Cell Prolif, 2025: e70028. DOI:10.1111/cpr.70028 . |
| [26] | MANOOK M, OLASO D, ANWAR I, et al. Prolonged xenokidney graft survival in sensitized NHP recipients by expression of multiple human transgenes in a triple knockout pig[J]. Sci Transl Med, 2024, 16(751): eadk6152. DOI:10.1126/scitranslmed.adk6152 . |
| [27] | YANG C, WEI Y F, LI X L, et al. Production of four-gene (GTKO/hCD55/hTBM/hCD39)-edited donor pigs and kidney xenotransplantation[J]. Xenotransplantation, 2024, 31(4): e12881. DOI:10.1111/xen.12881 . |
| [28] | ZHAO H, LI Y Y, WIRIYAHDAMRONG T, et al. Improved production of GTKO/hCD55/hCD59 triple-gene-modified Diannan miniature pigs for xenotransplantation by recloning[J]. Transgenic Res, 2020, 29(3):369-379. DOI:10.1007/s11248-020-00201-2 . |
| [1] | ZHU Chan, ZHANG Dongliang, ZHAO Deli, SHI Xueqin, QIAN Lei, ZHANG Xuan, JIN Yan, DUAN Wei, QI Ruocheng, LIU Chaohua, YANG Xuekang, HAN Juntao, PAN Dengke. Perioperative Animal Care for Xenotransplantation from Genetically Edited Pigs to Monkeys [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 495-501. |
| [2] | Jiaoxiang WANG, Yan WANG, Ke HU, Kaixiang XU, Taiyun WEI, Deling JIAO, Heng ZHAO, Hongye ZHAO, HongJiang WEI. Establishment of PCR Identification Method for Pig Blood Type [J]. Laboratory Animal and Comparative Medicine, 2023, 43(6): 585-594. |
| [3] | ZHANG Qi, WANG Jian-fei. Progress and Perspective for Xenotransplantation [J]. Laboratory Animal and Comparative Medicine, 2018, 38(6): 407-411. |
| [4] | ZHANG Zhen-hua, SHEN Zeng-li, HOU Chuan-ling, SONG Chun-jiao, SUN Ai-jing. Establishment of Different Malignant Potential Prostate Cancer Animal Model from Same Origination [J]. Laboratory Animal and Comparative Medicine, 2014, 34(2): 83-88. |
| [5] | YE Wen-xue,SHEN Zhen-ya,ZHANG Sheng-chao. Establish a High Rebeating Rate Mouse-to-rat Heterotopic Cardiac Transplantation Model [J]. Laboratory Animal and Comparative Medicine, 2008, 28(3): 148-150. |
| [6] | LU Xuan1, XIAO Ming-di2,LI Jing-lai2. Establishment of the Model for Acute Vascular Rejection in Heart Xenotransplantation [J]. Laboratory Animal and Comparative Medicine, 2003, 23(3): 143-145. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||