
Laboratory Animal and Comparative Medicine ›› 2024, Vol. 44 ›› Issue (5): 502-510.DOI: 10.12300/j.issn.1674-5817.2024.034
• Laboratory Animal Welfare and Ethics • Previous Articles Next Articles
TAN He( ), YANG Xiaohui, ZHANG Daxiu, WANG Guicheng(
), YANG Xiaohui, ZHANG Daxiu, WANG Guicheng( )(
)( )
)
Received:2024-02-29
Revised:2024-06-05
Online:2024-10-25
Published:2024-10-25
Contact:
WANG Guicheng
CLC Number:
TAN He,YANG Xiaohui,ZHANG Daxiu,et al. Optimal Adaptation Period for Metabolic Cage Experiments in Mice at Different Developmental Stages[J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 502-510. DOI: 10.12300/j.issn.1674-5817.2024.034.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2024.034
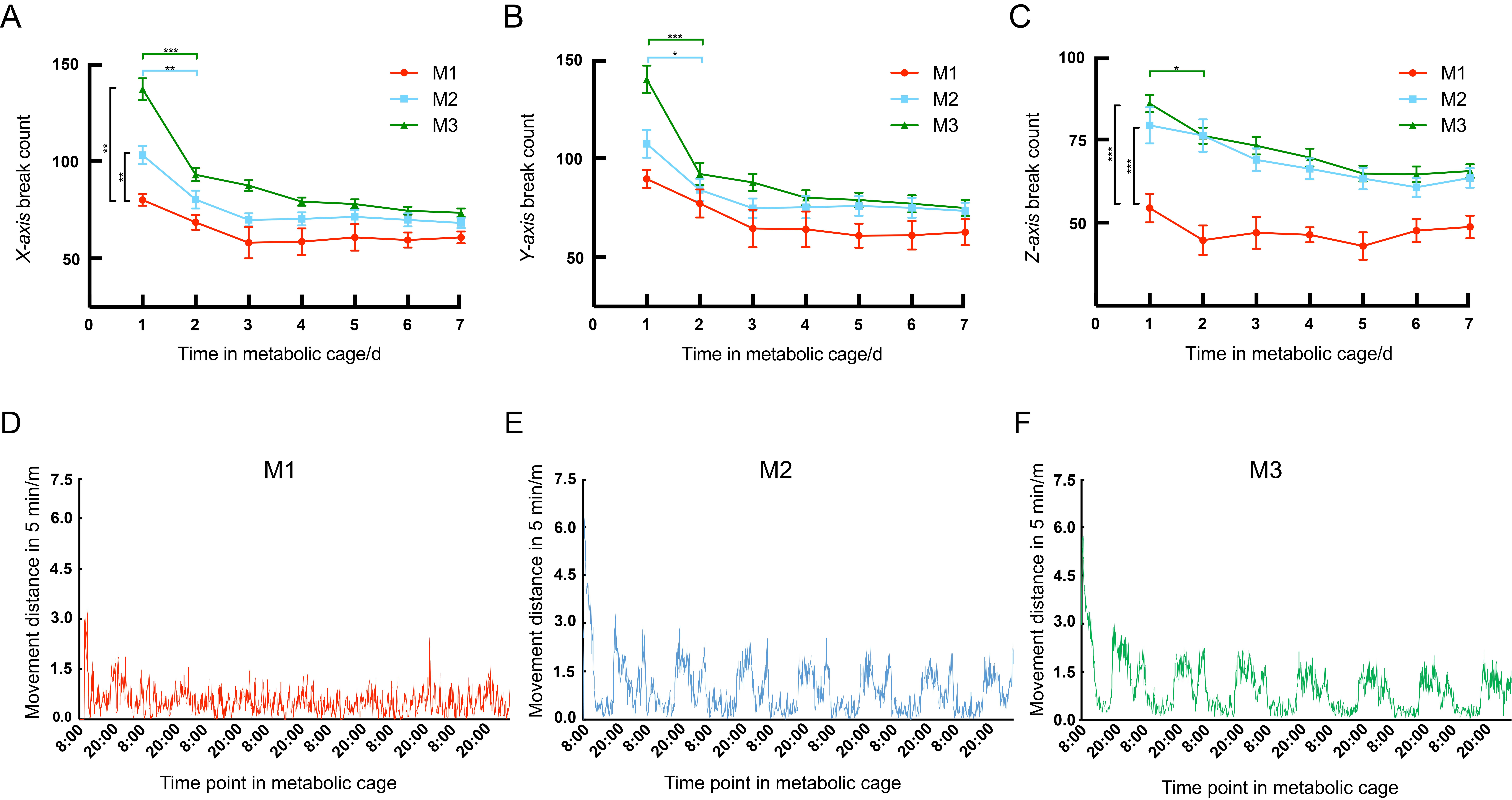
Figure 1 Comparison of activity levels of C57BL/6 mice at different developmental stages in metabolism cagesNote:A-C, Frequency of infrared beam interruptions on X, Y, Z axes, the movement frequencies of weaned mice (M1, n=8) on X and Z axes was significantly lower than that of adolescent (M2, n=16) and adult (M3, n=24) mice (*P<0.05,**P<0.01, ***P<0.001); D-F, Total movement distance of M1, M2, M3 mice in all directions every 5 minutes in metabolism cage.
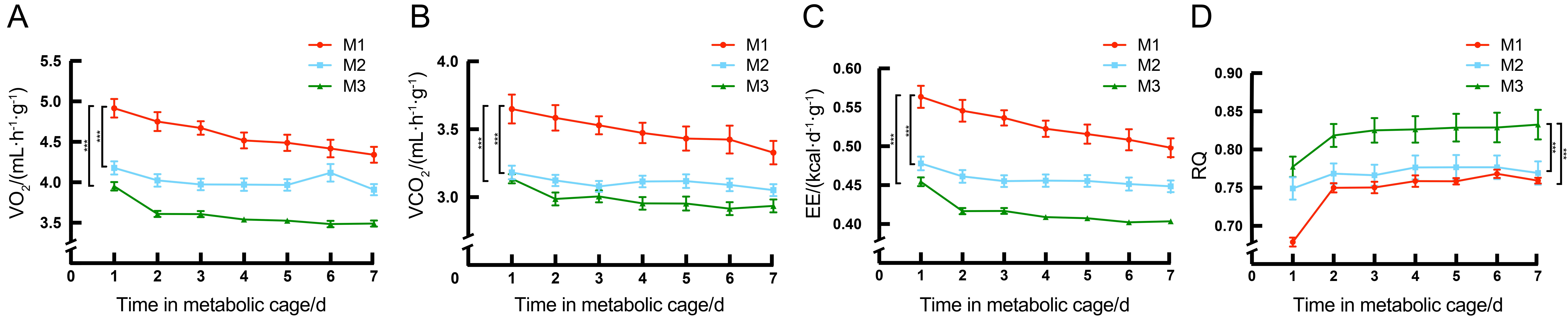
Figure 2 Metabolic activity changes of mice in metabolism cages across 3 age groupsNote:A, Changes in oxygen consumption; B, Changes in carbon dioxide exhalation; C, Changes in energy expenditure (EE) levels; D, Changes in metabolic cage respiratory quotient (RQ) in mice. O2 consumption, CO2 exhalation, and EE of weaned mice (M1, n=8) were significantly higher than those of adolescent (M2, n=16) and adult (M3, n=24) mice (***P<0.001). RQ of M3 mice was significantly higher than that of M1 and M2 mice (***P<0.001). Data were calibrated on body mass (g), and results were expressed as mean±SEM, statistically significant differences were shown by repeated measures ANOVA.
适应时间 Adaptation time | 指标 Indicators | 离乳期(M1) Weaned (M1) | 青春期(M2) Adolescent (M2) | 成年期(M3) Adult (M3) |
|---|---|---|---|---|
第1天 0-24 h | 摄食量/g | 4.43±1.12 | 4.27±1.02 | 5.02±1.51 |
| 饮水量/mL | 3.38±0.62 | 2.98±0.59 | 3.50±1.01 | |
| XYZ轴移动频次/次 | 222.21±23.20 | 289.41±40.00 | 368.90±39.49 | |
第2天 24-48 h | 摄食量/g | 4.69±0.80 | 4.25±0.99 | 4.50±1.21 |
| 饮水量/mL | 3.57±0.73 | 3.15±0.75 | 3.44±0.77 | |
| XYZ 轴移动频次/次 | 186.22±32.15 | 240.27±32.29*** | 264.98±36.63*** | |
第3天 48-72 h | 摄食量/g | 4.78±1.00 | 3.82±0.67 | 4.78±1.14 |
| 饮水量/mL | 4.78±1.00 | 3.05±0.66 | 3.43±0.82 | |
| XYZ 轴移动频次/次 | 168.21±54.76 | 213.24±24.71*** | 250.81±25.62*** |
Table 1 Evaluation of adaptation effects on various indicators in metabolic cages during the first three days
适应时间 Adaptation time | 指标 Indicators | 离乳期(M1) Weaned (M1) | 青春期(M2) Adolescent (M2) | 成年期(M3) Adult (M3) |
|---|---|---|---|---|
第1天 0-24 h | 摄食量/g | 4.43±1.12 | 4.27±1.02 | 5.02±1.51 |
| 饮水量/mL | 3.38±0.62 | 2.98±0.59 | 3.50±1.01 | |
| XYZ轴移动频次/次 | 222.21±23.20 | 289.41±40.00 | 368.90±39.49 | |
第2天 24-48 h | 摄食量/g | 4.69±0.80 | 4.25±0.99 | 4.50±1.21 |
| 饮水量/mL | 3.57±0.73 | 3.15±0.75 | 3.44±0.77 | |
| XYZ 轴移动频次/次 | 186.22±32.15 | 240.27±32.29*** | 264.98±36.63*** | |
第3天 48-72 h | 摄食量/g | 4.78±1.00 | 3.82±0.67 | 4.78±1.14 |
| 饮水量/mL | 4.78±1.00 | 3.05±0.66 | 3.43±0.82 | |
| XYZ 轴移动频次/次 | 168.21±54.76 | 213.24±24.71*** | 250.81±25.62*** |
适应时间 Adaptation time | 指标 Indicators | 离乳期(M1) Weaned (M1) | 青春期(M2) Adolescent (M2) | 成年期(M3) Adult (M3) |
|---|---|---|---|---|
第1个光周期 0-12 h | 摄食量/g | 1.52±0.44 | 1.16±0.47 | 1.58±0.72 |
| 饮水量/mL | 0.44±0.29 | 0.37±0.25 | 0.49±0.34 | |
第2个光周期 24-36 h | 摄食量/g | 2.03±0.45* | 1.05±0.47 | 0.95±0.42*** |
| 饮水量/mL | 1.29±0.43*** | 0.54±0.35 | 0.67±0.31 |
Table 2 Evaluation of adaptation effects on various indicators in metabolic cages during the first and second light periods
适应时间 Adaptation time | 指标 Indicators | 离乳期(M1) Weaned (M1) | 青春期(M2) Adolescent (M2) | 成年期(M3) Adult (M3) |
|---|---|---|---|---|
第1个光周期 0-12 h | 摄食量/g | 1.52±0.44 | 1.16±0.47 | 1.58±0.72 |
| 饮水量/mL | 0.44±0.29 | 0.37±0.25 | 0.49±0.34 | |
第2个光周期 24-36 h | 摄食量/g | 2.03±0.45* | 1.05±0.47 | 0.95±0.42*** |
| 饮水量/mL | 1.29±0.43*** | 0.54±0.35 | 0.67±0.31 |
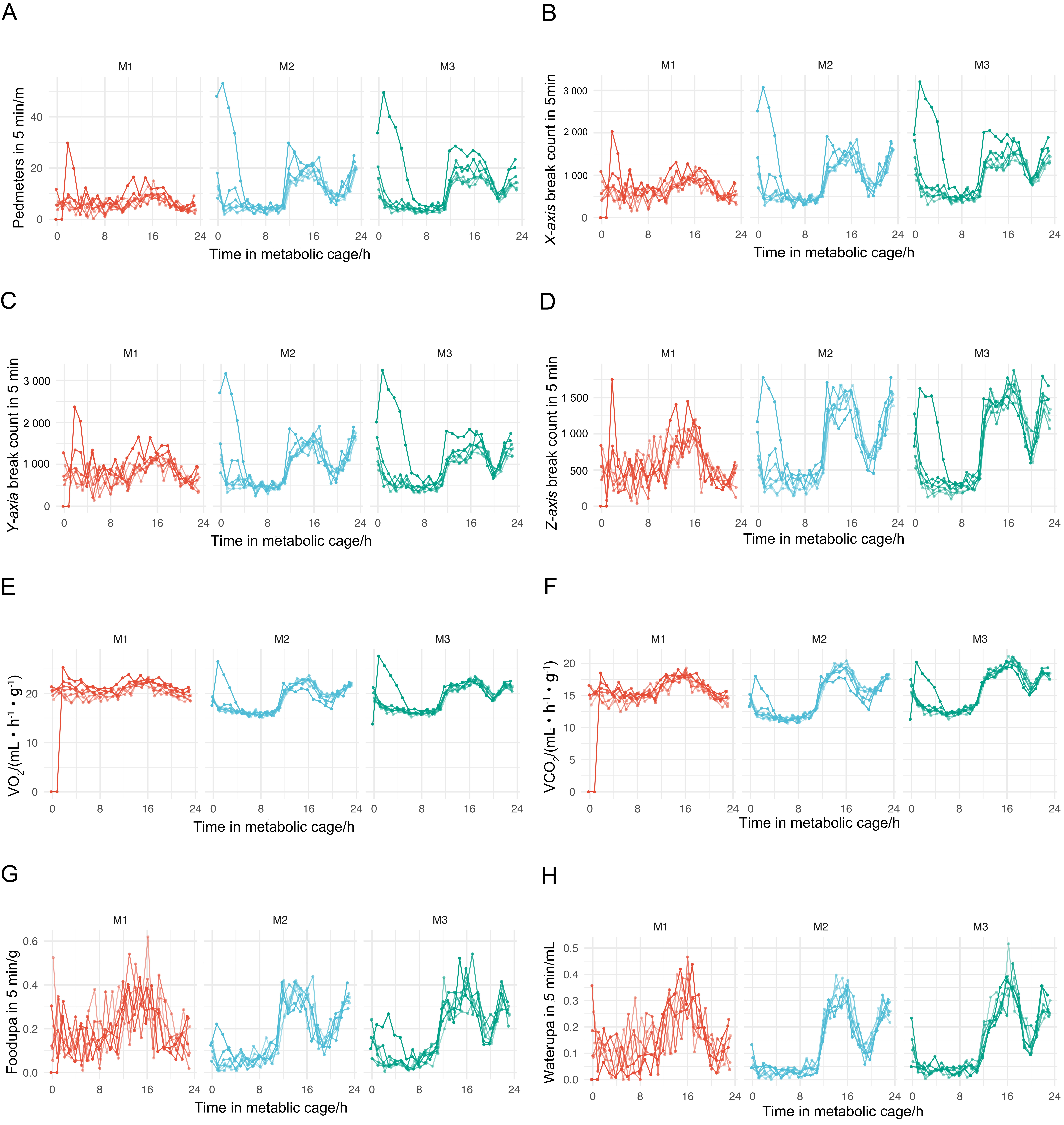
Figure 3 Visualization analysis of adaptation indicators for mice in metabolic cages at different developmental stagesNote:A, The total movements of the mice in all directions during 5 minutes in metabolic cages; B, The frequency of movement along the X axis; C, The frequency of movement along the Y axis; D, The frequency of movement along the Z axis; E, Changes in oxygen consumption in metabolic cages; F, Changes in carbon dioxide exhaled in metabolic cages; G, The amount of food intake every 5 minutes by mice in metabolic cages; H, The amount of water intake by mice every 5 minutes in metabolic cages. M1, weaned mice; M2, adolescent mice; M3, adult mice.
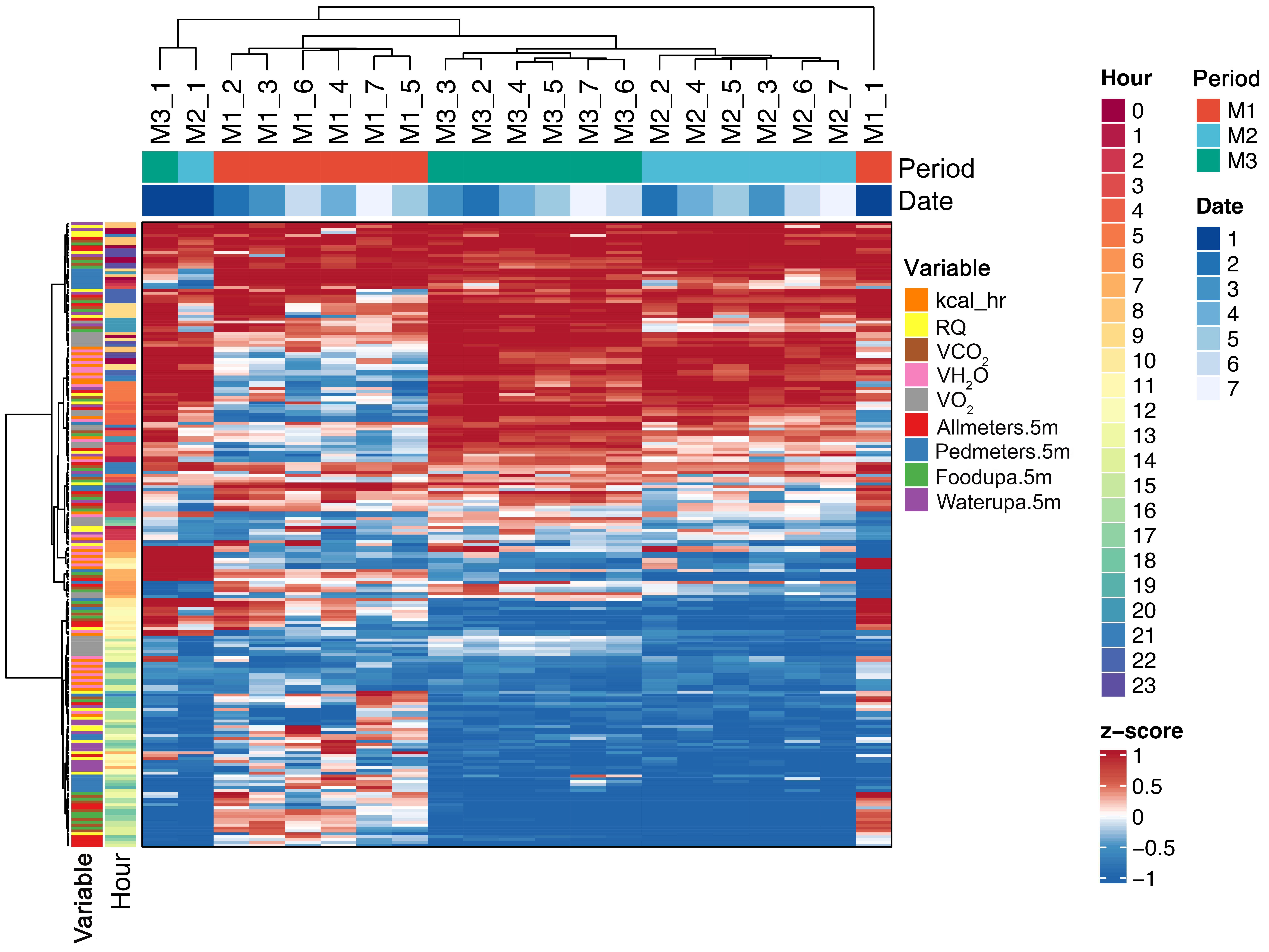
Figure 4 Multifactor clustering analysis of adaptation indicators for mice at different developmental stages in metabolic cagesNote:The data from day 1 in metabolic cages for weaned mice (M1_1), adolescent mice (M2_1), and adult mice (M3_1) were outliers and did not cluster with the data from the same group. Kcal_hr,EE per hour/mL;VCO2,mice carbon dioxide exhalation;VO2,oxygen consumption/mL;VH2O,water intake/mL;Allmeters.5m,total movement distance in 5 min/m;Pedmeters.5m,total directional movement distance along X, Y, Z axes in 5 min/m;Foodupa.5m,food intake in 5 min/g;Waterupa.5m,water intake in 5 min/mL。
| 1 | GRUNDY S M. Metabolic syndrome update[J]. Trends Cardiovasc Med, 2016, 26(4):364-373. DOI: 10.1016/j.tcm. 2015.10.004 . |
| 2 | GUPTA A, GUPTA V. Metabolic syndrome: what are the risks for humans?[J]. Biosci Trends, 2010, 4(5):204-212. |
| 3 | GRIFFIN J L. Understanding mouse models of disease through metabolomics[J]. Curr Opin Chem Biol, 2006, 10(4):309-315. DOI: 10.1016/j.cbpa.2006.06.027 . |
| 4 | KULKARNI N M, JAJI M S, SHETTY P, et al. A novel animal model of metabolic syndrome with non-alcoholic fatty liver disease and skin inflammation[J]. Pharm Biol, 2015, 53(8):1110-1117. DOI: 10.3109/13880209.2014.960944 . |
| 5 | LUO Y H, LU H C, PENG D Q, et al. Liver-humanized mice: a translational strategy to study metabolic disorders[J]. J Cell Physiol, 2022, 237(1):489-506. DOI: 10.1002/jcp.30610 . |
| 6 | EVANS C C, LEPARD K J, KWAK J W, et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity[J]. PLoS One, 2014, 9(3): e92193. DOI: 10.1371/journal.pone.0092193 . |
| 7 | RIDAURA V K, FAITH J J, REY F E, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice[J]. Science, 2013, 341(6150):1241214. DOI: 10.1126/science.1241214 . |
| 8 | TETRI L H, BASARANOGLU M, BRUNT E M, et al. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent[J]. Am J Physiol Gastrointest Liver Physiol, 2008, 295(5): G987-G995. DOI: 10.1152/ajpgi.90272.2008 . |
| 9 | LIU Z W, HUANG M L, WU X, et al. PER1 phosphorylation specifies feeding rhythm in mice[J]. Cell Rep, 2014, 7(5):1509-1520. DOI: 10.1016/j.celrep.2014.04.032 . |
| 10 | ZHU S W, YEE B K, NYFFELER M, et al. Influence of differential housing on emotional behaviour and neurotrophin levels in mice[J]. Behav Brain Res, 2006, 169(1):10-20. DOI: 10.1016/j.bbr.2005.11.024 . |
| 11 | ISHIDA H, MITSUI K, NUKAYA H, et al. Study of active substances involved in skin dysfunction induced by crowding stress. I. effect of crowding and isolation on some physiological variables, skin function and skin blood perfusion in hairless mice[J]. Biol Pharm Bull, 2003, 26(2):170-181. DOI: 10.1248/bpb.26.170 . |
| 12 | STEYERMARK A C, MUELLER P J. Cage size affects feeding and energetics of captive rodents[J]. Physiol Biochem Zool, 2002, 75(2):209-213. DOI: 10.1086/338689 . |
| 13 | BARTOLOMUCCI A, PALANZA P, SACERDOTE P, et al. Individual housing induces altered immuno-endocrine responses to psychological stress in male mice[J]. Psychoneuroendocrinology, 2003, 28(4):540-558. DOI: 10.1016/s0306-4530(02)00039-2 . |
| 14 | MANSER C E, ELLIOT H, MORRIS T H, et al. The use of a novel operant test to determine the strength of preference for flooring in laboratory rats[J]. Lab Anim, 1996, 30(1):1-6. DOI: 10.1258/002367796780744974 . |
| 15 | MANSER C E, MORRIS T H, BROOM D M. An investigation into the effects of solid or grid cage flooring on the welfare of laboratory rats[J]. Lab Anim, 1995, 29(4):353-363. DOI: 10.1258/002367795780740023 . |
| 16 | BRUNNER L J, DIPIRO J T, FELDMAN S. Metabolic cage isolation reduces antipyrine clearance in rats[J]. J Pharm Pharmacol, 1994, 46(7):581-584. DOI: 10.1111/j.2042-7158.1994.tb03861.x . |
| 17 | KALLIOKOSKI O, JACOBSEN K R, DARUSMAN H S, et al. Mice do not habituate to metabolism cage housing: a three week study of male BALB/c mice[J]. PLoS One, 2013, 8(3): e58460. DOI: 10.1371/journal.pone.0058460 . |
| 18 | DUTTA S, SENGUPTA P. Men and mice: relating their ages[J]. Life Sci, 2016, 152:244-248. DOI: 10.1016/j.lfs.2015.10.025 . |
| 19 | PALANZA P. Animal models of anxiety and depression: how are females different?[J]. Neurosci Biobehav Rev, 2001, 25(3):219-233. DOI: 10.1016/s0149-7634(01)00010-0 . |
| 20 | STECHMAN M J, AHMAD B N, LOH N Y, et al. Establishing normal plasma and 24-hour urinary biochemistry ranges in C3H, BALB/c and C57BL/6J mice following acclimatization in metabolic cages[J]. Lab Anim, 2010, 44(3):218-225. DOI: 10.1258/la.2010.009128 . |
| 21 | 尹媛, 陆璐, 王蕴, 等. 小鼠生活笼实验引入适应期的必要性研究[J]. 实验动物与比较医学, 2021, 41(4):358-362. DOI: 10.12300/j.issn.1674-5817.2020.154 . |
| YIN Y, LU L, WANG Y, et al. Necessity of an acclimatization period in a home cage experiment of mice[J]. Lab Anim Comp Med, 2021, 41(4):358-362. DOI: 10.12300/j.issn.1674-5817.2020.154 . | |
| 22 | ERIKSSON E, ROYO F, LYBERG K, et al. Effect of metabolic cage housing on immunoglobulin A and corticosterone excretion in faeces and urine of young male rats[J]. Exp Physiol, 2004, 89(4):427-433. DOI: 10.1113/expphysiol. 2004. 027656 . |
| 23 | TULI J S, SMITH J A, MORTON D B. Stress measurements in mice after transportation[J]. Lab Anim, 1995, 29(2):132-138. DOI: 10.1258/002367795780740249 . |
| 24 | Parliament European. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes [S/OL]. (2010-10-20) [2024-02-20].. |
| [1] | LIU Yueqin, XUE Weiguo, WANG Shuyou, SHEN Yaohua, JIA Shuyong, WANG Guangjun, SONG Xiaojing. Observation of Digestive Tract Tissue Morphology in Mice Using Probe-Based Confocal Laser Endomicroscopy [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 457-465. |
| [2] | KONG Zhihao, WEI Xiaofeng, YU Lingzhi, FENG Liping, ZHU Qi, SHI Guojun, WANG Chen. Isolation and Identification of Staphylococcus xylosus in Nude Mice with Squamous Skin Scurfs [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 368-375. |
| [3] | XU Qiuyu, YAN Guofeng, FU Li, FAN Wenhua, ZHOU Jing, ZHU Lian, QIU Shuwen, ZHANG Jie, WU Ling. A Mouse Model of Polycystic Ovary Syndrome Established Through Subcutaneous Administration of Letrozole Sustained-Release Pellets and Hepatic Transcriptome Analysis [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 119-129. |
| [4] | LIU Rongle, CHENG Hao, SHANG Fusheng, CHANG Shufu, XU Ping. Study on Cardiac Aging Phenotypes of SHJH hr Mice [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 13-20. |
| [5] | WU Zhihao, CAO Shuyang, ZHOU Zhengyu. Establishment of an Intestinal Fibrosis Model Associated with Inflammatory Bowel Disease in VDR-/- Mice Induced by Helicobacter hepaticus Infection and Mechanism Exploration [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 37-46. |
| [6] | ZHANG Nan, LI Huaiyin, LIAN Xiaodi, WEI Juanpeng, GAO Ming. Effects of Different Durations of Light Exposure on Body Weight and Learning and Memory Abilities of NIH Mice [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 73-78. |
| [7] | ZHAO Xiaona, WANG Peng, YE Maoqing, QU Xinkai. Establishment of a New Hyperglycemic Obesity Cardiac Dysfunction Mouse Model with Triacsin C [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 605-612. |
| [8] | MENG Yu, LIANG Dongli, ZHENG Linlin, ZHOU Yuanyuan, WANG Zhaoxia. Optimization and Evaluation of Conditions for Orthotopic Nude Mouse Models of Human Liver Tumor Cells [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 511-522. |
| [9] | Jing QIN, Yong ZHAO, Caiqin ZHANG, Bing BAI, Changhong SHI. Construction and Evaluation of Theranostic Near-infrared Fluorescent Probe for Targeting Inflammatory Brain Edema [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 243-250. |
| [10] | Yisu ZHANG, Xinru LIU, Ruojie WU, Rui LIU, Hong OUYANG, Xiaohong LI. Establishment and Evaluation of Mouse Model of Pregnancy Pain-depression Comorbidity Induced by Chronic Unpredictable Stress, Complete Freund's Adjuvant and Formalin [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 259-269. |
| [11] | Dong WU, Rui SHI, Peishan LUO, Ling'en LI, Xijing SHENG, Mengyang WANG, Lu NI, Sujuan WANG, Huixin YANG, Jing ZHAO. Effects of Different Pellet Feed Hardness on Growth and Reproduction, Feed Utilization Rate, and Environmental Dust in Laboratory Mice [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 313-320. |
| [12] | Yun LIU, Tingting FENG, Wei TONG, Zhi GUO, Xia LI, Qi KONG, Zhiguang XIANG. Glycyrrhizic Acid Showed Therapeutic Effects on Severe Pulmonary Damages in Mice Induced by Pneumonia Virus of Mice Infection [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 251-258. |
| [13] | Jinhua HU, Jingjie HAN, Min JIN, Bin HU, Yuefen LOU. Effects of Puerarin on Bone Density in Rats and Mice: A Meta-analysis [J]. Laboratory Animal and Comparative Medicine, 2024, 44(2): 149-161. |
| [14] | Min LIANG, Yang GUO, Jinjin WANG, Mengyan ZHU, Jun CHI, Yanjuan CHEN, Chengji WANG, Zhilan YU, Ruling SHEN. Construction of Dmd Gene Mutant Mice and Phenotype Verification in Muscle and Immune Systems [J]. Laboratory Animal and Comparative Medicine, 2024, 44(1): 42-51. |
| [15] | Jianhua ZHENG, Yunzhi FA, Qiaoyan DONG, Yefeng QIU, Jingqing CHEN. Construction and Evaluation of a Mouse Model with Intestinal Injury by Acute Hypoxic Stress in Plateau [J]. Laboratory Animal and Comparative Medicine, 2024, 44(1): 31-41. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||