
Laboratory Animal and Comparative Medicine ›› 2024, Vol. 44 ›› Issue (3): 251-258.DOI: 10.12300/j.issn.1674-5817.2024.014
• Animal Models of Human Diseases • Previous Articles Next Articles
Yun LIU, Tingting FENG, Wei TONG, Zhi GUO, Xia LI, Qi KONG, Zhiguang XIANG( )(
)( )
)
Received:2024-01-23
Revised:2024-04-12
Online:2024-06-25
Published:2024-07-06
Contact:
Zhiguang XIANG
CLC Number:
Yun LIU,Tingting FENG,Wei TONG,et al. Glycyrrhizic Acid Showed Therapeutic Effects on Severe Pulmonary Damages in Mice Induced by Pneumonia Virus of Mice Infection[J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 251-258. DOI: 10.12300/j.issn.1674-5817.2024.014.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2024.014
Figure 1 Animal experiment timelineNote: Three days before pneumonia virus of mice (PVM) infection, all mice were transferred to the ABSL-2 facility. On Day 0, the body weights of mice were measured, PVM intranasal inoculation was performed, and glycyrrhizic acid (GA) or normal saline (NS) was administered via gavage. Subsequently, GA or NS treatment was given daily and body weights were measured every 3 days. Each group consisted of 6 mice. The endpoint of the experiment was set at 15 dpi (day post infection).
目的基因 Target gene | 上游引物(5’-3’) Forward primer | 下游引物(5’-3’) Reverse primer |
|---|---|---|
| PVM | GCCTGCATCAACACAGTGTGT | GCCTGATGTGGCAGTGCTT |
| TLR-4 | ATGGCATGGCTTACACCACC | GAGGCCAATTTTGTCTCCACA |
| AGER | ACATGTGTGTCTGAGGGAAGC | AGCTCTGACCGCAGTGTAAAG |
| HMGB1 | CTTCGGCCTTCTTCTTGTTCT | GGCAGCTTTCTTCTCATAGGG |
| IL-2 | CCCAAGCAGGCCACAGAATTGAAA | AGTCAAATCCAGAACATGCCGCAG |
| IFN-γ | AGAGGATGGTTTGCATCTGGGTCA | ACAACGCTATGCAGCTTGTTCGTG |
| CCL2 | GGCTCAGCCAGATGCAGTTAA | CCTACTCATTGGGATCATCTTGCT |
| TNF-α | GACAAGGCTGCCCCGACTA | TTTCTCCTGGTATGAGATAGCAAATC |
| IL-1β | ATGAGGACATGAGCACCT TC | CATTGAGTTGGAGAGCTTTC |
| IL-6 | GACTTCCATCCAGTTGCCTTC TT | TCCACGATTTCCCAGAGAACA |
Table 1 Primer Sequence
目的基因 Target gene | 上游引物(5’-3’) Forward primer | 下游引物(5’-3’) Reverse primer |
|---|---|---|
| PVM | GCCTGCATCAACACAGTGTGT | GCCTGATGTGGCAGTGCTT |
| TLR-4 | ATGGCATGGCTTACACCACC | GAGGCCAATTTTGTCTCCACA |
| AGER | ACATGTGTGTCTGAGGGAAGC | AGCTCTGACCGCAGTGTAAAG |
| HMGB1 | CTTCGGCCTTCTTCTTGTTCT | GGCAGCTTTCTTCTCATAGGG |
| IL-2 | CCCAAGCAGGCCACAGAATTGAAA | AGTCAAATCCAGAACATGCCGCAG |
| IFN-γ | AGAGGATGGTTTGCATCTGGGTCA | ACAACGCTATGCAGCTTGTTCGTG |
| CCL2 | GGCTCAGCCAGATGCAGTTAA | CCTACTCATTGGGATCATCTTGCT |
| TNF-α | GACAAGGCTGCCCCGACTA | TTTCTCCTGGTATGAGATAGCAAATC |
| IL-1β | ATGAGGACATGAGCACCT TC | CATTGAGTTGGAGAGCTTTC |
| IL-6 | GACTTCCATCCAGTTGCCTTC TT | TCCACGATTTCCCAGAGAACA |
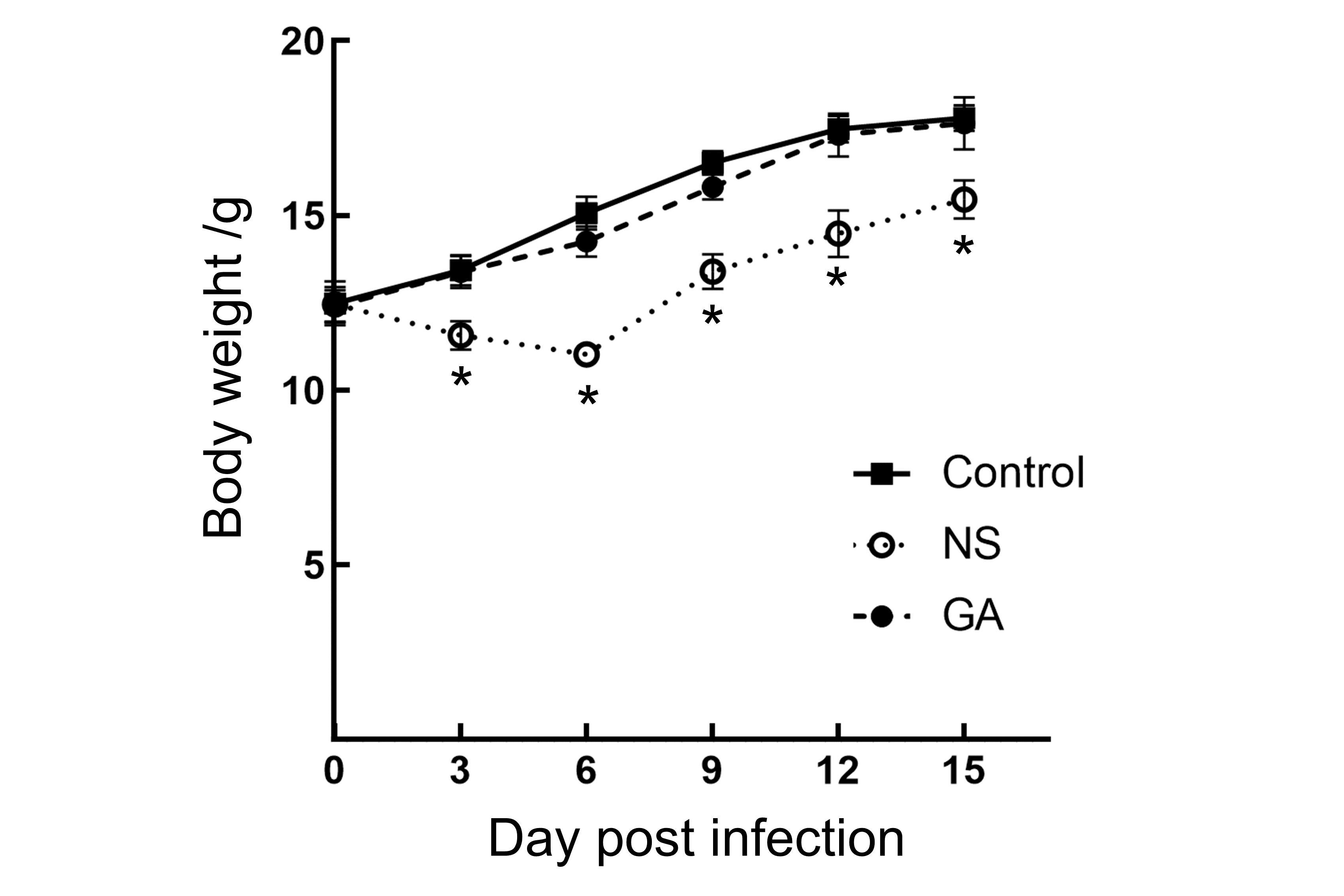
Figure 2 Body weight curves of mice after PVM infectionNote:The body weights of mice in the three groups were expressed as mean value±SD (n=6). Compared with the Control group, the NS group experienced weight loss at 3 and 6 dpi. Although weight gain in the NS group could be detected after 9 dpi, there were significant differences compared to the Control group (P<0.05). There were no significant weight differences in GA group compared to the Control group (P>0.05). Control: uninfected control group; NS: group treated with normal saline (NS) after infection; GA: group treated with glycyrrhizic acid (GA) after infection.
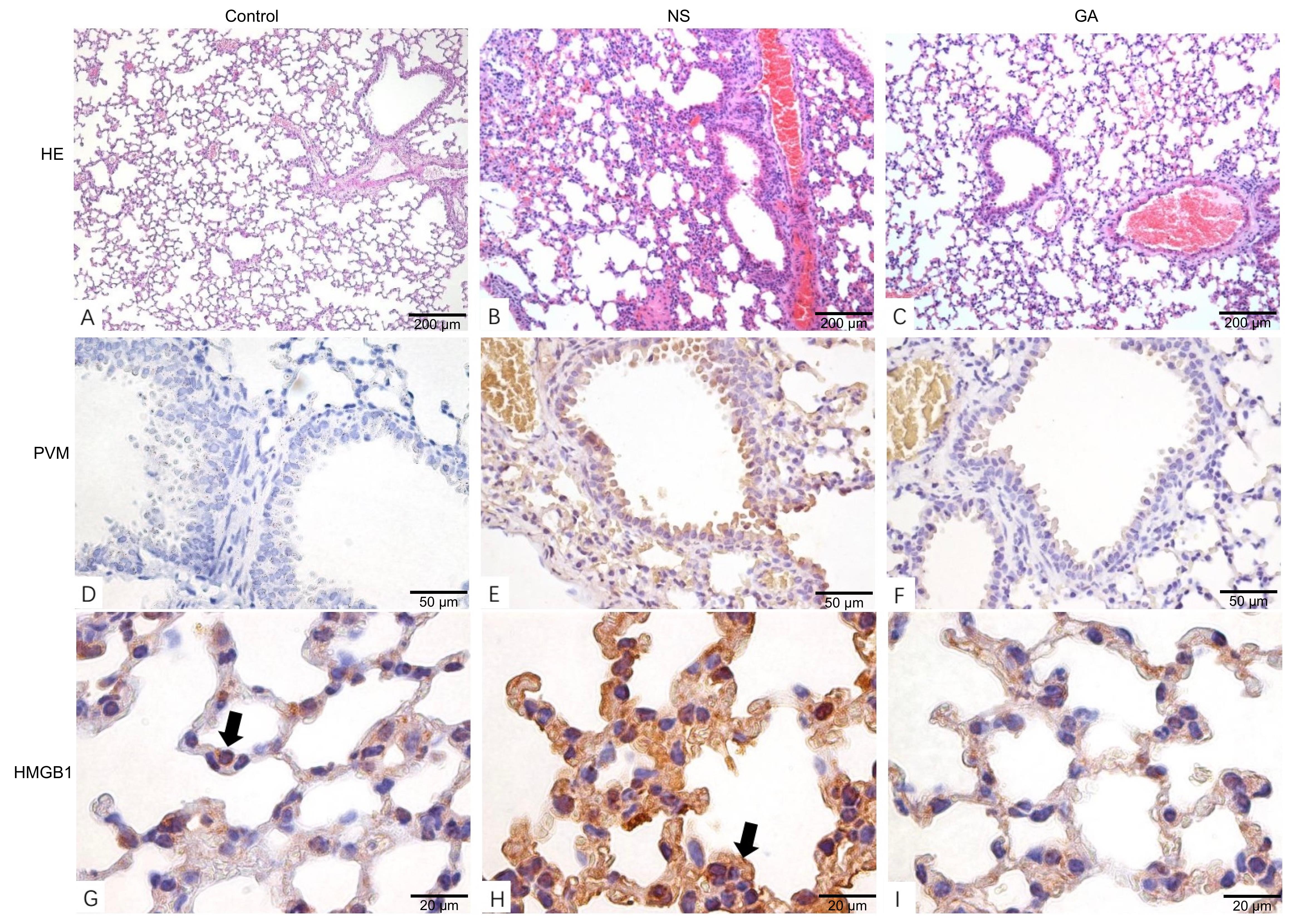
Figure 3 Pathological changes in the lungs of mice after PVM infection in three groupsNote:A-C, Histopathological analysis showed the pulmonary tissue pathological changes after PVM infection (HE staining, ×10). Compared with the Control group, thickened alveolar septa, hemorrhage in alveolar cavities, and vascular hyperemia were detected in the NS group; No significant pathological symptom was observed in the GA group. D-F, Immunohistochemical analysis demonstrated lower PVM viral load in pulmonary tissues in the GA group compared to the NS group (TMB staining, ×40). G-I, Immunohistochemical analysis showed the HMGB1 expression in pulmonary tissues (TMB staining, ×100). In the Control group, the cell nucleus locations of HMGB1 were indicated by arrows; in the NS group, HMGB1 was overexpressed and distributed in the cytoplasm. Control: uninfected control group; NS: group treated with normal saline(0.9%NaCl) after PVM infection; GA: group treated with glycyrrhizic acid after PVM infection. PVM: pneumonia virus of mice.
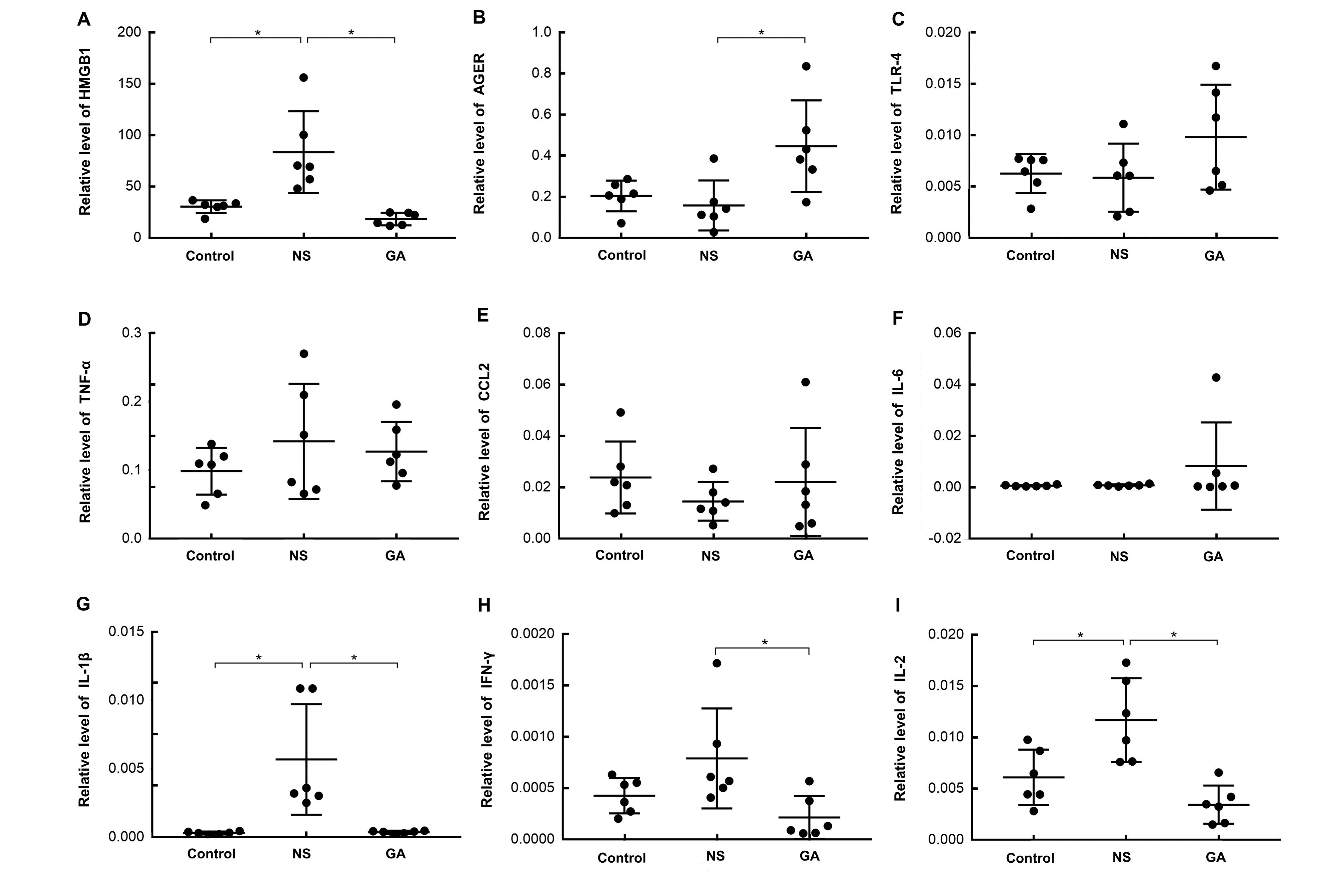
Figure 4 Expression of inflammatory factors and the alarmin HMGB1 and its receptor AGER after PVM infection in pulmonary tissueNote:Real-time fluorescence quantitative PCR was performed on the pulmonary tissues from mice in three groups. The expression of high mobility group box 1 (HMGB1), advanced glycosylation end-product-specific receptor (AGER), Toll-like receptor 4 (TLR4), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), C-C motif chemokine ligand 2 (CCL2), IL-6, interferon-γ (IFN-γ), and IL-2 were shown as ratios to GAPDH. n=6, *P<0.05. Control: uninfected control group; NS: group treated with normal saline(0.9%NaCl) after PVM infection; GA: group treated with glycyrrhizic acid after PVM infection. PVM: pneumonia virus of mice.
| 1 | ARANDA S S, POLACK F P. Prevention of pediatric respiratory syncytial virus lower respiratory tract illness: perspectives for the next decade[J]. Front Immunol, 2019, 10:1006. DOI: 10.3389/fimmu.2019.01006 . |
| 2 | 侯长春, 赵海金, 蔡绍曦, 等. 呼吸道合胞病毒增加小鼠肺组织的高迁移率族蛋白B1的表达和释放[J]. 南方医科大学学报, 2010, 30(4):700-703. DOI: 10.12122/j.issn.1673-4254.2010.04.010 . |
| HOU C C, ZHAO H J, CAI S X, et al. Respiratory syncytial virus increases the expression and release of high mobility group Box-1 protein in the lung tissue of mice[J]. J South Med Univ, 2010, 30(4):700-703. DOI: 10.12122/j.issn.1673-4254.2010.04.010 . | |
| 3 | RAYAVARA K, KUROSKY A, STAFFORD S J, et al. Proinflammatory effects of respiratory syncytial virus-induced epithelial HMGB1 on human innate immune cell activation[J]. J Immunol, 2018, 201(9):2753-2766. DOI: 10.4049/jimmunol.1800558 . |
| 4 | EASTON A J, DOMACHOWSKE J B, ROSENBERG H F. Animal pneumoviruses: molecular genetics and pathogenesis[J]. Clin Microbiol Rev, 2004, 17(2):390-412. DOI: 10.1128/cmr.17.2.390-412.2004 . |
| 5 | TAYLOR G. Animal models of respiratory syncytial virus infection[J]. Vaccine, 2017, 35(3):469-480. DOI: 10.1016/j.vaccine.2016.11.054 . |
| 6 | DYER K D, GARCIA-CRESPO K E, GLINEUR S, et al. The Pneumonia Virus of Mice (PVM) model of acute respiratory infection[J]. Viruses, 2012, 4(12):3494-3510. DOI: 10.3390/v4123494 . |
| 7 | ALTAMIRANO-LAGOS M J, DÍAZ F E, MANSILLA M A, et al. Current animal models for understanding the pathology caused by the respiratory syncytial virus[J]. Front Microbiol, 2019, 10:873. DOI: 10.3389/fmicb.2019.00873 . |
| 8 | SCAFFIDI P, MISTELI T, BIANCHI M E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation[J]. Nature, 2002, 418(6894):191-195. DOI: 10.1038/nature00858 . |
| 9 | LI G Q, LIANG X Y, LOTZE M T. HMGB1: the central cytokine for all lymphoid cells[J]. Front Immunol, 2013, 4:68. DOI: 10.3389/fimmu.2013.00068 . |
| 10 | LOTZE M T, TRACEY K J. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal[J]. Nat Rev Immunol, 2005, 5(4):331-342. DOI: 10.1038/nri1594 . |
| 11 | WANG L Q, YANG R, YUAN B C, et al. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb[J]. Acta Pharm Sin B, 2015, 5(4):310-315. DOI: 10.1016/j.apsb.2015.05.005 . |
| 12 | GOWDA P, PATRICK S, JOSHI S D, et al. Glycyrrhizin prevents SARS-CoV-2 S1 and Orf3a induced high mobility group box 1 (HMGB1) release and inhibits viral replication[J]. Cytokine, 2021, 142:155496. DOI: 10.1016/j.cyto.2021.155496 . |
| 13 | UTSUNOMIYA T, KOBAYASHI M, POLLARD R B, et al. Glycyrrhizin, an active component of licorice roots, reduces morbidity and mortality of mice infected with lethal doses of influenza virus[J]. Antimicrob Agents Chemother, 1997, 41(3):551-556. DOI: 10.1128/AAC.41.3.551 . |
| 14 | DENG Q P, WANG M J, ZENG X, et al. Effects of glycyrrhizin in a mouse model of lung adenocarcinoma[J]. Cell Physiol Biochem, 2017, 41(4):1383-1392. DOI: 10.1159/000467897 . |
| 15 | YAO D C, BROWNLEE M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands[J]. Diabetes, 2010, 59(1):249-255. DOI: 10.2337/db09-0801 . |
| 16 | SHARMA P, SHARMA A, VISHWAKARMA A L, et al. Host lung immunity is severely compromised during tropical pulmonary eosinophilia: role of lung eosinophils and macrophages[J]. J Leukoc Biol, 2016, 99(4):619-628. DOI: 10.1189/jlb.4A0715-309RR . |
| 17 | JANG S S, KIM H G, LEE J S, et al. Melatonin reduces X-ray radiation-induced lung injury in mice by modulating oxidative stress and cytokine expression[J]. Int J Radiat Biol, 2013, 89(2):97-105. DOI: 10.3109/09553002.2013.734943 . |
| 18 | DEANE R, SINGH I, SAGARE A P, et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease[J]. J Clin Invest, 2012, 122(4):1377-1392. DOI: 10.1172/jci58642 . |
| 19 | ORSATTI C L, MISSIMA F, PAGLIARONE A C, et al. Th1/Th2 cytokines' expression and production by propolis-treated mice[J]. J Ethnopharmacol, 2010, 129(3):314-318. DOI: 10.1016/j.jep.2010.03.030 . |
| 20 | TRACEY L, PÉREZ-ROSADO A, ARTIGA M J, et al. Expression of the NF-kappaB targets BCL2 and BIRC5/Survivin characterizes small B-cell and aggressive B-cell lymphomas, respectively[J]. J Pathol, 2005, 206(2):123-134. DOI: 10.1002/path.1768 . |
| 21 | HOU C H, FONG Y C, TANG C H. HMGB-1 induces IL-6 production in human synovial fibroblasts through c-Src, Akt and NF-κB pathways[J]. J Cell Physiol, 2011, 226(8):2006-2015. DOI: 10.1002/jcp.22541 . |
| 22 | CHEN R C, KANG R, TANG D L.The mechanism of HMGB1 secretion and release[J].Exp Mol Med, 2022, 54(2):91-102. DOI:10.1038/s12276-022-00736-w . |
| 23 | MAGNA M, PISETSKY D S.The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases[J]. Mol Med, 2014, 20(1):138-146. DOI:10.2119/molmed.2013.00164 . |
| 24 | NI Y F, KUAI J K, LU Z F, et al. Glycyrrhizin treatment is associated with attenuation of lipopolysaccharide-induced acute lung injury by inhibiting cyclooxygenase-2 and inducible nitric oxide synthase expression[J]. J Surg Res, 2011, 165(1): e29-e35. DOI: 10.1016/j.jss.2010.10.004 . |
| 25 | SITIA G, IANNACONE M, MÜLLER S, et al. Treatment with HMGB1 inhibitors diminishes CTL-induced liver disease in HBV transgenic mice[J]. J Leukoc Biol, 2007, 81(1):100-107. DOI: 10.1189/jlb.0306173 . |
| 26 | INGRAHAM N E, LOTFI-EMRAN S, THIELEN B K, et al. Immunomodulation in COVID-19[J]. Lancet Respir Med, 2020, 8(6):544-546. DOI: 10.1016/S2213-2600(20)30226-5 . |
| 27 | MURCK H. Symptomatic protective action of glycyrrhizin (licorice) in COVID-19 infection?[J]. Front Immunol, 2020, 11:1239. DOI: 10.3389/fimmu.2020.01239 . |
| 28 | SEO E H, SONG G Y, KWAK B O, et al. Effects of glycyrrhizin on the differentiation of myeloid cells of the heart and lungs in lipopolysaccharide-induced septic mice[J]. Shock, 2017, 48(3):371-376. DOI: 10.1097/SHK.0000000000000850 . |
| 29 | YU Z Q, OHTAKI Y, KAI K Z, et al. Critical roles of platelets in lipopolysaccharide-induced lethality: effects of glycyrrhizin and possible strategy for acute respiratory distress syndrome[J]. Int Immunopharmacol, 2005, 5(3):571-580. DOI: 10.1016/j.intimp.2004.11.004 . |
| [1] | Dan WANG, Xiaolu ZHANG, Yan WANG, Bo FU, Wendong WANG, Jing LIU, Suyin ZHANG, Yihe WU, Deguo WU, Xiaoyan DU, Dawei ZHAN, Xiulin ZHANG, Changlong LI. Study on the Antibody Production Efficiency in Modified Big-BALB/c Mice [J]. Laboratory Animal and Comparative Medicine, 2023, 43(6): 612-618. |
| [2] | CHENG Jishuai, MOU Tangwei, LIU Lei, WU Huaye, LI Qihan. Comparative Pathological Analysis of Herpes Simplex Virus Type 2 Infection in BALB/c Mice by Different Routes [J]. Laboratory Animal and Comparative Medicine, 2020, 40(1): 15-21. |
| [3] | LEI Shan, LIU Qiang, HUANG Wei-jin, WANG You-chun. Influence of Strain, Gender and Hair Coat of Mice on Establishing Bioluminescent Imaging Pseudovirus Mouse Model [J]. Laboratory Animal and Comparative Medicine, 2019, 39(6): 423-428. |
| [4] | ZHENG Hai-ming, ZHAO Hang, ZHENG Ping. A Novel Mouse Model for Ulcerative Colitis-associated Carcinogenesis Induced by Azoxymethane and Dextran Sulfate Sodium [J]. Laboratory Animal and Comparative Medicine, 2012, 32(1): 47-50. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||