
Laboratory Animal and Comparative Medicine ›› 2023, Vol. 43 ›› Issue (6): 595-603.DOI: 10.12300/j.issn.1674-5817.2023.093
• Animal Experimental Techniques and Methods • Previous Articles Next Articles
Xin LIU1,2,3, Shaobo SHI1,2,3, Cui ZHANG1,2,3, Bo YANG1,2,3, Chuan QU1,2,3( )(
)( )
)
Received:2023-06-30
Revised:2023-10-18
Online:2023-12-25
Published:2023-12-25
Contact:
Chuan QU
CLC Number:
Xin LIU,Shaobo SHI,Cui ZHANG,et al. Construction and Evaluation of End-to-side Anastomosis Model of Autologous Arteriovenous Fistula in Mice[J]. Laboratory Animal and Comparative Medicine, 2023, 43(6): 595-603. DOI: 10.12300/j.issn.1674-5817.2023.093.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2023.093
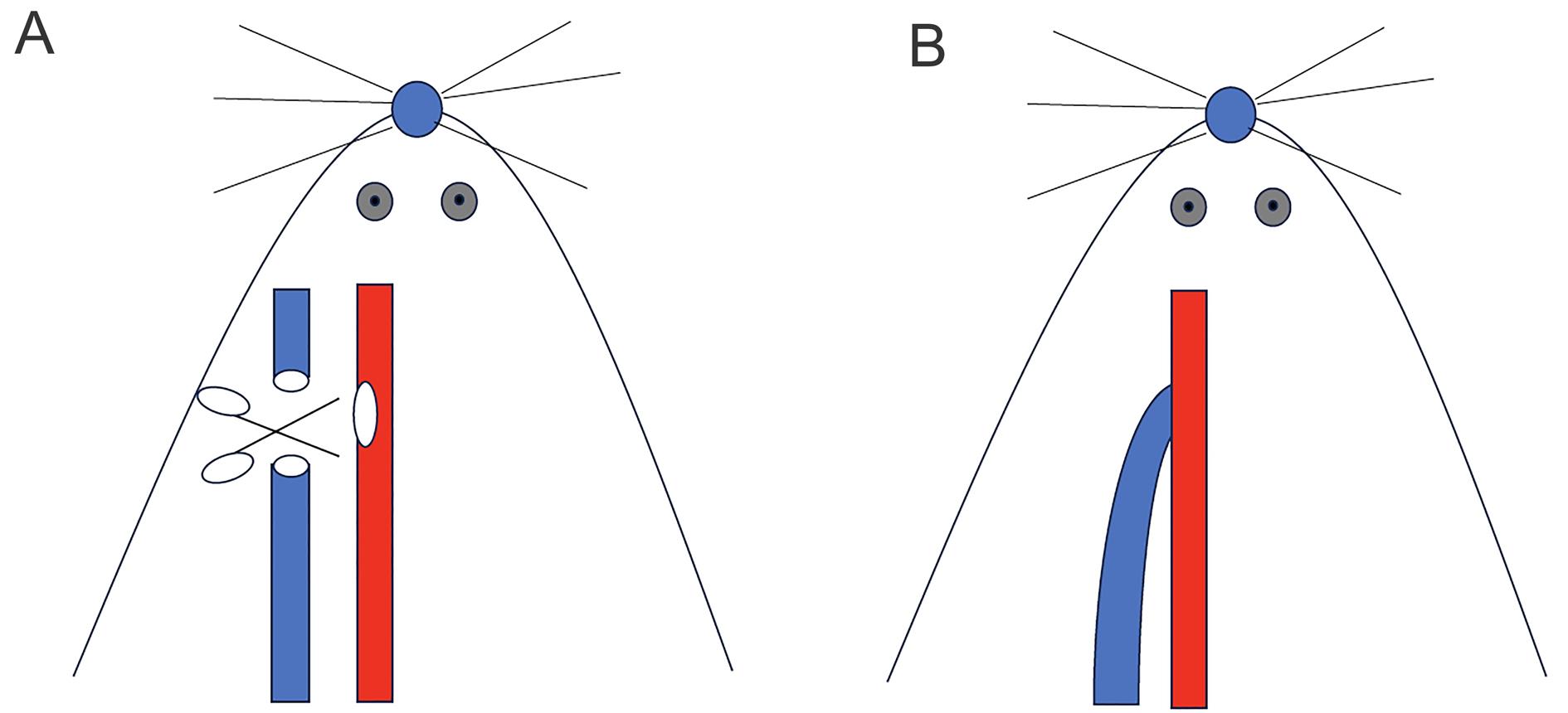
Figure 1 Operation diagram of autologous arteriovenous fistula in miceNote:The red blood vessel represents the common carotid artery and the blue blood vessel represents the external jugular vein. A, The external jugular vein and common carotid artery were separated, and incisions were made; B, Arteriovenous end-to-side anastomosis.
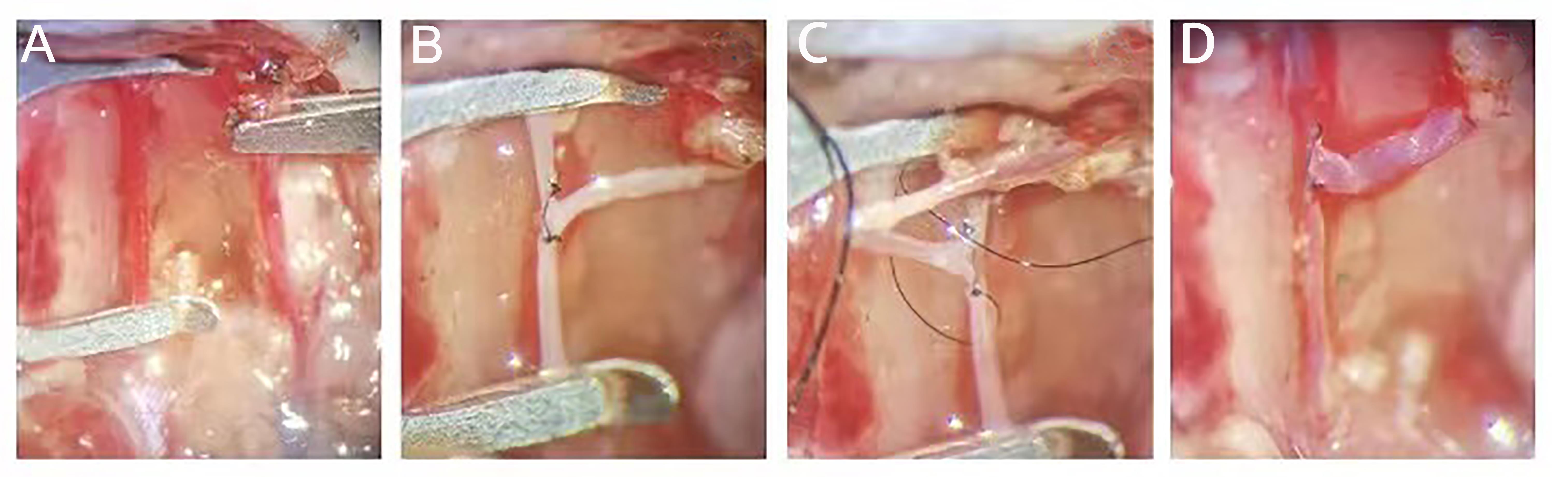
Figure 2 Procedures of end-to-side anastomosis of external jugular vein and common carotid artery in miceNote:A, The external jugular vein and the common carotid artery were separated, the distal end of the external jugular vein was ligated, and the proximal end of the external jugular vein as well as the proximal and distal end of the common carotid artery were closed with a microvascular clamp; B, Sutured the anterior wall of the anastomosis continuously at 0 and 12 points of the anastomosis; C, Lifted the inverted vein and sutured the posterior wall of the anastomosis continuously; D, After opening the vascular clamp, it could be observed that the end of the vein was full. Check for any blood seepage.
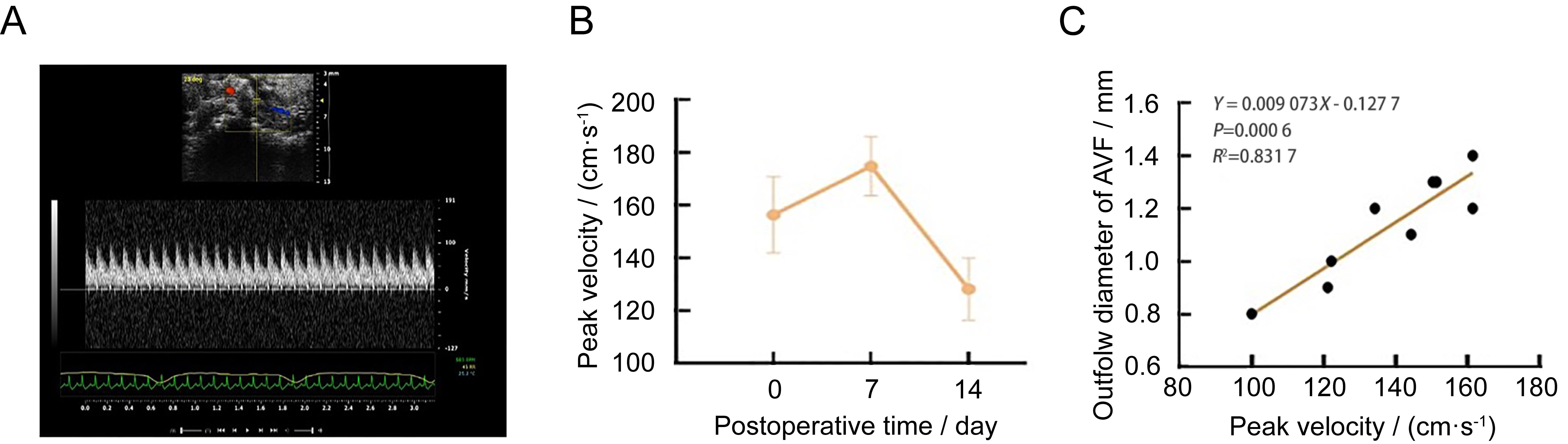
Figure 3 Noninvasive Doppler ultrasound assessment in mice autologous arteriovenous fistula (AVF) modelNote:A, Anastomotic ultrasound flow rate after surgery 14 days; B, The anastomotic flow rate was monitored on the day after surgery and 7 days and 14 days after surgery (n=9); C, The relationship between anastomotic flow velocity and outflow diameter of arteriovenous fistula after surgery 14 days (n=9).
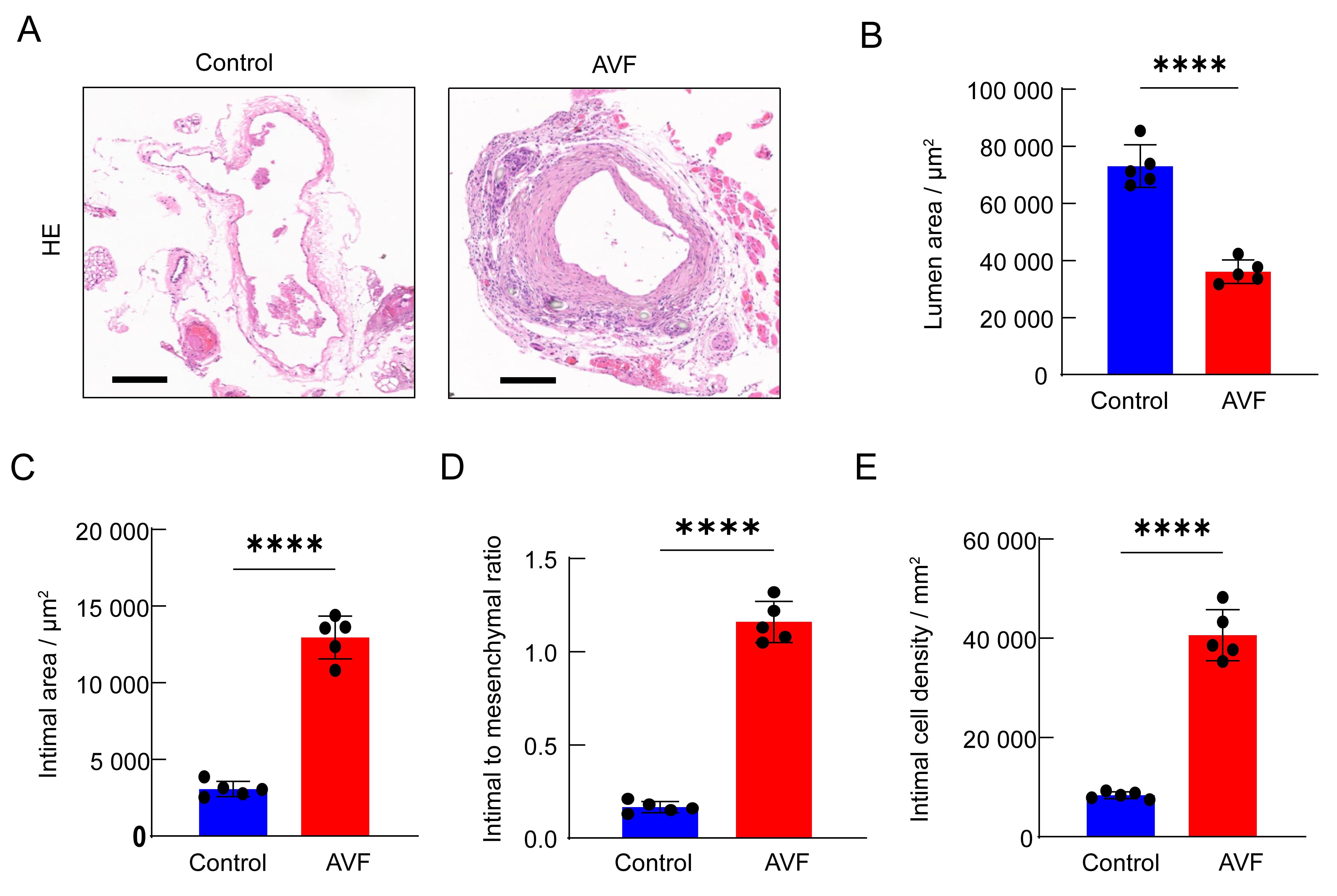
Figure 4 Analysis of vascular intima in mice autologous arteriovenous fistula (AVF) modelNote:A, the representative pictures of HE sections of the outflow veins on the control side and the AVF side at 14 days after surgery, the scale = 250 μm; B, the lumen area on the AVF side was significantly smaller than that on the control side at 14 days after surgery (n=5); C, the intimal area on the AVF side was significantly larger than that on the control side at 14 days after surgery (n=5); D, the intima-media ratio on the AVF side was significantly higher than that on the control side at 14 days after surgery (n=5); E, the intimal cell density on the AVF side was significantly higher than that on the control side at 14 days after surgery (n=5).
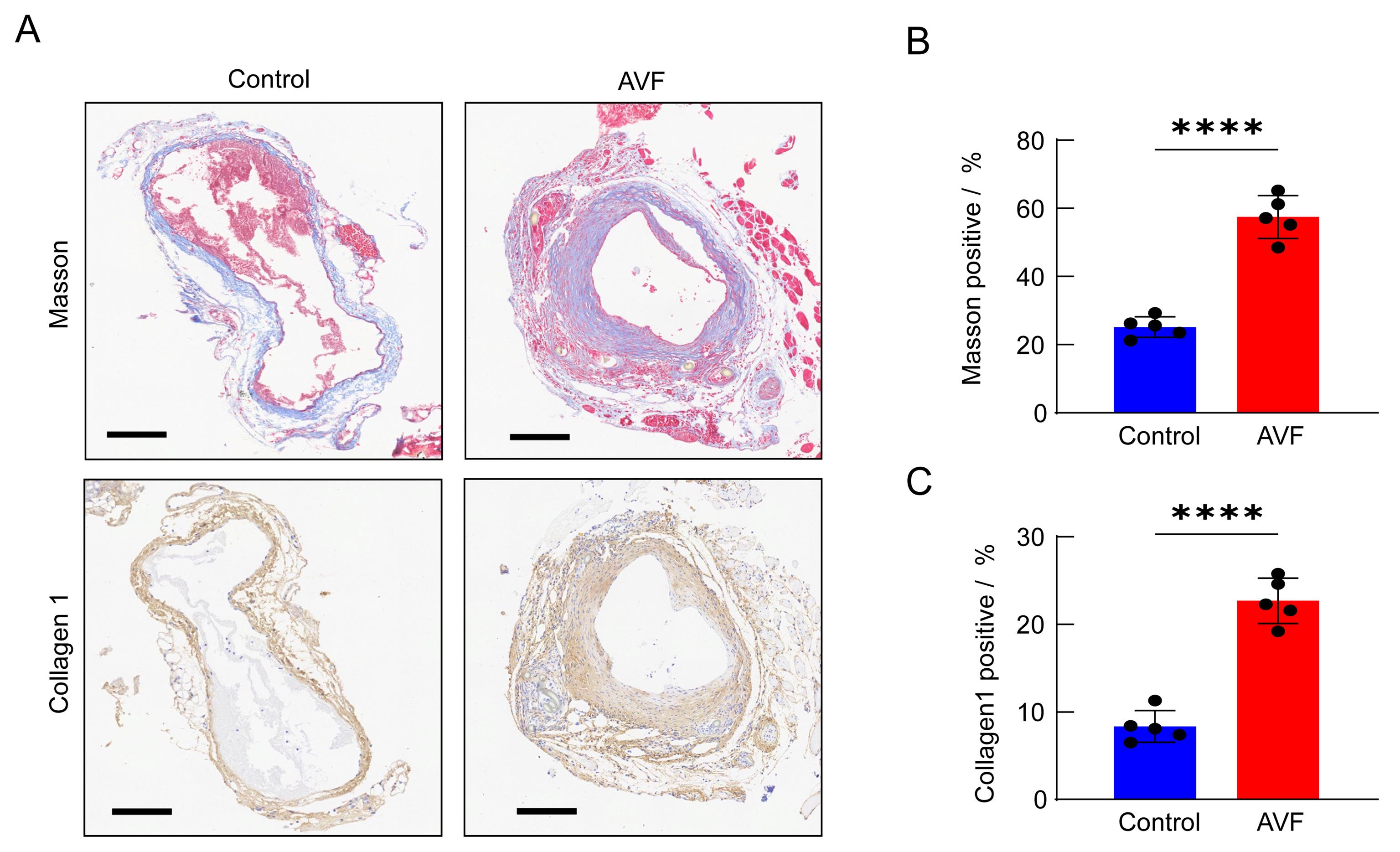
Figure 5 Analysis of collagen deposition in mice autologous arteriovenous fistula (AVF) modelNote: A. Masson staining and representative images of Collagen 1 immunohistochemical detection of outflow veins on the control side and AVF side at 14 days after surgery (scale = 250 μm); B, the proportion of MASSON-positive areas on AVF side at 14 days after surgery was significantly higher than that on the control side (n=5); C, the proportion of Collagen 1 positive areas on the AVF side was significantly higher than that on the control side at 14 days after surgery (n=5).
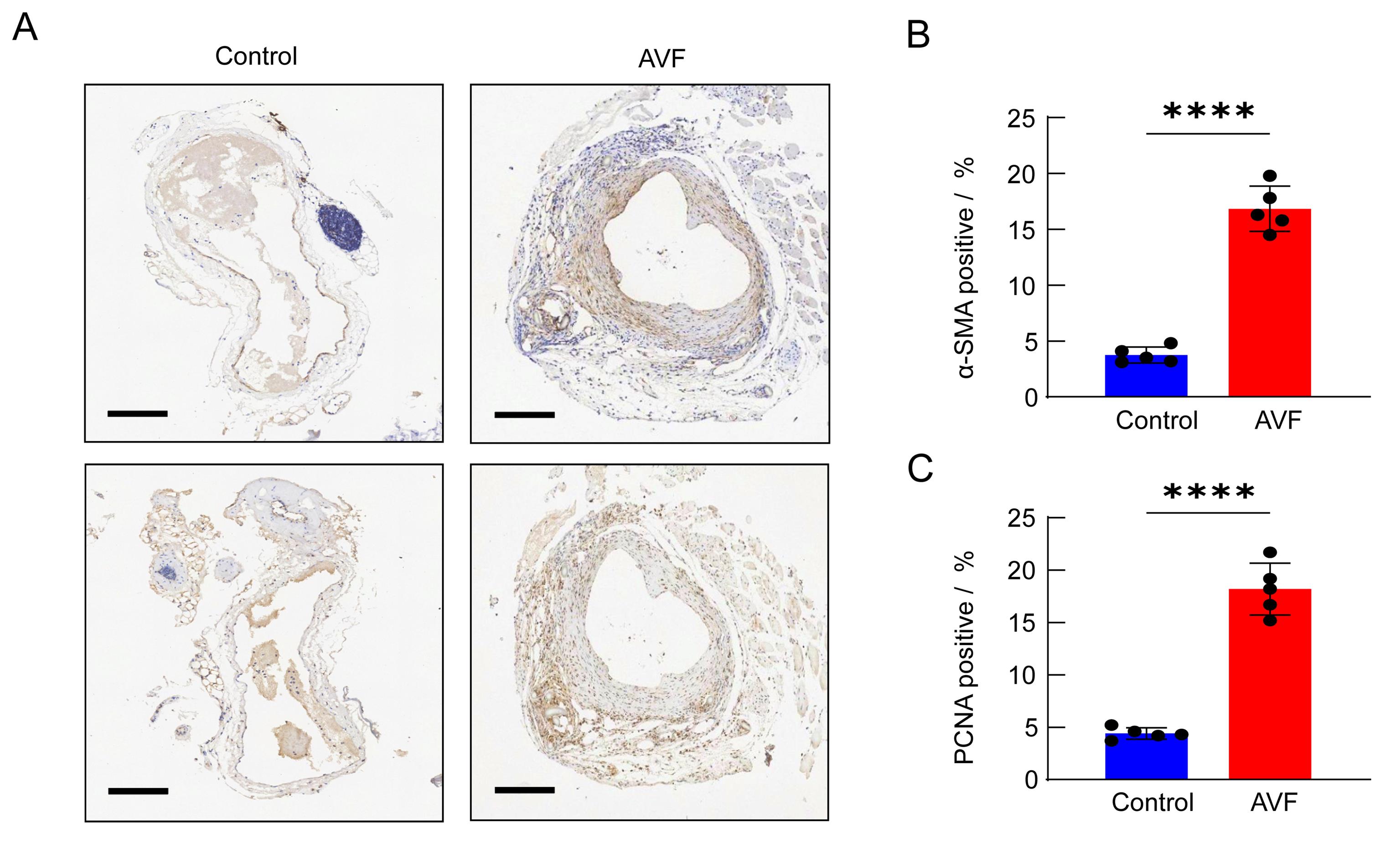
Figure 6 α-SMA and PCNA analysis in mice autologous arteriovenous fistula (AVF) modelNote: The expression of α-smooth muscle actin (α-SMA) and proliferating cell nuclear antigen (PCNA) in the outflow vein on the control side and AVF side at 14 days after surgery was detected by immunohistochemistry, scale =250 μm; B, the proportion of α-SMA positive cells on AVF side was significantly up-regulated at 14 days after surgery (n=5); C, the proportion of PCNA positive cells on AVF side increased significantly at 14 days after surgery (n=5).
| 1 | LIYANAGE T, NINOMIYA T, JHA V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review[J]. Lancet, 2015, 385(9981):1975-1982. DOI:10.1016/S0140-6736(14)61601-9 . |
| 2 | ROOIJENS P P G M, TORDOIR J H M, STIJNEN T, et al. Radiocephalic wrist arteriovenous fistula for hemodialysis: meta-analysis indicates a high primary failure rate[J]. Eur J Vasc Endovasc Surg, 2004, 28(6):583-589. DOI: 10.1016/j.ejvs.2004.08.014 . |
| 3 | HUIJBREGTS H J T, BOTS M L, WITTENS C H A, et al. Hemodialysis arteriovenous fistula patency revisited: results of a prospective, multicenter initiative[J]. Clin J Am Soc Nephrol, 2008, 3(3):714-719. DOI: 10.2215/CJN.02950707 . |
| 4 | AL-JAISHI A A, OLIVER M J, THOMAS S M, et al. Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis[J]. Am J Kidney Dis, 2014, 63(3):464-478. DOI: 10.1053/j.ajkd.2013.08.023 . |
| 5 | WASSE H, HUANG R, NAQVI N, et al. Inflammation, oxidation and venous neointimal hyperplasia precede vascular injury from AVF creation in CKD patients[J]. J Vasc Access, 2012, 13(2):168-174. DOI: 10.5301/jva.5000024 . |
| 6 | ROY-CHAUDHURY P, WANG Y, KRISHNAMOORTHY M, et al. Cellular phenotypes in human stenotic lesions from haemodialysis vascular access[J]. Nephrol Dial Transplant, 2009, 24(9):2786-2791. DOI: 10.1093/ndt/gfn708 . |
| 7 | ROY-CHAUDHURY P, AREND L, ZHANG J H, et al. Neointimal hyperplasia in early arteriovenous fistula failure[J]. Am J Kidney Dis, 2007, 50(5):782-790. DOI: 10.1053/j.ajkd. 2007. 07. 019 . |
| 8 | YANG B X, SHERGILL U, FU A A, et al. The mouse arteriovenous fistula model[J]. J Vasc Interv Radiol, 2009, 20(7):946-950. DOI: 10.1016/j.jvir.2009.03.044 . |
| 9 | YAMAMOTO K, PROTACK C D, TSUNEKI M, et al. The mouse aortocaval fistula recapitulates human arteriovenous fistula maturation[J]. Am J Physiol Heart Circ Physiol, 2013, 305(12): H1718-H1725. DOI: 10.1152/ajpheart.00590.2013 . |
| 10 | LIANG M, WANG Y, LIANG A L, et al. Migration of smooth muscle cells from the arterial anastomosis of arteriovenous fistulas requires Notch activation to form neointima[J]. Kidney Int, 2015, 88(3):490-502. DOI: 10.1038/ki.2015.73 . |
| 11 | MISRA S, KILARI S, YANG B X, et al. Anti human CX3CR1 VHH molecule attenuates venous neointimal hyperplasia of arteriovenous fistula in mouse model[J]. J Am Soc Nephrol, 2021, 32(7):1630-1648. DOI: 10.1681/ASN.2020101458 . |
| 12 | CAI C Q, KILARI S, ZHAO C L, et al. Therapeutic effect of adipose derived mesenchymal stem cell transplantation in reducing restenosis in a murine angioplasty model[J]. J Am Soc Nephrol, 2020, 31(8):1781-1795. DOI: 10.1681/ASN. 2019101042 . |
| 13 | ROY-CHAUDHURY P, KHAN R, CAMPOS B, et al. Pathogenetic role for early focal macrophage infiltration in a pig model of arteriovenous fistula (AVF) stenosis[J]. J Vasc Access, 2014, 15(1):25-28. DOI: 10.5301/jva.5000151 . |
| 14 | CHAN J S, CAMPOS B, WANG Y, et al. Proliferation patterns in a pig model of AV fistula stenosis: can we translate biology into novel therapies?[J]. Semin Dial, 2014, 27(6):626-632. DOI: 10.1111/sdi.12240 . |
| 15 | KOKOZIDOU M, KATSARGYRIS A, VERHOEVEN E L G, et al. Vascular access animal models used in research[J]. Ann Anat Anat Anz, 2019, 225:65-75. DOI: 10.1016/j.aanat.2019.06.002 . |
| 16 | FLORESCU M C, FOSTER K W, SACKS A R, et al. Sheep model of hemodialysis arteriovenous fistula using superficial veins[J]. Semin Dial, 2015, 28(6):687-691. DOI: 10.1111/sdi.12407 . |
| 17 | CASTIER Y, LEHOUX S, HU Y, et al. Characterization of neointima lesions associated with arteriovenous fistulas in a mouse model[J]. Kidney Int, 2006, 70(2):315-320. DOI: 10.1038/sj.ki.5001569 . |
| 18 | CAI C Q, ZHAO C L, KILARI S, et al. Experimental murine arteriovenous fistula model to study restenosis after transluminal angioplasty[J]. Lab Anim, 2020, 49(11):320-334. DOI: 10.1038/s41684-020-00659-x . |
| [1] | LIU Yayi, JIA Yunfeng, ZUO Yiming, ZHANG Junping, LÜ Shichao. Progress and Evaluation of Animal Model of Heart Qi-Yin Deficiency Syndrome [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 411-421. |
| [2] | ZHAO Xin, WANG Chenxi, SHI Wenqing, LOU Yuefen. Advances in the Application of Zebrafish in the Research of Inflammatory Bowel Disease Mechanisms and Drug Development [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 422-431. |
| [3] | LIU Yueqin, XUE Weiguo, WANG Shuyou, SHEN Yaohua, JIA Shuyong, WANG Guangjun, SONG Xiaojing. Observation of Digestive Tract Tissue Morphology in Mice Using Probe-Based Confocal Laser Endomicroscopy [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 457-465. |
| [4] | KONG Zhihao, WEI Xiaofeng, YU Lingzhi, FENG Liping, ZHU Qi, SHI Guojun, WANG Chen. Isolation and Identification of Staphylococcus xylosus in Nude Mice with Squamous Skin Scurfs [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 368-375. |
| [5] | PAN Yicong, JIANG Wenhong, HU Ming, QIN Xiao. Optimization of Surgical Procedure and Efficacy Evaluation of Aortic Calcification Model in Rats with Chronic Kidney Disease [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 279-289. |
| [6] | CHEN Yuhan, CHEN Jinling, LI Xin, OU Yanhua, WANG Si, CHEN Jingyi, WANG Xingyi, YUAN Jiali, DUAN Yuanyuan, YANG Zhongshan, NIU Haitao. Analysis of Animal Models of Myasthenia Gravis Based on Its Clinical Characteristics in Chinese and Western Medicine [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 176-186. |
| [7] | LIAN Hui, JIANG Yanling, LIU Jia, ZHANG Yuli, XIE Wei, XUE Xiaoou, LI Jian. Construction and Evaluation of a Rat Model of Abnormal Uterine Bleeding [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 130-146. |
| [8] | LUO Shixiong, ZHANG Sai, CHEN Hui. Research Progress in Establishment and Evaluation of Common Asthma Animal Models [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 167-175. |
| [9] | WANG Biying, LU Jiashuo, ZAN Guiying, CHEN Ruosong, CHAI Jingrui, LIU Jinggen, WANG Yujun. Establishment Methods and Application Progress of Rodent Models for Drug Addiction [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 158-166. |
| [10] | XU Qiuyu, YAN Guofeng, FU Li, FAN Wenhua, ZHOU Jing, ZHU Lian, QIU Shuwen, ZHANG Jie, WU Ling. A Mouse Model of Polycystic Ovary Syndrome Established Through Subcutaneous Administration of Letrozole Sustained-Release Pellets and Hepatic Transcriptome Analysis [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 119-129. |
| [11] | WU Zhihao, CAO Shuyang, ZHOU Zhengyu. Establishment of an Intestinal Fibrosis Model Associated with Inflammatory Bowel Disease in VDR-/- Mice Induced by Helicobacter hepaticus Infection and Mechanism Exploration [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 37-46. |
| [12] | ZHANG Nan, LI Huaiyin, LIAN Xiaodi, WEI Juanpeng, GAO Ming. Effects of Different Durations of Light Exposure on Body Weight and Learning and Memory Abilities of NIH Mice [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 73-78. |
| [13] | FEI Bin, GUO Wenke, GUO Jianping. Research Progress on Animal Models for Hernia Diseases and New Hernia Repair Materials [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 55-66. |
| [14] | LIU Rongle, CHENG Hao, SHANG Fusheng, CHANG Shufu, XU Ping. Study on Cardiac Aging Phenotypes of SHJH hr Mice [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 13-20. |
| [15] | YANG Jiahao, DING Chunlei, QIAN Fenghua, SUN Qi, JIANG Xusheng, CHEN Wen, SHEN Mengwen. Research Progress on Animal Models of Sepsis-Related Organ Injury [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 636-644. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||