
Laboratory Animal and Comparative Medicine ›› 2025, Vol. 45 ›› Issue (2): 130-146.DOI: 10.12300/j.issn.1674-5817.2024.132
• Animal Models of Human Diseases • Previous Articles Next Articles
LIAN Hui1, JIANG Yanling1, LIU Jia1, ZHANG Yuli2, XIE Wei2, XUE Xiaoou2, LI Jian1( )(
)( )
)
Received:2024-09-05
Revised:2025-01-07
Online:2025-04-25
Published:2025-04-25
Contact:
LI Jian
CLC Number:
LIAN Hui,JIANG Yanling,LIU Jia,et al. Construction and Evaluation of a Rat Model of Abnormal Uterine Bleeding[J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 130-146. DOI: 10.12300/j.issn.1674-5817.2024.132.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2024.132
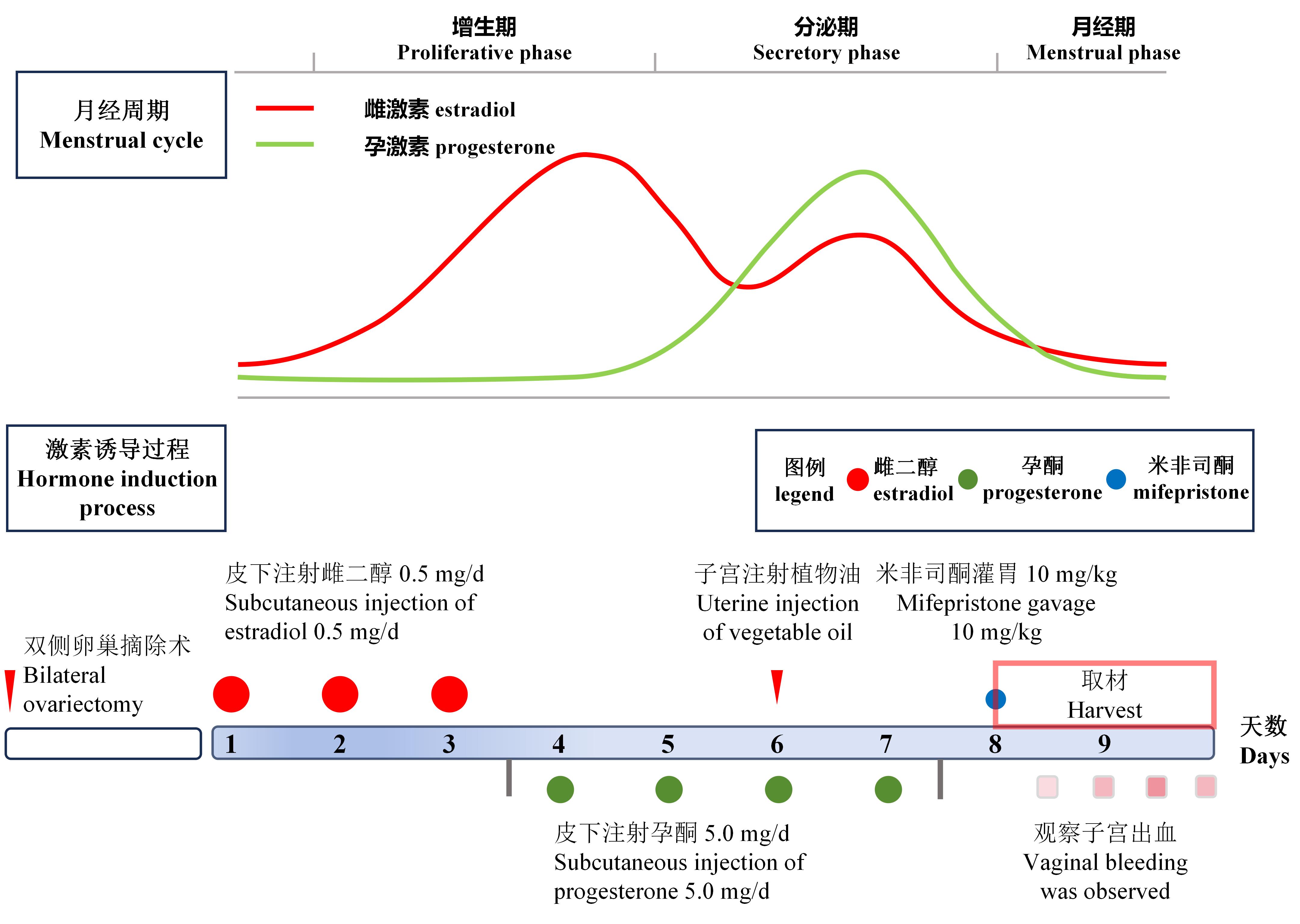
Figure 1 Schematic diagram of estrogen and progesterone level fluctuations during the menstrual cycle and flowchart of the preparation process for a rat model of abnormal uterine bleedingNote: Curves in the figure represent the changes in estrogen and progesterone levels during the normal human menstrual cycle. The process in the figure illustrates the protocol for preparing a rat model of abnormal uterine bleeding.
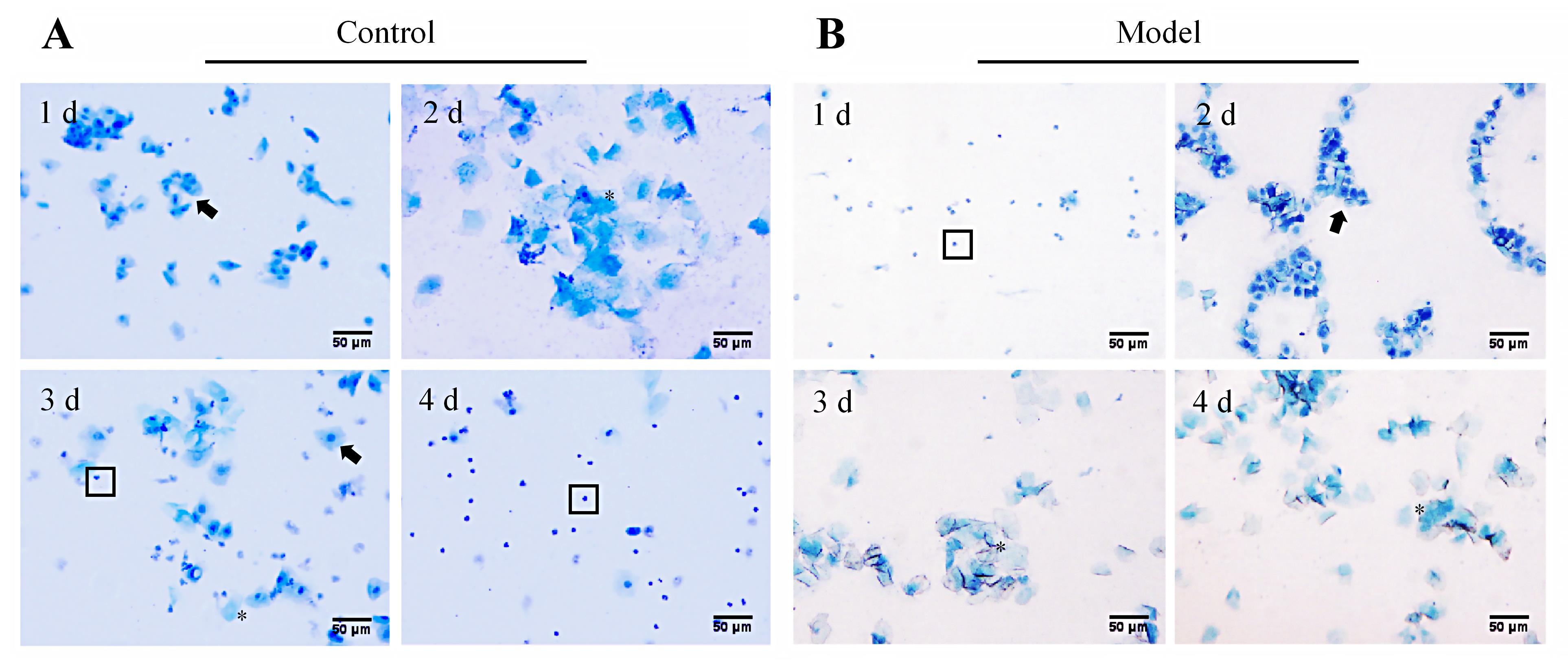
Figure 2 Detection of changes in the estrous cycle in abnormal uterine bleeding model rats using vaginal exfoliated cell smear (methylene blue staining, ×200)Note: A, Morphological changes of vaginal exfoliated cells of a representative rat in the normal control group during one estrous cycle (Days 1, 2, 3, and 4 correspond to proestrus, estrus, metestrus, and diestrus, respectively, showing cyclical changes); B, Morphological changes of vaginal exfoliated cells of a representative rat in the model group from ovariectomy, hormone induction to progesterone withdrawal (Days 1, 2, 3, and 4 correspond to diestrus, proestrus, estrus, and estrus, respectively, showing a non-cyclic pattern). In figures, black arrows (?) indicate nucleated epithelial cells, asterisks (*) mark keratinized epithelial cells, and boxes (□) denote white blood cells. In figures, the scale bar is 50 μm.
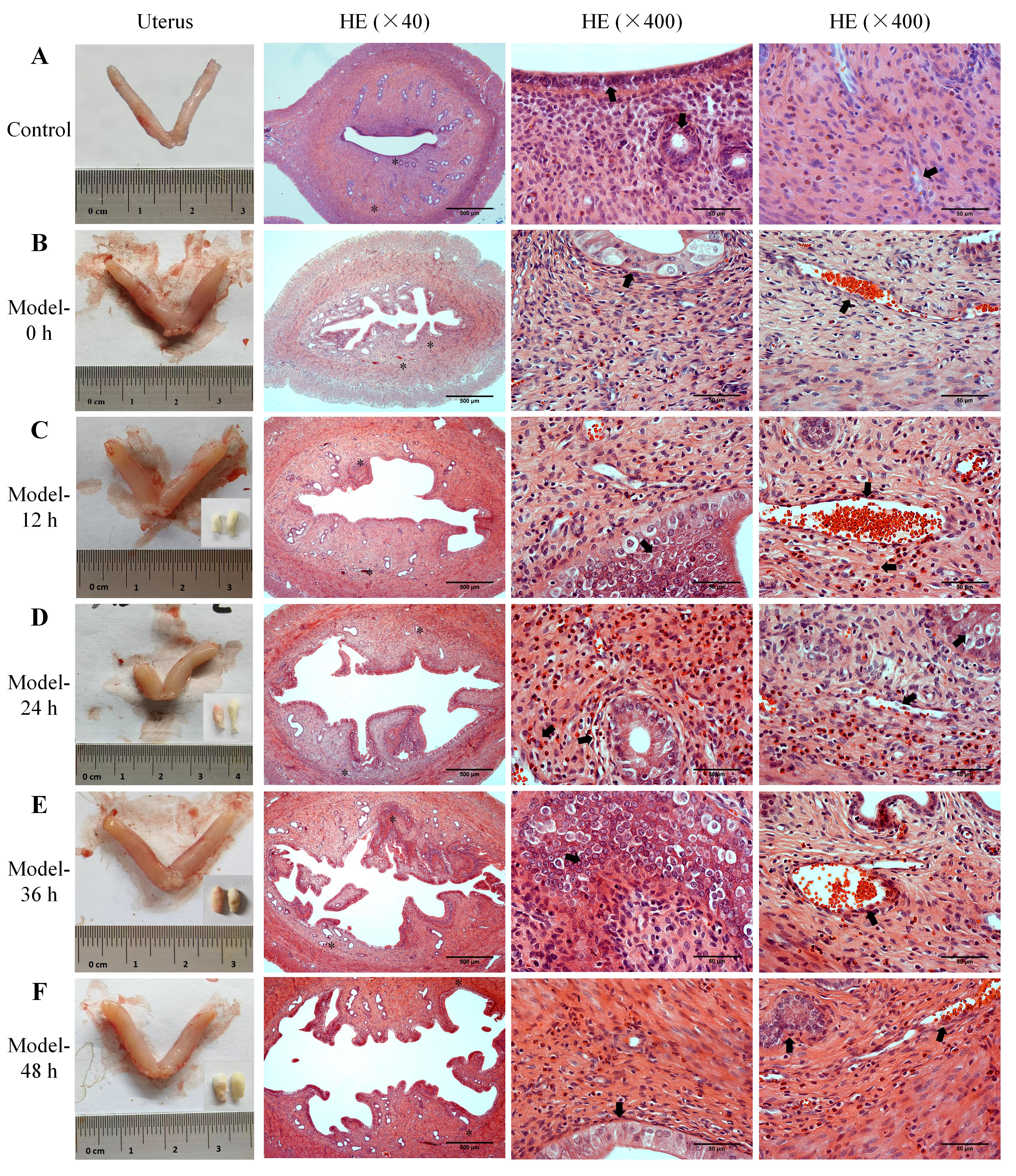
Figure 3 Uterine morphology, bleeding patterns, and histopathological changes of endometrial tissues in abnormal uterine bleeding model ratsNote: A, Uterine morphology and the HE-stained images of endometrial tissue in normal control rats; B-F, The uterine morphology in AUB model rats at 0 h, 12 h, 24 h, 36 h, and 48 h after progesterone withdrawal, along with vaginal cotton balls collected for uterine bleeding assessment every 12 hours (lower right corner), and the HE-stained images of endometrial tissue at the corresponding time points. The two asterisks (*) in each low-magnification HE-stained image indicate the two areas observed under high magnification, and the black arrows (?) point to the structures and pathological phenomena such as the endometrial epithelium, glands, necrotic stroma, blood vessels, and bleeding. In the HE-stained images, the scale bars from left to right column are 500 μm, 50 μm and 50 μm, respectively.
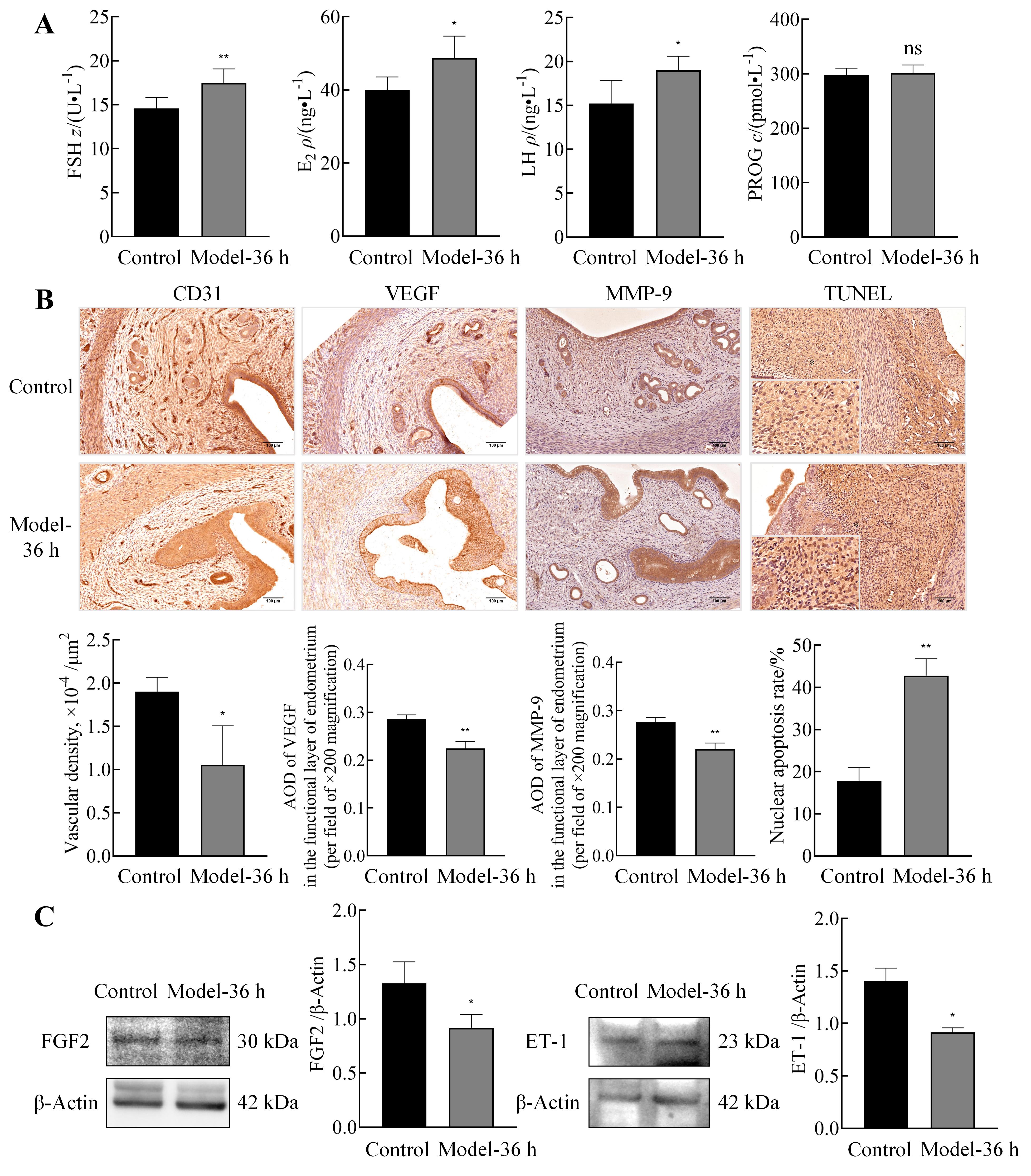
Figure 4 Changes in serum sex hormone levels, injury and repair of uterus blood vessels and stroma, and vascular contractile function of uterus in abnormal uterine bleeding model ratsNote: A, ELISA was used to measure serum levels of sex hormones, including FSH, E2, LH, and PROG, in normal control rats and the AUB model rats at 36 h after progesterone withdrawal (n=6). B, Immunohistochemistry was performed to assess the expression of vascular endothelial marker platelet endothelial cell adhesion molecule-1 (CD31), angiogenesis-related protein vascular endothelial growth factor (VEGF), and stromal injury/repair protein matrix metalloproteinase-9 (MMP-9) in uterine tissues of both groups,along with statistical analysis of the results (n=3; scale bars: 100 μm). Additionally, TUNEL staining of rat endometrium was conducted, and the percentage of positive cells was quantified [n=3; asterisks (*) in low-magnification images (scale bars: 100 μm) indicate regions selected for high-magnification observation (scale bars: 25 μm)]. C, Western blotting was employed to detect the expression of fibroblast growth factor 2 (FGF2) and endothelin-1 (ET-1) in uterine tissues of both groups, along with relative quantification (n=3; data presented as mean ± standard error (SE); Compared with normal control group, nsP>0.05, *P<0.05, **P<0.01).
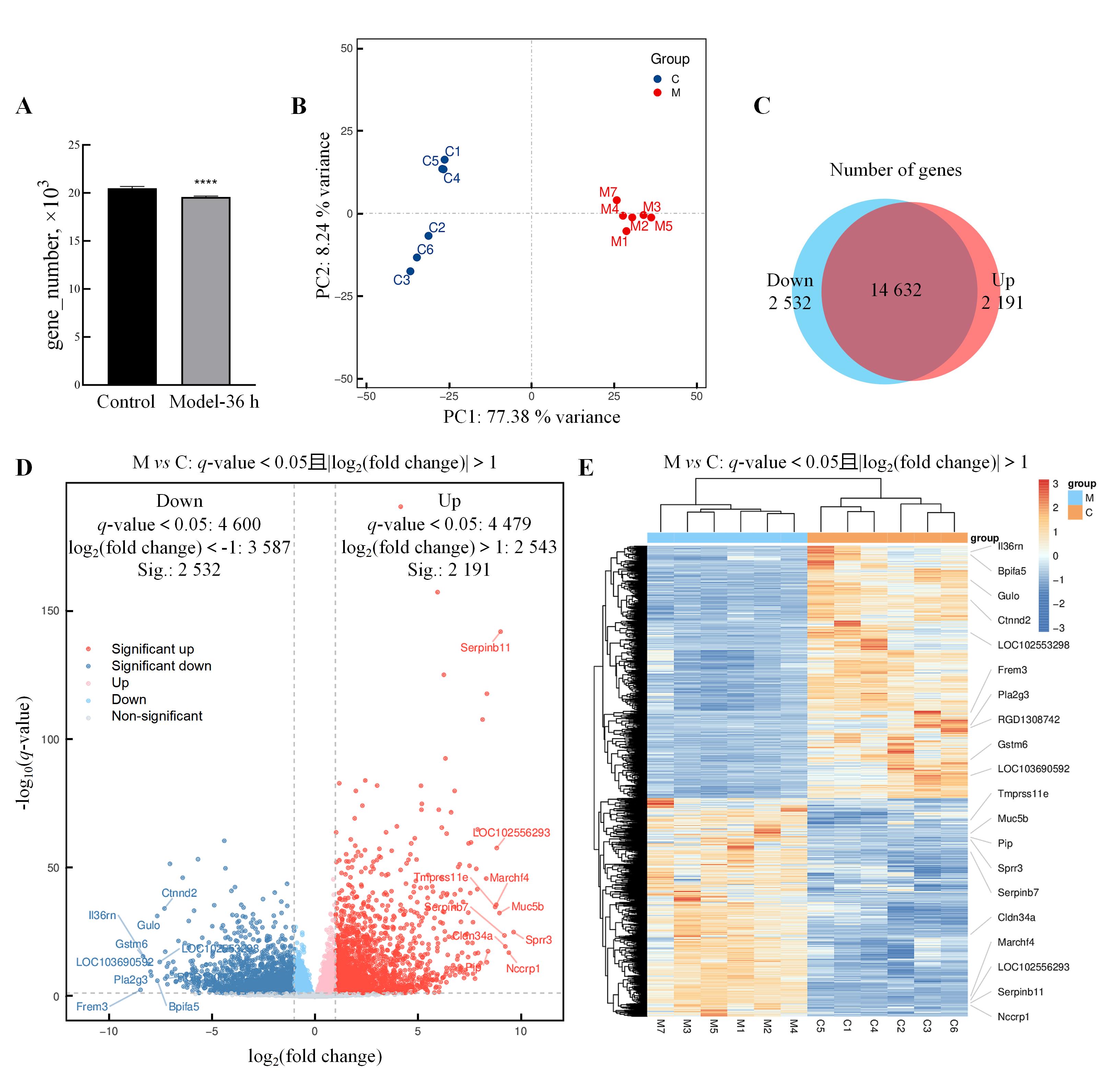
Figure 5 Transcriptomic analysis of differentially expressed genes in uterine tissue of abnormal uterine bleedingmodel ratsNote: A, The number of genes in rat samples from the normal control rats and the AUB model rats at 36 h after progesterone withdrawal (Model-36 h)(compared with normal control group, ****P<0.000 1); B, Results of principal component analysis; C, Venn diagram showing the differences in expressed genes between the two groups [The blue area represents the gene set with significantly decreased expression (Down) in the Model-36 h compared to normal control group, while the red area represents the gene set with significantly increased expression (Up) in the Model-36 h compared to normal control group]; D, Volcano plot of differentially expressed genes (down-regulated differentially expressed genes meeting fold change criteria in blue, non-meeting fold change criteria in light blue; up-regulated differentially expressed genes meeting criteria in red, non-meeting criteria in light red; non-significantly expressed genes in gray. The plot labels some of the differentially expressed genes with higher fold changes, including both down-regulated and up-regulated ones); E, Differentially expressed gene clustering heatmap by group, where the intensity of color represents the value of data points (grouping and clustering are based on similarity, and genes with higher fold changes in up-regulation and down-regulation are labeled in the heatmap).
编号 No. | 基因符号 Gene symbol | log2差异倍数 log2(fold change) | q值 q-value | 功能概述 Summary |
|---|---|---|---|---|
| Up-regulated genes | ||||
| 1 | Scin | 4.173 | 4.546×10-191 | Predicted to enable actin filament binding activity and phosphatidylinositol-4,5-bisphosphate binding activity. etc. |
| 2 | Btc | 5.961 | 7.347×10-158 | Enables epidermal growth factor receptor binding activity and growth factor activity. etc. |
| 3 | Serpinb11 | 9.021 | 1.630×10-142 | Predicted to enable serine-type endopeptidase inhibitor activity. etc. |
| 4 | Slc16a12 | 6.266 | 9.424×10-126 | Enables creatine transmembrane transporter activity. etc. |
| 5 | Tprg1 | 8.356 | 2.229×10-118 | Predicted to be active in cytoplasm. etc. |
| 6 | Olfm4 | 8.147 | 2.346×10-108 | Predicted to enable cadherin binding activity and structural molecule activity. etc. |
| 7 | Tnfaip6 | 6.344 | 3.099×10-93 | Predicted to enable several functions, including carboxylesterase activity. etc. |
| 8 | Nck1 | 2.451 | 1.127×10-84 | Predicted to enable several functions, including eukaryotic initiation factor eIF2 binding activity. etc. |
| 9 | Actn4 | 1.188 | 1.391×10-83 | Enables ubiquitin protein ligase binding activity. etc. |
| 10 | Tmbim1 | 3.027 | 1.062×10-82 | Predicted to enable calcium channel activity and death receptor binding activity. etc. |
| Down-regulated genes | ||||
| 1 | Gstm5 | -4.393 | 3.077×10-61 | Enables glutathione transferase activity and identical protein binding activity. etc. |
| 2 | Armh4 | -5.671 | 4.422×10-54 | Predicted to enable TORC2 complex binding activity. etc. |
| 3 | LOC102548820 | -7.032 | 2.774×10-52 | - |
| 4 | Arg2 | -4.338 | 1.561×10-50 | Enables arginase activity and nitric-oxide synthase binding activity. etc. |
| 5 | Frem2 | -6.413 | 6.963×10-47 | Predicted to be involved in anatomical structure morphogenesis and cell adhesion. etc. |
| 6 | Mppe1 | -1.346 | 1.575×10-44 | Predicted to enable GPI anchor binding activity. etc. |
| 7 | Lef1 | -3.875 | 3.022×10-43 | Predicted to enable several functions, including DNA binding activity. etc. |
| 8 | Smad9 | -4.048 | 8.538×10-42 | Predicted to enable DNA-binding transcription factor activity, RNA polymerase Ⅱ-specific. etc. |
| 9 | Miga1 | -1.944 | 4.052×10-41 | Predicted to enable protein heterodimerization activity and protein homodimerization activity. etc. |
| 10 | Bmpr1b | -2.515 | 1.387×10-38 | Predicted to enable several functions, including ATP binding activity. etc. |
Table 1 Basic information of the top 10 up-regulated and the top 10 down-regulated differentially expressed genes in uterine tissues between abnormal uterine bleeding model rats and normal control rats
编号 No. | 基因符号 Gene symbol | log2差异倍数 log2(fold change) | q值 q-value | 功能概述 Summary |
|---|---|---|---|---|
| Up-regulated genes | ||||
| 1 | Scin | 4.173 | 4.546×10-191 | Predicted to enable actin filament binding activity and phosphatidylinositol-4,5-bisphosphate binding activity. etc. |
| 2 | Btc | 5.961 | 7.347×10-158 | Enables epidermal growth factor receptor binding activity and growth factor activity. etc. |
| 3 | Serpinb11 | 9.021 | 1.630×10-142 | Predicted to enable serine-type endopeptidase inhibitor activity. etc. |
| 4 | Slc16a12 | 6.266 | 9.424×10-126 | Enables creatine transmembrane transporter activity. etc. |
| 5 | Tprg1 | 8.356 | 2.229×10-118 | Predicted to be active in cytoplasm. etc. |
| 6 | Olfm4 | 8.147 | 2.346×10-108 | Predicted to enable cadherin binding activity and structural molecule activity. etc. |
| 7 | Tnfaip6 | 6.344 | 3.099×10-93 | Predicted to enable several functions, including carboxylesterase activity. etc. |
| 8 | Nck1 | 2.451 | 1.127×10-84 | Predicted to enable several functions, including eukaryotic initiation factor eIF2 binding activity. etc. |
| 9 | Actn4 | 1.188 | 1.391×10-83 | Enables ubiquitin protein ligase binding activity. etc. |
| 10 | Tmbim1 | 3.027 | 1.062×10-82 | Predicted to enable calcium channel activity and death receptor binding activity. etc. |
| Down-regulated genes | ||||
| 1 | Gstm5 | -4.393 | 3.077×10-61 | Enables glutathione transferase activity and identical protein binding activity. etc. |
| 2 | Armh4 | -5.671 | 4.422×10-54 | Predicted to enable TORC2 complex binding activity. etc. |
| 3 | LOC102548820 | -7.032 | 2.774×10-52 | - |
| 4 | Arg2 | -4.338 | 1.561×10-50 | Enables arginase activity and nitric-oxide synthase binding activity. etc. |
| 5 | Frem2 | -6.413 | 6.963×10-47 | Predicted to be involved in anatomical structure morphogenesis and cell adhesion. etc. |
| 6 | Mppe1 | -1.346 | 1.575×10-44 | Predicted to enable GPI anchor binding activity. etc. |
| 7 | Lef1 | -3.875 | 3.022×10-43 | Predicted to enable several functions, including DNA binding activity. etc. |
| 8 | Smad9 | -4.048 | 8.538×10-42 | Predicted to enable DNA-binding transcription factor activity, RNA polymerase Ⅱ-specific. etc. |
| 9 | Miga1 | -1.944 | 4.052×10-41 | Predicted to enable protein heterodimerization activity and protein homodimerization activity. etc. |
| 10 | Bmpr1b | -2.515 | 1.387×10-38 | Predicted to enable several functions, including ATP binding activity. etc. |
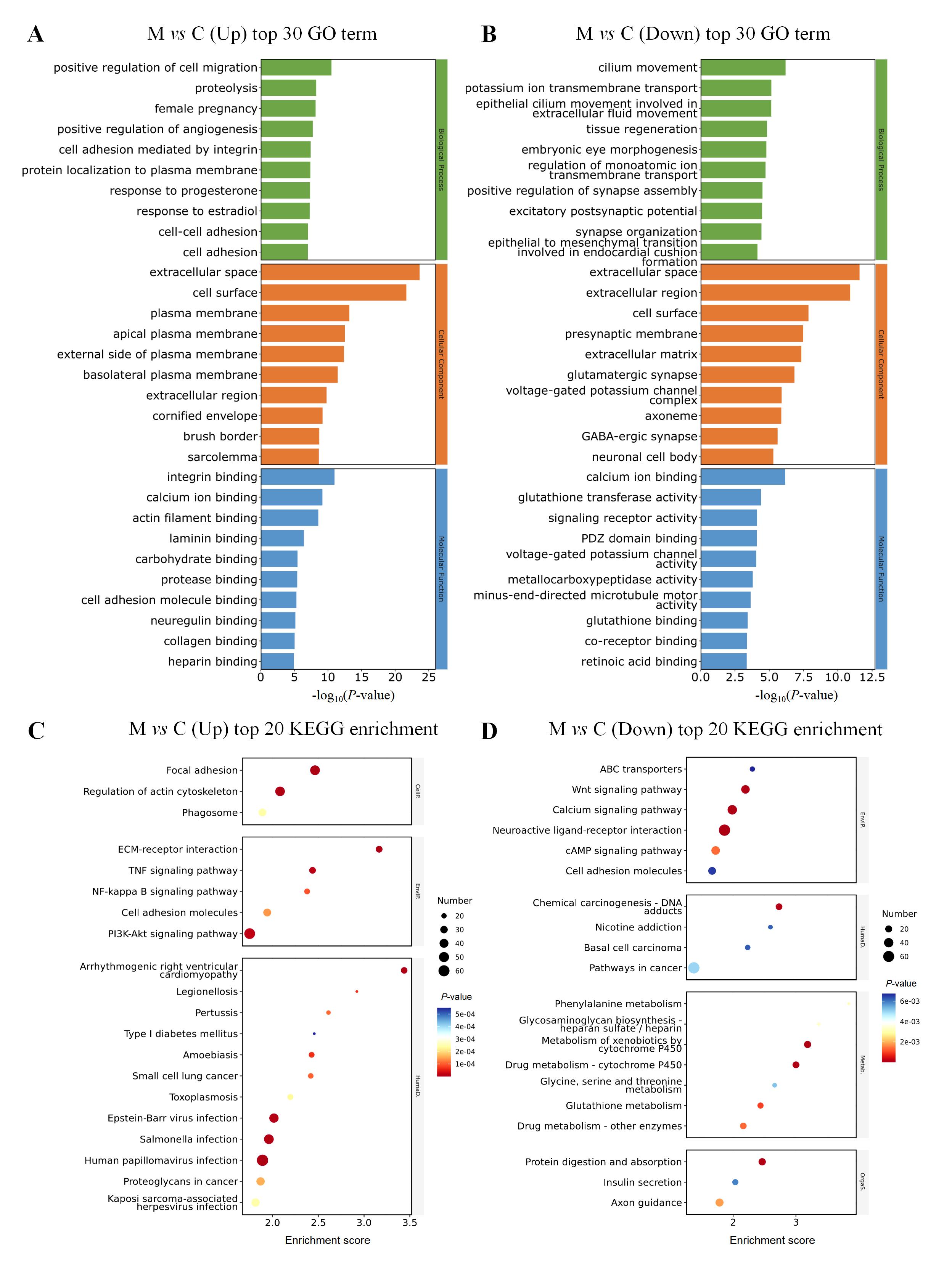
Figure 6 Transcriptomic analysis of differentially expressed genes in uterine tissue of model rats with abnormal uterine bleedingNote: A, GO functional analysis of up-regulated differentially expressed genes; B, GO functional analysis of down-regulated differentially expressed genes; C, KEGG enrichment analysis of up-regulated differentially expressed genes; D, KEGG enrichment analysis of down-regulated differentially expressed genes.
| 1 | 中华医学会妇产科学分会妇科内分泌学组. 异常子宫出血诊断与治疗指南(2022更新版)[J]. 中华妇产科杂志, 2022, 57(7):481-490. DOI: 10.3760/cma.j.cn112141-20220421-00258 . |
| Gynecologic Endocrinology Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association. Guideline on diagnosis and treatment of abnormal uterine bleeding: 2022 revisions[J]. Chin J Obstet Gynecol, 2022, 57(7):481-490. DOI: 10.3760/cma.j.cn112141-20220421-00258 . | |
| 2 | JAIN V, CHODANKAR R R, MAYBIN J A, et al. Uterine bleeding: how understanding endometrial physiology underpins menstrual health[J]. Nat Rev Endocrinol, 2022, 18(5):290-308. DOI:10.1038/s41574-021-00629-4 . |
| 3 | MUNRO M G, CRITCHLEY H O D, FRASER I S, et al. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions[J]. Int J Gynaecol Obstet, 2018, 143(3):393-408. DOI:10.1002/ijgo.12666 . |
| 4 | KITAHARA Y, HIRAIKE O, ISHIKAWA H, et al. National survey of abnormal uterine bleeding according to the FIGO classification in Japan[J]. J Obstet Gynaecol Res, 2023, 49(1):321-330. DOI:10.1111/jog.15464 . |
| 5 | LI Q H, REN J L, YANG L, et al. Parsing the Q-markers of Baoyin Jian to treat abnormal uterine bleeding by high-throughput chinmedomics strategy[J]. Pharmaceuticals, 2023, 16(5):719. DOI:10.3390/ph16050719 . |
| 6 | 高兰, 王侃, 贾媛, 等. 茜草不同炮制品对子宫内膜出血模型大鼠的影响[J]. 中医学报, 2020, 35(1):144-148. DOI: 10.16368/j.issn.1674-8999.2020.01.033 . |
| GAO L, WANG K, JIA Y, et al. Effects of different processed medicine of Rubia cordifolia L on endometrial hemorrhage model rats[J]. Acta Chin Med, 2020, 35(1):144-148. DOI: 10.16368/j.issn.1674-8999.2020.01.033 . | |
| 7 | KRIKUN G, BOOTH C J, BUCHWALDER L, et al. Long-term progestin-only contraception in humans versus animal models[J]. Ann N Y Acad Sci, 2011, 1221:119-123. DOI:10.1111/j.1749-6632.2010.05930.x . |
| 8 | 陈钢, 薛红, 孟宪丽, 等. 柴胡止血液对家兔置Cu-IUD的实验研究[J]. 辽宁中医学院学报, 1999, 1(3):200-201. DOI: 10.13194/j.jlunivtcm.1999.03.56.cheng.035 . |
| CHEN G, XUE H, MENG X L, et al. Experimental study on the effect of Chaihu Hemostatic solution on the placement of Cu-IUD in rabbits [J]. J Liaoning Coll Tradit Chin Med, 1999, 1(3):200-201. DOI: 10.13194/j.jlunivtcm.1999.03.56.cheng.035 . | |
| 9 | 尤昭玲, 马红霞, 陈俊明, 等. 恒河猴子宫内膜炎性出血模型的建立[J]. 中国比较医学杂志, 2003, 13(5): 310-312. DOI: 10.3969/j.issn.1671-7856.2003.05.015 . |
| YOU Z L, MA H X, CHEN J M, et al. Establishment of animal model with metrorrhagia induced by endometritis in experimental Rhesus monkey[J]. Chin J Comp Med, 2003, 13(5): 310-312. DOI: 10.3969/j.issn.1671-7856.2003.05.015 . | |
| 10 | FINN C A, POPE M. Vascular and cellular changes in the decidualized endometrium of the ovariectomized mouse following cessation of hormone treatment: a possible model for menstruation[J]. J Endocrinol, 1984, 100(3):295-300. DOI:10.1677/joe.0.1000295 . |
| 11 | CATALINI L, FEDDER J. Characteristics of the endometrium in menstruating species: lessons learned from the animal Kingdom[J]. Biol Reprod, 2020, 102(6):1160-1169. DOI:10.1093/biolre/ioaa029 . |
| 12 | BELLOFIORE N, RANA S, DICKINSON H, et al. Characterization of human-like menstruation in the spiny mouse: comparative studies with the human and induced mouse model[J]. Hum Reprod, 2018, 33(9):1715-1726. DOI:10.1093/humrep/dey247 . |
| 13 | J J 4th RASWEILER. Spontaneous decidual reactions and menstruation in the black mastiff bat, Molossus ater[J]. Am J Anat, 1991, 191(1):1-22. DOI:10.1002/aja.1001910102 . |
| 14 | CARTER A M. Classics revisited: C. J. van der Horst on pregnancy and menstruation in elephant shrews[J]. Placenta, 2018, 67:24-30. DOI:10.1016/j.placenta.2018.05.010 . |
| 15 | MORISON N B, ZHANG J, KAITU'U-LINO T J, et al. The long-term actions of etonogestrel and levonorgestrel on decidualized and non-decidualized endometrium in a mouse model mimic some effects of progestogen-only contraceptives in women[J]. Reproduction, 2007, 133(1):309-321. DOI:10.1530/rep.1.01171 . |
| 16 | 王晓东, 赵军宁, 张白嘉, 等. 药物致早孕大鼠子宫出血模型的建立[J]. 中国药理学通报, 1999, 15(2):182-184. DOI: 10.3321/j.issn: 1001-1978.1999.02.025 . |
| WANG X D, ZHAO J N, ZHANG B J, et al. Establishment of uterine bleeding model by mifepristoneand misoprostol in eraly-pregnancy rats[J]. Chin Pharmacol Bull, 1999, 15(2):182-184. DOI: 10.3321/j.issn: 1001-1978.1999.02.025 . | |
| 17 | TSOLOVA A O, AGUILAR R M, MAYBIN J A, et al. Pre-clinical models to study abnormal uterine bleeding (AUB)[J]. EBioMedicine, 2022, 84:104238. DOI:10.1016/j.ebiom. 2022. 104238 . |
| 18 | TERKEL J. Neuroendocrine processes in the establishment of pregnancy and pseudopregnancy in rats[J]. Psychoneuro-endocrinology, 1988, 13(1-2):5-28. DOI:10.1016/0306-4530(88)90004-2 . |
| 19 | 孔令伶俐, 许良智. 青春期排卵障碍性异常子宫出血的诊疗策略[J]. 实用妇产科杂志, 2022, 38(10):731-733. |
| KONG L L L, XU L Z. Diagnostic and therapeutic strategies for ovulatory dysfunctional uterine bleeding in adolescence [J]. J Pract Obstet Gynecol, 2022, 38(10):731-733. | |
| 20 | 朱小明, 徐君碧, 何人可, 等. 围绝经期妇女内分泌变化及相关疾病[J]. 山东大学学报(医学版), 2019, 57(2):6-10, 15. DOI: 10.6040/j.issn.1671-7554.0.2018.1459 . |
| ZHU X M, XU J B, HE R K, et al. Endocrine changes in perimenopausal women and the related diseases[J]. J Shandong Univ Health Sci, 2019, 57(2):6-10, 15. DOI: 10.6040/j.issn.1671-7554.0.2018.1459 . | |
| 21 | 李升华, 何涓, 唐健, 等. 自拟加味生化汤联合安宫黄体酮治疗围绝经期排卵障碍性异常子宫出血的临床研究[J/OL]. 中华中医药学刊, 2024:1-7. (2024-08-06)[2025-01-06]. https://link.cnki.net/urlid/21.1546.r.20240805.1120.004. |
| LI S H, HE J, TANG J, et al. Clinical study of self-made modified shenghua decoctioncombined with medroxy-progesteronein treatment of patients with perimenopausal abnormal uterine bleeding-ovulatory dysfunction [J/OL]. China Arch Trad Chin Med, 2024:1-7. (2024-08-06)[2025-01-06]. https://link.cnki.net/urlid/21.1546.r.20240805.1120.004. | |
| 22 | BRASTED M, WHITE CA, KENNEDY TG, et al. Mimicking the events of menstruation in the murine uterus[J]. Biol Reprod, 2003, 69(4):1273-1280. DOI: 10.1095/biolreprod.103.016550 . |
| 23 | MAYBIN J A, MURRAY A A, SAUNDERS P T K, et al. Hypoxia and hypoxia inducible factor-1α are required for normal endometrial repair during menstruation[J]. Nat Commun, 2018, 9(1):295. DOI:10.1038/s41467-017-02375-6 . |
| 24 | 郎建霞, 毛晓红, 徐翠萍. 排卵障碍性异常子宫出血患者神经内分泌功能及变化研究[J]. 现代实用医学, 2018, 30(10):1327-1329. DOI: 10.3969/j.issn.1671-0800.2018.10.036 . |
| LANG J X, MAO X H, XU C P. Research on neuroendocrine function and changes in patients with abnormal uterine bleeding-ovulatory dysfunction [J]. Mod Pract Med, 2018, 30(10):1327-1329. DOI: 10.3969/j.issn.1671-0800.2018.10.036 . | |
| 25 | 杨尚芝, 金红, 李娟, 等. 坤泰胶囊联合米非司酮治疗围绝经期异常子宫出血的疗效及对bFGF、VEGF水平的影响[J]. 现代生物医学进展, 2023, 23(17):3320, 3340-3344. DOI: 10.13241/j.cnki.pmb.2023.17.027 . |
| YANG S Z, JIN H, LI J, et al. Therapeutic effects of Kuntai capsule combined with mifepristone on perimenopausal abnormal uterine bleeding and its influence on bFGF and VEGF levels[J]. Prog Mod Biomed, 2023, 23(17):3320, 3340-3344. DOI: 10.13241/j.cnki.pmb.2023.17.027 . | |
| 26 | MARSH M M, MALAKOOTI N, TAYLOR N H, et al. Endothelin and neutral endopeptidase in the endometrium of women with menorrhagia[J]. Hum Reprod, 1997, 12(9):2036-2040. DOI:10.1093/humrep/12.9.2036 . |
| 27 | CHODANKAR R, CRITCHLEY H O D. Biomarkers in abnormal uterine bleeding[J]. Biol Reprod, 2019, 101(6):1155-1166. DOI:10.1093/biolre/ioy231 . |
| 28 | SCHATZ F, GUZELOGLU-KAYISLI O, ARLIER S, et al. The role of decidual cells in uterine hemostasis, menstruation, inflammation, adverse pregnancy outcomes and abnormal uterine bleeding[J]. Hum Reprod Update, 2016, 22(4):497-515. DOI:10.1093/humupd/dmw004 . |
| 29 | MILLING SMITH O P, JABBOUR H N, CRITCHLEY H D. Cyclooxygenase enzyme expression and E series prostaglandin receptor signalling are enhanced in heavy menstruation[J]. Hum Reprod, 2007, 22(5):1450-1456. DOI:10.1093/humrep/del503 . |
| 30 | MALIK S, DAY K, PERRAULT I, et al. Reduced levels of VEGF-A and MMP-2 and MMP-9 activity and increased TNF-alpha in menstrual endometrium and effluent in women with menorrhagia[J]. Hum Reprod, 2006, 21(8):2158-2166. DOI:10.1093/humrep/del089 . |
| 31 | CRITCHLEY H O D, MAYBIN J A, ARMSTRONG G M, et al. Physiology of the endometrium and regulation of menstruation[J]. Physiol Rev, 2020, 100(3):1149-1179. DOI:10.1152/physrev.00031.2019 . |
| 32 | ARMSTRONG G M, MAYBIN J A, MURRAY A A, et al. Endometrial apoptosis and neutrophil infiltration during menstruation exhibits spatial and temporal dynamics that are recapitulated in a mouse model[J]. Sci Rep, 2017, 7(1):17416. DOI:10.1038/s41598-017-17565-x . |
| 33 | 于明明, 谢卓, 金华, 等. 孕酮回植通过调节内质网应激诱导的细胞自噬与凋亡阻止小鼠月经发生[J]. 遵义医学院学报, 2019, 42(3):276-281. DOI: 10.14169/j.cnki.zunyixuebao.2019.0057 . |
| YU M M, XIE Z, JIN H, et al. Progesterone replantation to prevent menstruation in mice by regulating endoplasmic reticulum stress-induced autophagy and apoptosis[J]. J Zunyi Med Univ, 2019, 42(3):276-281. DOI: 10.14169/j.cnki.zunyixuebao.2019.0057 . | |
| 34 | LI J M, LIAO C C, HUANG HC, et al. Regulation effect and mechanism of Sheng-Hua-Tang on female reproductive system: From experimental transcriptomic analysis to clinical applications[J]. J Ethnopharmacol, 2020, 249:112431. DOI: 10.1016/j.jep.2019.112431 . |
| 35 | LIU T, SHI F L, YING Y, et al. Mouse model of menstruation: an indispensable tool to investigate the mechanisms of menstruation and gynaecological diseases (Review)[J]. Mol Med Rep, 2020, 22(6):4463-4474. DOI:10.3892/mmr.2020.11567 . |
| 36 | MENNING A, WALTER A, RUDOLPH M, et al. Granulocytes and vascularization regulate uterine bleeding and tissue remodeling in a mouse menstruation model[J]. PLoS One, 2012, 7(8): e41800. DOI:10.1371/journal.pone.0041800 . |
| 37 | PETERSE D, CLERCQ K, GOOSSENS C, et al. Optimization of endometrial decidualization in the menstruating mouse model for preclinical endometriosis research[J]. Reprod Sci, 2018, 25(11):1577-1588. DOI:10.1177/1933719118756744 . |
| 38 | TONG M, KAYANI T, JONES D M, et al. Antiphospholipid antibodies increase endometrial stromal cell decidualization, senescence, and inflammation via toll-like receptor 4, reactive oxygen species, and p38 MAPK signaling[J]. Arthritis Rheumatol, 2022, 74(6):1001-1012. DOI:10.1002/art.42068 . |
| 39 | XU X B, HE B, WANG J D. Menstrual-like changes in mice are provoked through the pharmacologic withdrawal of progesterone using mifepristone following induction of decidualization[J]. Hum Reprod, 2007, 22(12):3184-3191. DOI:10.1093/humrep/dem312 . |
| 40 | ZOOK B C, JÄNNE O A, ABRAHAM A A, et al. The development and regression of deciduosarcomas and other lesions caused by estrogens and progestins in rabbits[J]. Toxicol Pathol, 2001, 29(4):411-416. DOI:10.1080/0192623 0152499755 . |
| [1] | LIU Yayi, JIA Yunfeng, ZUO Yiming, ZHANG Junping, LÜ Shichao. Progress and Evaluation of Animal Model of Heart Qi-Yin Deficiency Syndrome [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 411-421. |
| [2] | QIN Chao, LI Shuangxing, ZHAO Tingting, JIANG Chenchen, ZHAO Jing, YANG Yanwei, LIN Zhi, WANG Sanlong, WEN Hairuo. Study on the 90-day Feeding Experimental Background Data of SD Rats for Drug Safety Evaluation [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 439-448. |
| [3] | LIU Liyu, JI Bo, LIU Xiaoxuan, FANG Yang, ZHANG Ling, GUO Tingting, QUAN Ye, LI Hewen, LIU Yitian. Exploration of Rat Fetal Lung Tissue Fixation Methods [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 432-438. |
| [4] | LIU Zhiwei, YANG Ran, LIAN Hao, ZHANG Yu, JIN Lilun. Cartilage Protection and Anti-Inflammatory Effects of Fraxetin on Monosodium Iodoacetate-Induced Rat Model of Osteoarthritis [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 259-268. |
| [5] | JIANG Meng, HAO Shulan, TONG Liguo, ZHONG Qiming, GAO Zhenfei, WANG Yonghui, WANG Xixing, JI Haijie. Dynamic Evaluation of Vinorelbine-Induced Phlebitis of Dorsalis Pedis Vein in a Rat Model [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 251-258. |
| [6] | PAN Yicong, JIANG Wenhong, HU Ming, QIN Xiao. Optimization of Surgical Procedure and Efficacy Evaluation of Aortic Calcification Model in Rats with Chronic Kidney Disease [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 279-289. |
| [7] | YANG Jiahao, DING Chunlei, QIAN Fenghua, SUN Qi, JIANG Xusheng, CHEN Wen, SHEN Mengwen. Research Progress on Animal Models of Sepsis-Related Organ Injury [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 636-644. |
| [8] | YIN Yulian, MA Lina, TU Siyuan, CHEN Ling, YE Meina, CHEN Hongfeng. Establishment and Evaluation of a Rat Model of Non-Puerperal Mastitis [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 587-596. |
| [9] | YANG Jin, YU Shiya, LIN Nan, FANG Yongchao, ZHAO Hu, QIU Jinwei, LIN Hongming, CHEN Huiyan, WANG Yu, WU Weihang. Effect of Modified Duodenal Exclusion Surgery on Glucose Metabolism in Rats with Type 2 Diabetes Mellitus [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 523-530. |
| [10] | QI Longju, CHEN Shiyuan, LIAO Zehua, SHI Yuanhu, SUN Yuyu, WANG Qinghua. Transcriptomic Analysis of Menstrual Blood-Derived Stem Cells Transplantation Combined with Exercise Training in Promoting Spinal Cord Injury Recovery in Rats [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 531-542. |
| [11] | ZHANG Naiqun, YUAN Piaopiao, CAO Linrong, YING Na, YANG Taotao. Application of PNR Detection in the Diagnosis and Drug-efficacy Evaluation of Diabetic Kidney Disease in Rats [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 543-549. |
| [12] | HUANG Dongyan, WU Jianhui. Establishment Methods and Application Evaluation of Animal Models in Reproductive Toxicology Research [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 550-559. |
| [13] | ZHENG Yiqing, DENG Yasheng, FAN Yanping, LIANG Tianwei, HUANG Hui, LIU Yonghui, NI Zhaobing, LIN Jiang. Application Analysis of Animal Models for Pelvic Inflammatory Disease Based on Data Mining [J]. Laboratory Animal and Comparative Medicine, 2024, 44(4): 405-418. |
| [14] | WU Yue, LI Lu, ZHANG Yang, WANG Jue, FENG Tingting, LI Yitong, WANG Kai, KONG Qi. Integrative Analysis of Omics Data in Animal Models of Coronavirus Infection [J]. Laboratory Animal and Comparative Medicine, 2024, 44(4): 357-373. |
| [15] | XIAO Pan, WANG Hongyi, LU Lu, ZHANG Mei, CHEN Keming, SHEN Dongshuai, NIU Tingxian. Screening of Hypoxia-Sensitive and Hypoxia-Tolerant Wistar Rats and Preliminary Exploration of Hypoxia Sensitivity in Their G1 Generation [J]. Laboratory Animal and Comparative Medicine, 2024, 44(4): 374-383. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||