
Laboratory Animal and Comparative Medicine
• XXXX XXXX •
CAO Xingxin1( ), LI Aiyi2, HOU Jinghan1, LI Mingxue1, LI Yanyan1, JIN Weihua1, YANG Fengmei1, DUAN Suqin1, HE Zhanlong1(
), LI Aiyi2, HOU Jinghan1, LI Mingxue1, LI Yanyan1, JIN Weihua1, YANG Fengmei1, DUAN Suqin1, HE Zhanlong1( )(
)( )
)
Online:2025-05-15
Contact:
HE Zhanlong
CLC Number:
CAO Xingxin,LI Aiyi,HOU Jinghan,et al. Preclinical Meta-analysis of Astragalus and Its ingredients in Acute Pancreatitis[J]. Laboratory Animal and Comparative Medicine. DOI: 10.12300/j.issn.1674-5817.2025.026.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2025.026
第一作者/ 发表年份 First author & Publication date | 研究数量 黄芪组/对照组 Research volume Astragalus group & Control group | 实验动物/ 造模方法 Experimental animal & modeling method | 干预措施 黄芪组/对照组 Intervene measure Astragalus group & Control group | 采样 时间 Sampling time | SYRCLE 评分 Score of SYRCLE | 研究 指标 Research indicators | |
|---|---|---|---|---|---|---|---|
孙海燕 2006 SUN HY 2006 | 10/10 | SD大鼠,雌性 14~15周龄,体重(200±20)g;胆胰管开口处逆行注入脱氧胆酸钠0.25%, 0.5 ml*kg-1 | 黄芪总皂苷80 mg*kg-1造模前sc给药2次,间隔1h,末次给药30min后造模 | sc生理盐水 10 ml*kg-1 | 造模后6h和12h | 4 | ①② |
傅强 2006 FU Q 2006 | 20/20 | 昆明小鼠,雌性,体重25±3g;腹腔注射雨蛙素50g/kg,间隔1小时,连续6次,末次注射后,腹腔注射脂多糖10mg/kg | 末次造模注射后经尾静脉注射黄芪注射液0.5ml/只 | 末次造模注射后经尾静脉注射生理盐水0.5ml/只 | 给药2/6/12/24h后 | 3 | ① |
郭文学 2008 GUO WX 2008 | 8/8 | SD大鼠,雄性,体重250±30g;,经胰胆管逆行注射5%牛磺胆酸钠0.11ml/100g,速度为0.24ml/min | 术后即刻给药 黄芪注射液3ml/100g,并以2ml/100g•h维持 | 相同方法注射 生理盐水 | 给药2/6/12h后 | 3 | ①②⑦⑧ |
朱渊红 2009 ZHU YH 2009 | 24/24 | SD大鼠,雌雄各半,体重(250 ±20) g;经胰胆管逆行注射3.5%牛磺胆酸钠(1ml/ kg) | 造模前24、12小时及造模后黄芪注射液0.4ml/100g | 无 | 造模后6/9/12h | 3 | ① |
朱渊红 2012 ZHU YH | 8/8 | SD大鼠,雌雄各半,体重(250 ±20) g;胰胆管穿刺+3.5%磺胆酸钠(1ml/ kg) 注射 | 造模前24、12小时及造模后即刻分别于大鼠尾静脉注射黄芪注射液0.2ml/100g 及0.4ml/100g | 无 | 造模后6h | 3 | ⑦⑧ |
邱雷 2014 QIU L 2014 | 21/21 | SD大鼠,雄性,体重180~200g;胰胆管穿刺+4%磺胆酸钠(1ml/ kg) 注射 | 造模前12和2h黄芪甲苷40mg/kg腹腔注射 | 无 | 造模后24h | 3 | ①②③④⑤ |
王少言 2014 WANG SY 2014 | 15/15 | SD大鼠,雄性,鼠龄12~16周,体重250~300g;胰胆管穿刺+5%磺胆酸钠(1ml/ kg) 注射 | 造模前12h黄芪注射液0.2ml/100g | 等体积 生理盐水 | 造模后6h | 3 | ⑦⑧⑨ |
邱雷 2015 Lei Qiu 2015 | 18/18 | SD大鼠,雄性,体重250 ±30g; 4%磺胆酸钠(0.1ml/100 g)逆行胆胰管注射;腹腔注射20% L-Arg(3 mg/kg)2次,间隔1h | 黄芪甲苷(50 mg/kg) | 溶媒 | 造模后12、24、48 h | 3 | ①②③④⑤ |
王秉均 2016 WANG BJ | 8/8 | SD大鼠,雌雄各半,体质量180~220g;胰胆管穿刺+5%磺胆酸钠(1ml/ kg)注射 | 造模后立即单次黄芪多糖800mg/Kg灌胃 | 等体积 生理盐水 | 造模后12h | 4 | ①③⑦ |
郑洋 2016 ZHENG Y 2016 | 10/10 | SD大鼠50只,雌 雄各半,体重(180 ±20) g;胰胆管穿刺+5%磺胆酸钠注射 | 造模后黄芪注射液2ml/kg注射每6 h 1 次,共4 次 | 等体积 生理盐水 | 造模后24h | 3 | ③④⑥⑦⑧ |
郑蓓蓓 2017 ZHENG BB 2017 | 15/15 | SD大鼠,成年雄性,体重220g-250g;胰胆管穿刺+5%磺胆酸钠注射 | 造模后黄芪多糖300mg/Kg 0.5ml腹腔注射 | 0.5ml 生理盐水 | 造模后24h | 3 | ③④ |
Ran Ma 2018 | 20/20 | Balb/C小鼠,雄性,鼠龄8~10周,体重(20 ±2) g;腹腔内注射雨蛙肽(50 μg/ kg),每小时1次,连续8小时 | 雨蛙肽给药前1h腹腔注射25 mg/kg 、50 mg/kg毛蕊异黄酮 | 等量 生理盐水 | 注射雨蛙肽后16h | 3 | ①②③④⑥⑦⑧ |
吴爱详 2019 WU AX 2019 | 20/20 | 昆明小鼠,6~8周龄,体重( 20. 02 ±2. 65) g;腹腔注射20% L Arg 3 mg·kg-1,首次注射1 h后再注射1次 | 灌胃黄芪甲苷200 mg·kg-1,每天2 次,连续干预7 d | 等量 生理盐水 | 给药结束后 | 3 | ①②④ |
Chang-Ju Zhu 2021 | 6/6 | C57BL/6 N小鼠,雄性,鼠龄8~10周,体重18~22g;小鼠每小时腹腔注射4g/kg L-Arg,持续2h | 第一次注射L-Arg前1h,腹腔注射毛蕊异黄酮25或50 mg/kg | 等量 生理盐水 | 造模后 | 3 | ①②④ |
金玲玲 2021 JIN LL 2021 | 20/20 | SD大鼠,6周 龄,体质量220~250g,雌雄不限;胰胆管注射3.5%牛磺胆酸钠 | 造模后腹腔注射 20 mg/kg、40 mg/kg黄芪总皂苷,给药体积为10mL/kg | 等体积 生理盐水 | 给药后24h | 3 | ① |
王欣 2022 WANG X 2022 | 20/20 | C57BL/6 小鼠,6 ~ 8周龄,体质量为18 ~ 22 g;腹腔注射pH = 7,20% L-精氨酸2.5 mg/kg,以此间隔1h注射2次 | 造模后黄芪总皂100mg/kg 灌胃1次/d,连续7 d | 无 | 末次给药后24h | 3 | ①⑦⑧⑨ |
洪春霞 2023 HONG CX 2023 | 8/8 | SD大鼠,体质量(220-250)g;造模前禁食12h, 不禁水,麻醉经胰胆管穿刺后, 3.5%牛黄胆酸按1 mL/kg在微量泵恒压下匀速注射 | 造模前12 h、24 h、造模后即刻、造模后12 h及24 h分别给予(0.10、0.15、0.20) mL/(100 g·d)黄芪注射液经尾静脉注射 | 无 | 末次给药后6h | 3 | ①③ |
李振国 2024 LI ZG 2024 | 20/20 | SD大鼠,成年雄性,体重200g-220g;胰胆管穿刺+5%磺胆酸钠(1ml/ kg) 注射 | 造模后黄芪多糖400mg/(kg*d)灌胃2周 | 等体积 生理盐水 | 造模后 2周 | 3 | ③④⑤ |
Xueting Hou 2024 | 6/6 | 昆明小鼠,7周龄,体质量35 ± 2.5 g;胆胰管开口经十二指肠壁逆行注射脱氧胆酸钠(2.5%,0.5 mL/kg) | 小鼠后肢皮下注射黄芪总皂苷(20/40/80 mg/kg)两次,每次给药间隔1小时 | 10 mL/kg生理盐水 | 造模后6、12和24 h | 3 | ①⑦⑧ |
Table 1 Basic characteristic of included studies
第一作者/ 发表年份 First author & Publication date | 研究数量 黄芪组/对照组 Research volume Astragalus group & Control group | 实验动物/ 造模方法 Experimental animal & modeling method | 干预措施 黄芪组/对照组 Intervene measure Astragalus group & Control group | 采样 时间 Sampling time | SYRCLE 评分 Score of SYRCLE | 研究 指标 Research indicators | |
|---|---|---|---|---|---|---|---|
孙海燕 2006 SUN HY 2006 | 10/10 | SD大鼠,雌性 14~15周龄,体重(200±20)g;胆胰管开口处逆行注入脱氧胆酸钠0.25%, 0.5 ml*kg-1 | 黄芪总皂苷80 mg*kg-1造模前sc给药2次,间隔1h,末次给药30min后造模 | sc生理盐水 10 ml*kg-1 | 造模后6h和12h | 4 | ①② |
傅强 2006 FU Q 2006 | 20/20 | 昆明小鼠,雌性,体重25±3g;腹腔注射雨蛙素50g/kg,间隔1小时,连续6次,末次注射后,腹腔注射脂多糖10mg/kg | 末次造模注射后经尾静脉注射黄芪注射液0.5ml/只 | 末次造模注射后经尾静脉注射生理盐水0.5ml/只 | 给药2/6/12/24h后 | 3 | ① |
郭文学 2008 GUO WX 2008 | 8/8 | SD大鼠,雄性,体重250±30g;,经胰胆管逆行注射5%牛磺胆酸钠0.11ml/100g,速度为0.24ml/min | 术后即刻给药 黄芪注射液3ml/100g,并以2ml/100g•h维持 | 相同方法注射 生理盐水 | 给药2/6/12h后 | 3 | ①②⑦⑧ |
朱渊红 2009 ZHU YH 2009 | 24/24 | SD大鼠,雌雄各半,体重(250 ±20) g;经胰胆管逆行注射3.5%牛磺胆酸钠(1ml/ kg) | 造模前24、12小时及造模后黄芪注射液0.4ml/100g | 无 | 造模后6/9/12h | 3 | ① |
朱渊红 2012 ZHU YH | 8/8 | SD大鼠,雌雄各半,体重(250 ±20) g;胰胆管穿刺+3.5%磺胆酸钠(1ml/ kg) 注射 | 造模前24、12小时及造模后即刻分别于大鼠尾静脉注射黄芪注射液0.2ml/100g 及0.4ml/100g | 无 | 造模后6h | 3 | ⑦⑧ |
邱雷 2014 QIU L 2014 | 21/21 | SD大鼠,雄性,体重180~200g;胰胆管穿刺+4%磺胆酸钠(1ml/ kg) 注射 | 造模前12和2h黄芪甲苷40mg/kg腹腔注射 | 无 | 造模后24h | 3 | ①②③④⑤ |
王少言 2014 WANG SY 2014 | 15/15 | SD大鼠,雄性,鼠龄12~16周,体重250~300g;胰胆管穿刺+5%磺胆酸钠(1ml/ kg) 注射 | 造模前12h黄芪注射液0.2ml/100g | 等体积 生理盐水 | 造模后6h | 3 | ⑦⑧⑨ |
邱雷 2015 Lei Qiu 2015 | 18/18 | SD大鼠,雄性,体重250 ±30g; 4%磺胆酸钠(0.1ml/100 g)逆行胆胰管注射;腹腔注射20% L-Arg(3 mg/kg)2次,间隔1h | 黄芪甲苷(50 mg/kg) | 溶媒 | 造模后12、24、48 h | 3 | ①②③④⑤ |
王秉均 2016 WANG BJ | 8/8 | SD大鼠,雌雄各半,体质量180~220g;胰胆管穿刺+5%磺胆酸钠(1ml/ kg)注射 | 造模后立即单次黄芪多糖800mg/Kg灌胃 | 等体积 生理盐水 | 造模后12h | 4 | ①③⑦ |
郑洋 2016 ZHENG Y 2016 | 10/10 | SD大鼠50只,雌 雄各半,体重(180 ±20) g;胰胆管穿刺+5%磺胆酸钠注射 | 造模后黄芪注射液2ml/kg注射每6 h 1 次,共4 次 | 等体积 生理盐水 | 造模后24h | 3 | ③④⑥⑦⑧ |
郑蓓蓓 2017 ZHENG BB 2017 | 15/15 | SD大鼠,成年雄性,体重220g-250g;胰胆管穿刺+5%磺胆酸钠注射 | 造模后黄芪多糖300mg/Kg 0.5ml腹腔注射 | 0.5ml 生理盐水 | 造模后24h | 3 | ③④ |
Ran Ma 2018 | 20/20 | Balb/C小鼠,雄性,鼠龄8~10周,体重(20 ±2) g;腹腔内注射雨蛙肽(50 μg/ kg),每小时1次,连续8小时 | 雨蛙肽给药前1h腹腔注射25 mg/kg 、50 mg/kg毛蕊异黄酮 | 等量 生理盐水 | 注射雨蛙肽后16h | 3 | ①②③④⑥⑦⑧ |
吴爱详 2019 WU AX 2019 | 20/20 | 昆明小鼠,6~8周龄,体重( 20. 02 ±2. 65) g;腹腔注射20% L Arg 3 mg·kg-1,首次注射1 h后再注射1次 | 灌胃黄芪甲苷200 mg·kg-1,每天2 次,连续干预7 d | 等量 生理盐水 | 给药结束后 | 3 | ①②④ |
Chang-Ju Zhu 2021 | 6/6 | C57BL/6 N小鼠,雄性,鼠龄8~10周,体重18~22g;小鼠每小时腹腔注射4g/kg L-Arg,持续2h | 第一次注射L-Arg前1h,腹腔注射毛蕊异黄酮25或50 mg/kg | 等量 生理盐水 | 造模后 | 3 | ①②④ |
金玲玲 2021 JIN LL 2021 | 20/20 | SD大鼠,6周 龄,体质量220~250g,雌雄不限;胰胆管注射3.5%牛磺胆酸钠 | 造模后腹腔注射 20 mg/kg、40 mg/kg黄芪总皂苷,给药体积为10mL/kg | 等体积 生理盐水 | 给药后24h | 3 | ① |
王欣 2022 WANG X 2022 | 20/20 | C57BL/6 小鼠,6 ~ 8周龄,体质量为18 ~ 22 g;腹腔注射pH = 7,20% L-精氨酸2.5 mg/kg,以此间隔1h注射2次 | 造模后黄芪总皂100mg/kg 灌胃1次/d,连续7 d | 无 | 末次给药后24h | 3 | ①⑦⑧⑨ |
洪春霞 2023 HONG CX 2023 | 8/8 | SD大鼠,体质量(220-250)g;造模前禁食12h, 不禁水,麻醉经胰胆管穿刺后, 3.5%牛黄胆酸按1 mL/kg在微量泵恒压下匀速注射 | 造模前12 h、24 h、造模后即刻、造模后12 h及24 h分别给予(0.10、0.15、0.20) mL/(100 g·d)黄芪注射液经尾静脉注射 | 无 | 末次给药后6h | 3 | ①③ |
李振国 2024 LI ZG 2024 | 20/20 | SD大鼠,成年雄性,体重200g-220g;胰胆管穿刺+5%磺胆酸钠(1ml/ kg) 注射 | 造模后黄芪多糖400mg/(kg*d)灌胃2周 | 等体积 生理盐水 | 造模后 2周 | 3 | ③④⑤ |
Xueting Hou 2024 | 6/6 | 昆明小鼠,7周龄,体质量35 ± 2.5 g;胆胰管开口经十二指肠壁逆行注射脱氧胆酸钠(2.5%,0.5 mL/kg) | 小鼠后肢皮下注射黄芪总皂苷(20/40/80 mg/kg)两次,每次给药间隔1小时 | 10 mL/kg生理盐水 | 造模后6、12和24 h | 3 | ①⑦⑧ |
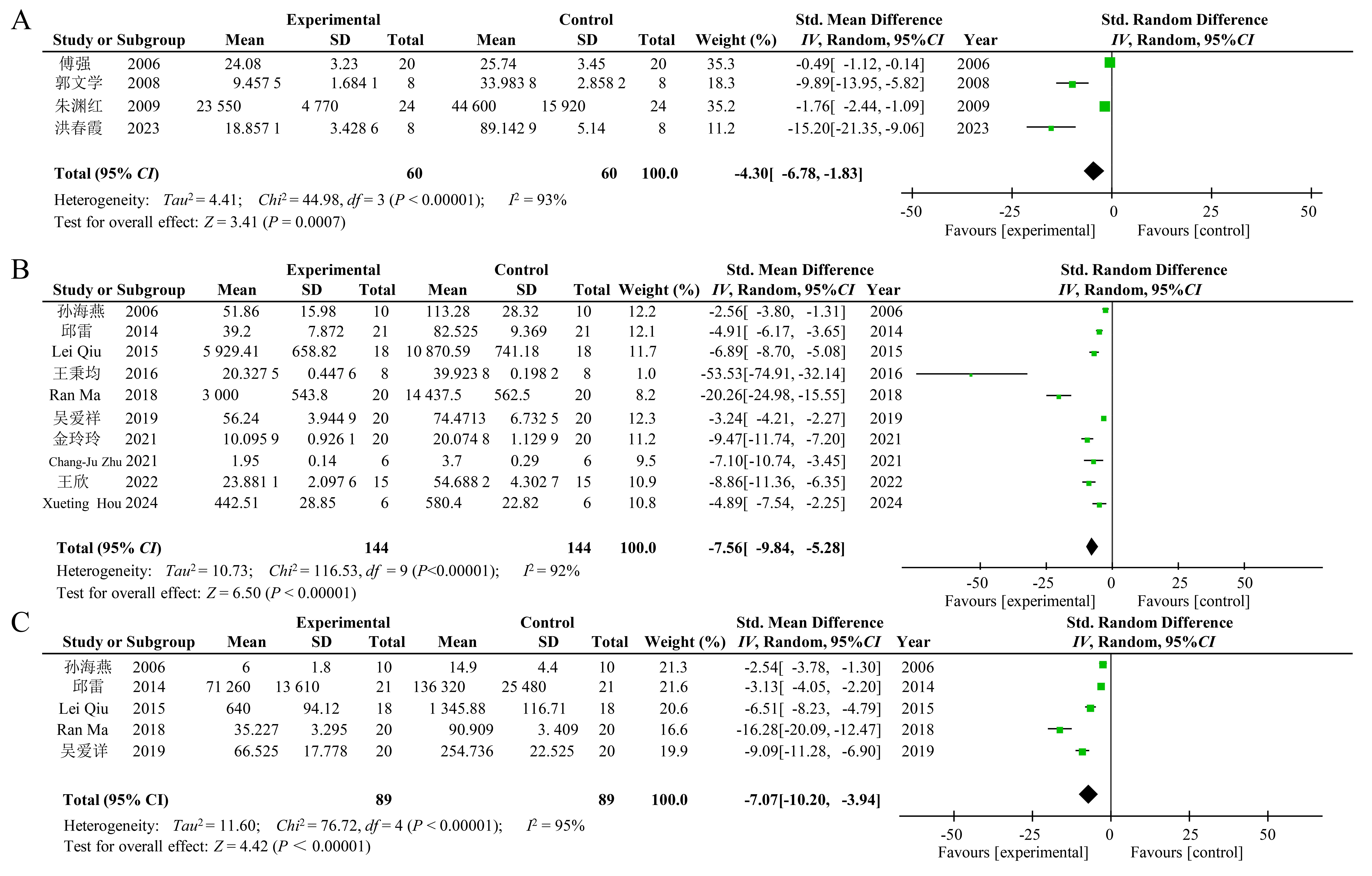
Figure 3 Forest plots of astragalus for clinical parameters of acute pancreatitisNote:A to D, forest plot of astragalus for clinical parameters of acute pancreatitis: astragalus injection for AMS, astragalus components for AMS, astragalus components for LPS.
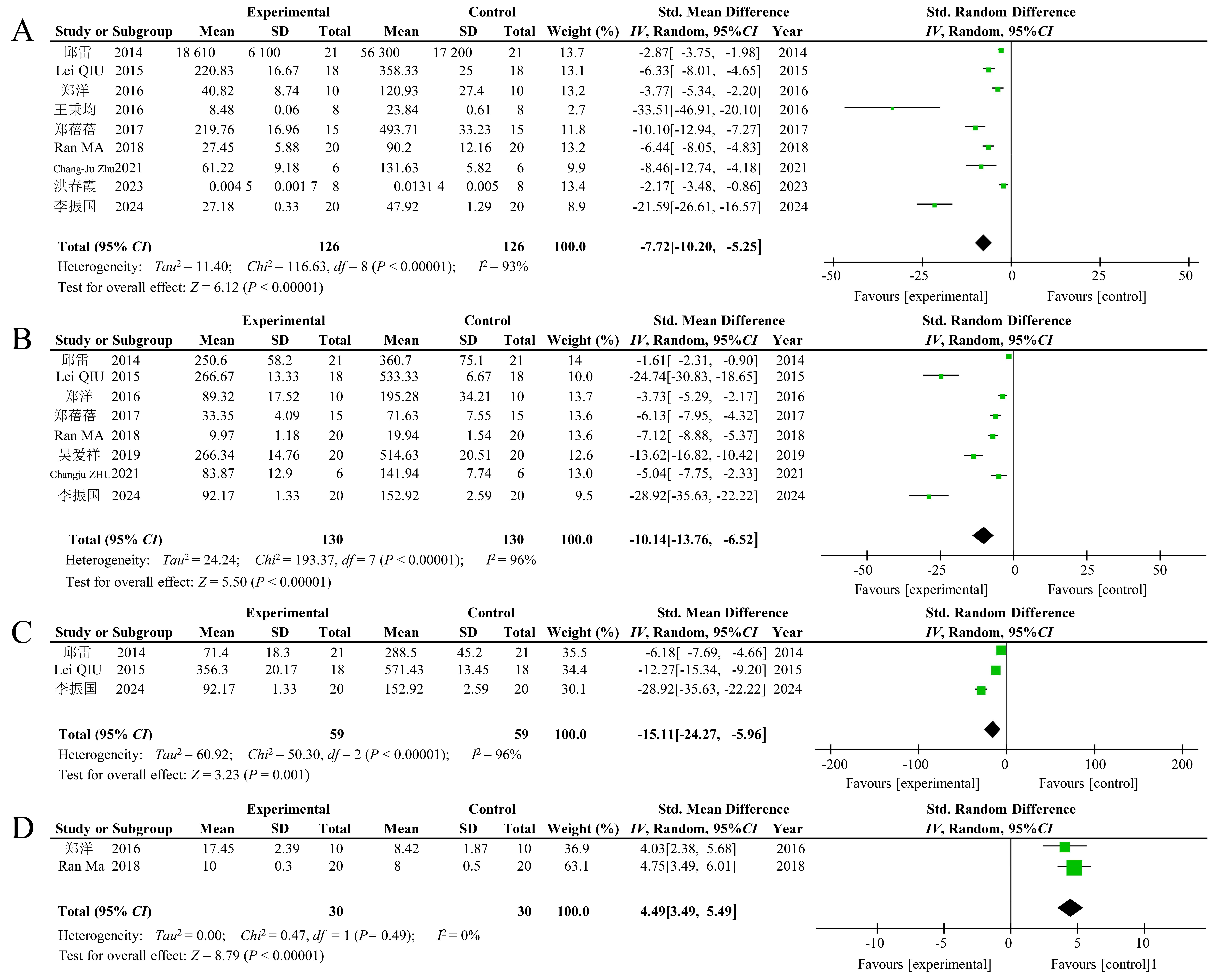
Figure 4 Forest plot of astragalus for inflammatory mediatorNote:A to D, forest plot of astragalus for inflammatory mediator: TNF-α, IL-6, IL-1β, IL-10.
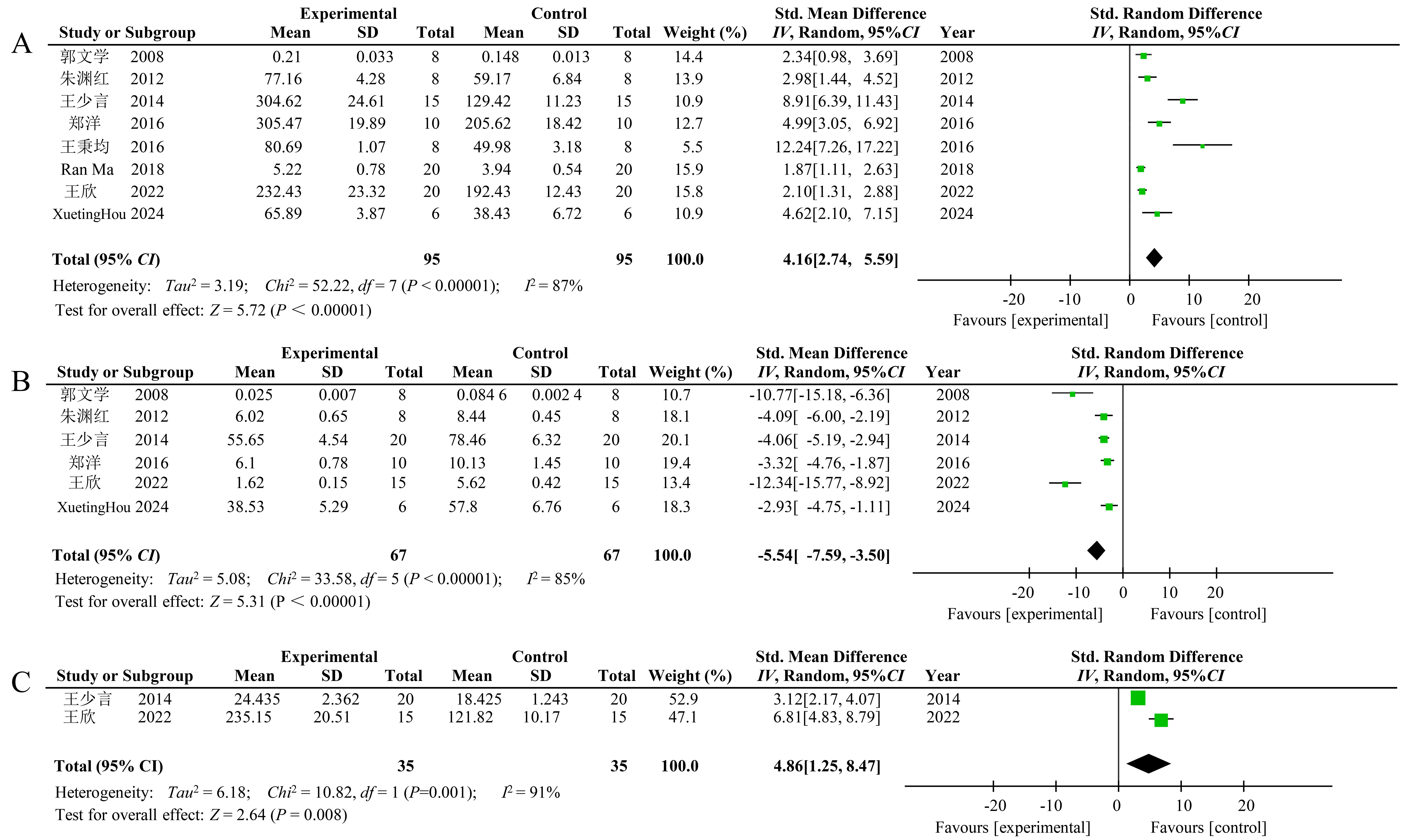
Figure 5 Effect of astragalus for oxidative & antioxidant equilibriumNote:A to C, forest plots of astragalus for oxidative & antioxidant equilibrium: SOD, MDA, GSH-PX.
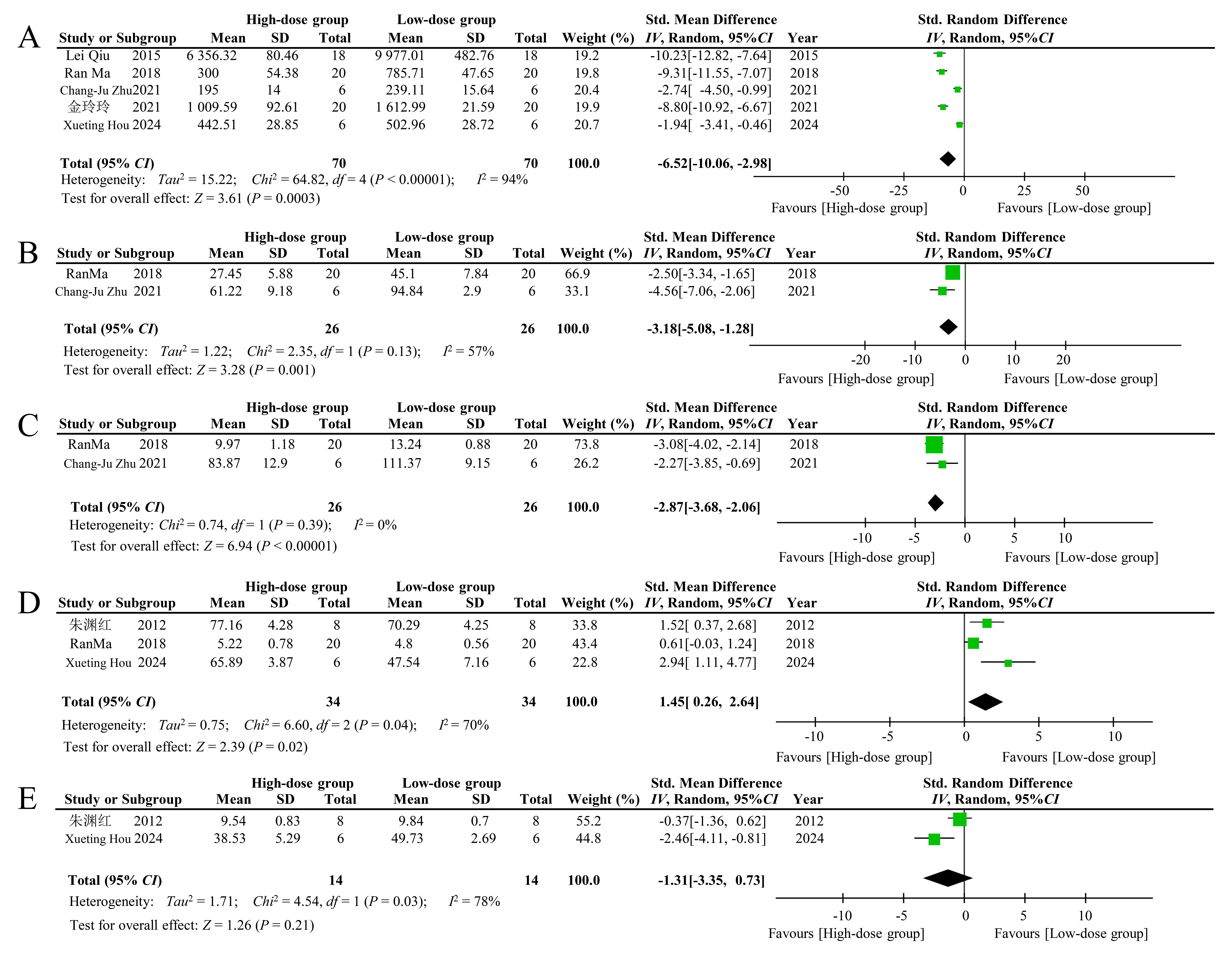
Figure 6 Effects of high- and low-dose Astragalus groups on multiple parametersNote:A to E, forest plots of high- and low-dose Astragalus groups on multiple parameters: AMS, TNF-α, IL-6, SOD, (MDA).
| 1 | MEDEROS MA, REBER HA, GIRGIS MD. Acute pancreatitis: a review[J]. JAMA, 2021, 325(4):382-390. DOI: 10.1001/jama.2021.5789 . |
| 2 | SCHEPERS N J, BAKKER O J, BESSELINK M G, et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis[J]. Gut, 2019, 68(6):1044-1051. DOI: 10.1136/gutjnl-2017-314657 . |
| 3 | HAN K, CHEN S, SONG Y, et al. Burden of pancreatitis and associated risk factors in China, 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019[J]. Chin Med J (Engl), 2022, 135(11):1340-1347. DOI: 10.1097/CM9.0000000000002164 . |
| 4 | GARDNER TB. Acute Pancreatitis[J]. Ann Intern Med, 2021, 174(2):17-32. DOI: 10.7326/AITC202102160 . |
| 5 | 李芝萍, 汪娅君, 张菊萍, 等. 通腑逐瘀方联合常规西药治疗腑实热结证急性胰腺炎临床研究[J]. 新中医, 2024, 56 (10): 81-85. DOI: 10.13457/j.cnki.jncm.2024.10.016 . |
| LI ZP, WANG YJ, ZHANG JP, et al. Clinical Study on Tongfu Zhuyu Decoction Combined with Conventional Western Medicine for Acute Pancreatitis with Syndrome of Bowel Excess and Heat Bind[J]. New J Tradit Chin Med, 2024, 56 (10): 81-85. DOI: 10.13457/j.cnki.jncm.2024.10.016 . | |
| 6 | BOXHOORN L, VOERMANS R P, BOUWENSE S A, et al. Acute pancreatitis[J]. Lancet, 2020, 396(10252):726-734. DOI: 10.1016/S0140-6736(21)02377-1 . |
| 7 | WANG P, WANG Z, ZHANG Z, et al. A review of the botany, phytochemistry, traditional uses, pharmacology, toxicology, and quality control of the Astragalus memeranaceus[J]. Front Pharmacol, 2023, 23(14):1242318. DOI: 10.3389/fphar.2023.1242318 . |
| 8 | 张琪, 张会芳, 李兆洋, 等.黄芪注射液对急性胰腺炎患者氧化应激状态及肠黏膜屏障功能的影响[J]. 吉林中医药, 2021, 41 (12): 1616-1619. DOI: 10.13463/j.cnki.jlzyy.2021.12.021 . |
| ZHANG Q, ZHANG H F, LI Z Y, et al. Effects of Huangqi Injection on the oxidative stress and intestinal mucosal barrier function in patients with acute pancreatitis [J]. Jilin J Chin Med, 2021, 41(12): 1616-1619. DOI: 10.13463/j.cnki.jlzyy.2021.12.021 . | |
| 9 | 王嫄嫄, 郑洋, 周哲, 等. 黄芪注射液对急性胰腺炎肠道屏障功能和微炎性反应状态的影响[J]. 世界中医药, 2018, 13 (4): 818-821. DOI: 10.3969/j.issn.1673-7202.2018.04.008 . |
| WANG Y Y, ZHENG Y, ZHOU Z, et al. Clinical Study on Huangqi Injection on Intestinal Barrier Function and Micro Inflammatory State in Patients with Acute Pancreatitis[J]. World Chin Med, 2018, 13 (4): 818-821. DOI: 10.3969/j.issn.1673-7202.2018.04.008 . | |
| 10 | AL-MASAWA M E, ALSHAWSH M A, NG C Y, et al. Efficacy and safety of small extracellular vesicle interventions in wound healing and skin regeneration: A systematic review and meta-analysis of animal studies. Theranostics, 2022, 12(15):6455-6508. DOI: 10.7150/thno.73436 . |
| 11 | LIU D, SONG D, NING W, et al. Efficacy and safety of prophylaxis for venous thromboembolism in brain neoplasm patients undergoing neurosurgery: a systematic review and Bayesian network meta-analysis[J]. J Thromb Thrombolysis, 2023, 55(4):710-720. DOI: 10.1007/s11239-023-02780-3 . |
| 12 | 孙海燕, 黄民, 关溯. 黄芪总苷对实验性急性胰腺炎的保护作用[J]. 时珍国医国药, 2006, (10):1980-1981. DOI: 10.3969/j.issn.1008-0805.2006.10.044 . |
| SUN HY, HUANG M, GUAN S. Effects of astragalosides on experimental acute pancreatitis[J]. Lishizhen Med Mater Med. Res, 2006, (10):1980-1981. DOI: 1008-0805 (2006) 10-1980-02. DOI: 10.3969/j.issn.1008-0805.2006.10.044 . | |
| 13 | 傅强, 崔乃强, 邵伟, 等. 急性出血坏死性胰腺炎小鼠辅助性T细胞亚群1/2的变化规律及中药干预作用研究[J]. 中国中西医结合急救杂志, 2006, (04):214-217. DOI: 10.3321/j.issn:1008-9691.2006.04.006 . |
| FU Q, CUI N Q, SHAO W, et al. Study on regularity of changes of helper T cell subgroup Th1/Th2 and interference effect of traditional Chinese medicine in mice with acute hemorrhage necrotizing pancreatitis[J]. Chin J Integr Tradit West Med Intensive Crit Car, 2006, (04):214-217. DOI: 10.3321/j.issn:1008-9691.2006.04.006 . | |
| 14 | 郭文学. 重症急性胰腺炎时MDA、SOD与肝脏微循环变化及黄芪干预的实验研究[D]. 河北医科大学, 2008. DOI: 10.7666/d.y1353969 . |
| GUO W X. Severe acute pancreatitis MDA, SOD and liver microcirculation changes and the experimental intervention study of Astragalus[D]. Hebei Medical University, 2008. DOI: 10.7666/d.y1353969 . | |
| 15 | 朱渊红, 王真, 蔡宛如. 黄芪注射液对SAP肺损伤大鼠的实验研究[J]. 中华中医药学刊, 2009, 27(12):2608-2609. DOI: 10.13193/j.archtcm.2009.12.146.zhuyh.041 . |
| ZHU Y H, WANG Z, CAI WR. The Experimental study on astragalus to rats with lung injury associated with severe acute pancreatitis[J]. Chin Arch Tradit Chin Med, 2009, 27(12):2608-2609. DOI: 10.13193/j.archtcm.2009.12.146.zhuyh.041 . | |
| 16 | 朱渊红, 王新华, 陈芝芸. 黄芪注射液对SAP肺损伤大鼠SOD、MDA及肺组织bcl-2、bax蛋白表达的影响[J]. 中国中医药科技, 2012, 19(5):416-417+441. DOI: 10.3969/j.issn.1005-7072.2012.05.022 . |
| ZHU Y H, WANG X H, CHEN Z Y. Impact of astragalus injection on SOD and MDA levels, and bcl-2/bax protein expression in lung tissue of rats with SAP-induced pulmonary injury[J]. Chin J Tradit Med Sci Technol, 2012, 19(5):416-417+441. DOI: 10.3969/j.issn.1005-7072.2012.05.022 . | |
| 17 | 邱雷, 史建华, 殷国建, 等. 黄芪甲苷对实验性大鼠重症急性胰腺炎的治疗作用[J]. 上海医学, 2014, 37(3):227-230. |
| QIU L, SHI J H, YIN G J, et al. Therapeutic effects of astragaloside Ⅳ on severe acute pancreatitis in rats[J]. Shanghai Med J, 2014, 37(03):227-230. | |
| 18 | 王少言, 初巍巍, 霍阳, 等. 黄芪注射液对重症急性胰腺炎大鼠外周血清氧化相关物质的影响[J]. 解放军医药杂志, 2014, 26(12):5-7. DOI: 10.3969/j.issn.2095-140X.2014.12.002 . |
| WANG S Y, CHU W W, HUO Y, et al. Effect of Huangqi Injection on Oxidative Relevant Materials of Peripheral Serum in Rats with Severe Acute Pancreatitis[J]. Med Pharm J Chin People's Liberation Army, 2014, 26(12):5-7. DOI: 10.3969/j.issn.2095-140X.2014.12.002 . | |
| 19 | Qiu L, Yin G, Cheng L, et al. Astragaloside IV ameliorates acute pancreatitis in rats by inhibiting the activation of nuclear factor-κB[J]. Int J Mol Med. 2015, 35(3):625-36. DOI: 10.3892/ijmm.2015.2070 . |
| 20 | 王秉钧, 王先坤, 晏波, 等. 黄芪多糖对大鼠重症急性胰腺炎的作用及机制[J]. 中国医院药学杂志, 2016, 36(16):1366-1368. DOI: 10.13286/j.cnki.chinhosppharmacyj.2016.16.08 . |
| WANG B J, WANG X K, YAN B, et al. Effects of astragalus polysaccharides on rats with severe acute pancreatitis and underlying mechanisms[J]. Chin J Hosp Pharm, 2016, 36(16):1366-1368. DOI: 10.13286/j.cnki.chinhosppharmacyj.2016.16.08 . | |
| 21 | 郑洋, 于晶晶, 杜鹃, 等. 黄芪注射液对急性胰腺炎大鼠肠道屏障功能的影响[J]. 中国临床研究, 2016, 29(12):1614-1617. DOI: 10.13429/j.cnki.cjcr.2016.12.006 . |
| ZHENG Y, YU J J, DU J, et al. Effects of astragalus injection on intestinal barrier functions in rats with acute pancreatitis[J]. Chin J Clin Res, 2016, 29(12):1614-1617. DOI: 10.13429/j.cnki.cjcr.2016.12.006 . | |
| 22 | 邓蓓蓓. 黄芪多糖对重症急性胰腺炎大鼠肠粘膜屏障损伤的保护作用[D]. 吉林大学, 2017. |
| ZHENG B B. Protection of astragalus polysacharin on intestinal barrier dysfunction in rats with severe acute pancreatitis[D]. Jilin University, 2017. | |
| 23 | MA R, YUAN F, WANG S, et al. Calycosin alleviates cerulein-induced acute pancreatitis by inhibiting the inflammatory response and oxidative stress via the p38 MAPK and NF-κB signal pathways in mice. Biomed Pharmacother, 2018, 105:599-605. DOI: 10.1016/j.biopha.2018.05.080 . |
| 24 | 吴爱祥. 黄芪甲苷对重症急性胰腺炎小鼠胰腺的影响[J]. 中国临床药理学杂志, 2019, 35(18):2065-2067. DOI: 10.13699/j.cnki.1001-6821.2019.18.028 . |
| WU A X. Effects of astragalus glycoside in severe acute pancreatitis mice[J]. Chin J Clin Pharmacol, 2019, 35(18):2065-2067. DOI: 10.13699/j.cnki.1001-6821.2019.18.028 . | |
| 25 | ZHU C J, YANG W G, LI D J, et al. Calycosin attenuates severe acute pancreatitis-associated acute lung injury by curtailing high mobility group box 1 - induced inflammation[J]. World J Gastroenterol, 2021, 27(44):7669-7686. DOI: 10.3748/wjg.v27.i44.7669 . |
| 26 | 金玲玲, 汤斌斌, 周晶晶, 等. 黄芪总皂苷对重症急性胰腺炎大鼠肠道损伤的保护作用及对肠组织p38MAPK/NF-κB表达的影响[J]. 新中医, 2021, 53(07):6-10. DOI: 10.13457/j.cnki.jncm.2021.07.002 . |
| JIN L L, TANG B B, ZHOU J J, et al. Total saponins of astragalus has protective effect on intestinal injury in rats with severe acute pancreatitis and its effect on P38MAPK/NF-κB expressions in intestinal tissues[J]. New J Tradit Chin Med, 2021, 53(7):6-10. DOI: 10.13457/j.cnki.jncm.2021.07.002 . | |
| 27 | 王欣, 周文勇, 孙月. 黄芪总皂苷通过激活Nrf2/HO-1信号通路缓解L-精氨酸诱导的小鼠急性胰腺炎氧化损伤的研究[J]. 现代中药研究与实践, 2022, 36(03):38-42. DOI: 10.13728/j.1673-6427.2022.03.008 . |
| WANG X, ZHOU W Y, SUN Y. Study on the effect of astragalus saponins on oxidative damage of l-arginine induced acute pancreatitis in mice by activating Nrf2/HO-1 signaling pathway[J]. Res Pract Chin Med, 2022, 36(03):38-42. DOI: 10.13728/j.1673-6427.2022.03.008 . | |
| 28 | 洪春霞, 张照伟, 陈伟前. 黄芪注射液通过调控mTOR/p70S6K信号通路对急性出血坏死型胰腺炎肺损伤炎症反应的影响[J]. 世界华人消化杂志, 2023, 31(5): 184-192. DOI: 10.11569/wcjd.v31.i5.184 . |
| HONG C X, ZHANG Z W, CHEN W Q. Astragalus injection improves inflammatory response in lung injury in acute hemorrhagic necrotizing pancreatitis by regulating the mTOR/p70S6K signal pathway[J]. World Chin J Digestol, 2023, 31(5): 184-192. DOI: 10.11569/wcjd.v31.i5.184 . | |
| 29 | 李振国, 孔德生, 李文科. 黄芪多糖对大鼠急性胰腺炎的作用及其机制研究[J]. 海南医学, 2024, 35(01):6-9. DOI: 10.3969/j.issn.1003-6350.2024.01.002 . |
| LI Z G, KONG D S, LI W K. Effect of astragalus polysaccharides on acute pancreatitis in rats and its mechanism[J]. Hainan Med J, 2024, 35(1):6-9. DOI: 10.3969/j.issn.1003-6350.2024.01.002 . | |
| 30 | HOU X, YU M, XU Y, et al. Antioxidative effect of astragalosides on acute pancreatitis in mice[J]. Front Vet Sci. 2024, 11:1418899. DOI: 10.3389/fvets.2024.1418899 . |
| 31 | 尉洪利, 单连美, 孙玉欣, 等. 柴胡龙骨牡蛎汤对肝胆湿热证急性胰腺炎患者的临床疗效[J]. 中成药, 2025, 47(01):338-341. DOI: 10.3969/j.issn.100-1528.2025.01.056 . |
| YU H L, SHAN L M, SUN Y X, et al. Clinical Efficacy of Chaihu-Longgu-Muli Decoction in Patients with Acute Pancreatitis of Liver-Gallbladder Damp-Heat Syndrome[J]. Chin Tradit Pat Med, 2025, 47(01):338-341. DOI: 10.3969/j.issn.100-1528.2025.01.056 . | |
| 32 | 林杉, 秦文昊, 代崧霖, 等. 中药辅助治疗经内镜逆行胰胆管造影术后急性胰腺炎研究进展[J]. 中国中西医结合杂志, 2024, 44(11):1400-1404. DOI: 10.7661/j.cjim.20240910.272 . |
| LIN S, QIN W H, DAI S L, et al. Research progress on traditional Chinese medicine adjuvant therapy for acute pancreatitis after endoscopic retrograde cholangiopancreatography[J]. Chin J Integr Tradit West Med, 2024, 44(11):1400-1404. DOI: 10.7661/j.cjim.20240910.272 . | |
| 33 | 谢红英, 罗晓丽, 卢慧. 黄芪注射液对病毒性心肌炎患者外周血NLRP3炎性小体表达的影响[J]. 西部中医药, 2021, 34(7):94-97. DOI: 10.12174/j.issn.2096-9600.2021.07.24 . |
| XIE H Y, LUO X L, LU H. Influence of huangqi injection on the expressions of nlrp3 inflammasome in peripheral blood of patients with viral myocarditis[J]. Western Journal of Traditional Chinese Medicine, 2021, 34(7):94-97. DOI: 10.12174/j.issn.2096-9600.2021.07.24 . | |
| 34 | 张燕. 黄芪注射液联合布地奈德对支气管哮喘急性发作患者肺功能的影响[J]. 实用中西医结合临床, 2021, 21(17):71-72. DOI: 10.13638/j.issn.1671-4040.2021.17.033 . |
| ZHANG Y. Impact of astragalus injection combined with budesonide on pulmonary function in patients with acute exacerbation of bronchial asthma[J]. Practical Clinical Journal of Integrated Traditional Chinese and Western Medicine, 2021, 21(17):71-72. DOI: 10.13638/j.issn.1671-4040.2021.17.033 . | |
| 35 | 中华医学会外科学分会胰腺外科学组. 中国急性胰腺炎诊治指南(2021)[J].. 浙江实用医学, 2021, 26(06):511-519, 535. DOI: 10.16794/j.cnki.cn33-1207/r.2021.06.003 . |
| STUDY GROUP OF PANCREATIC SURGERY IN CHINA SOCIETY OF SURGERY OF CHINESE MEDICAL ASSOCIATION. Chinese guidelines for the diagnosis and treatment of acute pancreatitis (2021) [J]. Zhejiang Practical Medicine, 2021, 26(06):511-519+535. DOI: 10.16794/j.cnki.cn33-1207/r.2021.06.003 . | |
| 36 | LEPPÄNIEMI A, TOLONEN M, TARASCONI A, et al. 2019 WSES guidelines for the management of severe acute pancreatitis[J]. World J Emerg Surg. 2019, 14:27. DOI: 10.1186/s13017-019-0247-0 . |
| 37 | 宋艳, 何永恒, 杨芳, 等. 黄芪多糖调节脂联素/TLR/NF-κB信号通路对溃疡性结肠炎小鼠的治疗作用[J]. 中国免疫学杂志, 2021, 37(11):1319-1324. DOI: 0.3969/j.issn.000484X.2021.11.008 . |
| SONG Y, HE Y H, YANG F, et al. Regulatory effect of astragalus polysaccharide on adiponectin/ TLR/ NFκB signal pathway in mice with ulcerative colitis[J]. Chin J Immunol, 2021, 37(11):1319-1324. DOI: 10.3969/j.issn.000484X.2021.11.008 . | |
| 38 | HABTEZION A, GUKOVSKAYA AS, PANDOL SJ. Acute Pancreatitis: A Multifaceted Set of Organelle and Cellular Interactions[J]. Gastroenterology, 2019, 156(7):1941-1950. DOI: 10.1053/j.gastro.2018.11.082 . |
| 39 | LIU D, WEN L, WANG Z, et al. The Mechanism of Lung and Intestinal Injury in Acute Pancreatitis: A Review[J]. Front Med (Lausanne). 2022, 9:904078. DOI: 10.3389/fmed.2022.904078 . |
| 40 | JIAO J, LIU J, LUO F, et al. Qingyi granules ameliorate severe acute pancreatitis in rats by modulating the gut microbiota and serum metabolic aberrations[J]. Pharm Biol. 2023, 61(1):927-937. DOI: 10.1080/13880209.2023.2222755 . |
| 41 | WANG L J, JIN Y L, PEI W L, et al. Amuc_1100 pretreatment alleviates acute pancreatitis in a mouse model through regulating gut microbiota and inhibiting inflammatory infiltration[J]. Acta Pharmacol Sin. 2024, 45(3):570-580. DOI: 10.1038/s41401-023-01186-4 . |
| 42 | PALATHINGAL BAVA E, GEORGE J, TARIQUE M, et al. Pirfenidone increases IL-10 and improves acute pancreatitis in multiple clinically relevant murine models[J]. JCI Insight. 2022, 7(2):e141108. DOI: 10.1172/jci.insight.141108 . |
| 43 | LI H, WU D, ZHANG H, et al. Autophagy-mediated ferroptosis is involved in development of severe acute pancreatitis[J]. BMC Gastroenterol, 2024, 24(1):245. DOI: 10.1186/s12876-024-03345-1 . |
| 44 | 周龙云, 田子睿, 刘书芬, 等. 黄芪对中枢神经系统的药理作用及毒理研究现状[J]. 中草药, 2018, 49(20):4935-4944. DOI: 10.7501/j.issn.0253-2670.2018.20.034 . |
| ZHOU L Y, TIAN Z R, LIU S F, et al. Review on neuroprotection effect of Astragali Radix on central nervous system and related toxicology[J]. Chin Tradit Herb Drugs, 2018, 49(20):4935-4944. DOI: 10.7501/j.issn.0253-2670.2018.20.034 . |
| [1] | ZHENG Qingyong, YANG Donghua, MA Zhichao, ZHOU Ziyu, LU Yang, WANG Jingyu, XING Lina, KANG Yingying, DU Li, ZHAO Chunxiang, DI Baoshan, TIAN Jinhui. Recommendations for Standardized Reporting of Systematic Reviews and Meta-Analysis of Animal Experiments [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 496-507. |
| [2] | MIN Fangui, FU Hongkun, LIU Yonggang, LIU Xiangmei, LIU Zhonghua, LI Yao, TAO Yufeng. Special Welfare and Ethical Requirements for Infectious Animal Experiments [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 239-246. |
| [3] | LI Tengfei, ZHENG Qingyong, XU Jianguo, LI Yiyi, ZHOU Yongjia, XU Caihua, ZHANG Mingyue, TIAN Jiexiang, WANG Gang, TIAN Jinhui. Improving the Certainty of Evidence in Animal Experiment Systematic Review/Meta-Analysis: An Empirical Study of the GRADE Method [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 101-111. |
| [4] | ZHENG Qingyong, LI Tengfei, XU Jianguo, ZHOU Yongjia, MA Zhichao, WANG Na, LI Molan, YANG Wenjing, WU Peirun, WANG Haidong, TIAN Jinhui. Advances and Challenges in the Research of Integration Methods of Animal Experimental Evidence [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 567-576. |
| [5] | Editorial Board of Laboratory Animal and Comparative Medicine . Guideline Checklist for Publishing Research Papers on Animal Experimentation and Comparative Medicine in China (2024 Edition) [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 577-582. |
| [6] | Jinhua HU, Jingjie HAN, Min JIN, Bin HU, Yuefen LOU. Effects of Puerarin on Bone Density in Rats and Mice: A Meta-analysis [J]. Laboratory Animal and Comparative Medicine, 2024, 44(2): 149-161. |
| [7] | Zhengwen MA, Xiaying LI, Xiaoyu LIU, Yao LI, Jian WANG, Jin LU, Guoyuan CHEN, Xiao LU, Yu BAI, Xuancheng LU, Yonggang LIU, Wanyong PANG, Yufeng TAO. Interpretation and Elaboration for the ARRIVE Guidelines 2.0—Animal Research: Reporting In Vivo Experiments (V) [J]. Laboratory Animal and Comparative Medicine, 2024, 44(1): 105-114. |
| [8] | Jinhuan MIAO, Xia XU, Lu ZHOU, Haiyan CHENG, Yan HE. Visual Analysis of Animal Experiments on Traditional Chinese Medicine (TCM) Nursing Technology Based on VOSviewer [J]. Laboratory Animal and Comparative Medicine, 2023, 43(6): 626-635. |
| [9] | Xiaying LI, Yonglu TIAN, Xiaoyu LIU, Xuancheng LU, Guoyuan CHEN, Xiao LU, Yu BAI, Jing GAO, Yao LI, Yusheng WEI, Wanyong PANG, Yufeng TAO. Explanation and Elaboration for the ARRIVE Guidelines 2.0—Reporting Animal Research and In Vivo Experiments (Ⅳ) [J]. Laboratory Animal and Comparative Medicine, 2023, 43(6): 659-668. |
| [10] | Shuo WANG, Yunhui LÜ, Xiaokang WANG, Zhenhao ZHANG, Yongchun CUI. Construction and Verification of Quality Evaluation Indicator System for Extracorporeal Membrane Oxygenation Animal Experimental Platform [J]. Laboratory Animal and Comparative Medicine, 2023, 43(6): 604-611. |
| [11] | Xiaoyu LIU, Xuancheng LU, Xiaomeng SHI, Yuzhou ZHANG, Chao LÜ, Guoyuan CHEN, Xiao LU, Yu BAI, Jing GAO, Yao LI, Yonggang LIU, Yufeng TAO, Wanyong PANG. Explanation and Elaboration for the ARRIVE Guidelines 2.0—Reporting Animal Research and In Vivo Experiments (Ⅲ) [J]. Laboratory Animal and Comparative Medicine, 2023, 43(4): 446-456. |
| [12] | Guoyuan CHEN, Xiao LU, Yu BAI, Lingzhi YU, Ying QIAO, Jian WANG, Jin LU, Xiaoyu LIU, Xuancheng LU, Jing GAO, Yao LI, Wanyong PANG. Explanation and Elaboration of the ARRIVE Guidelines 2.0—Reporting Animal Research and In Vivo Experiments (Ⅱ) [J]. Laboratory Animal and Comparative Medicine, 2023, 43(3): 323-331. |
| [13] | Jian WANG, Jin LU, Zhengwen MA, Guoyuan CHEN, Xiao LU, Yu BAI, Xiaoyu LIU, Xuancheng LU, Jing GAO, Yao LI, Wanyong Pang. Explanation and Elaboration for the ARRIVE Guidelines 2.0—Reporting Animal Research and In Vivo Experiments (Ⅰ) [J]. Laboratory Animal and Comparative Medicine, 2023, 43(2): 213-224. |
| [14] | Xiangrong DING, Shurui HUO, Jiejie DAI. Research Progress on Influenza A Virus and Nervous System Disease of Human and Experimental Animals [J]. Laboratory Animal and Comparative Medicine, 2023, 43(2): 180-185. |
| [15] | Junyan ZHANG, Xiaoyu LIU, Yao LI, Guoyuan CHEN, Xiao LU, Yu BAI, Xuancheng LU, Wanyong PANG, Baojin WU. Introduction to the International Guide for Animal Research Reporting ARRIVE 2.0, and Its Implementation Plan in the Journal [J]. Laboratory Animal and Comparative Medicine, 2023, 43(1): 86-94. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||