
Laboratory Animal and Comparative Medicine ›› 2025, Vol. 45 ›› Issue (4): 449-456.DOI: 10.12300/j.issn.1674-5817.2024.177
• Animal Experimental Techniques and Methods • Previous Articles Next Articles
LIU Kun, LAN Qing, YI Bing, XIE Xiaojie( )(
)( )
)
Received:2024-11-26
Revised:2025-04-10
Online:2025-08-25
Published:2025-09-01
Contact:
XIE Xiaojie
CLC Number:
LIU Kun,LAN Qing,YI Bing,et al. Key Challenges and Mitigation Strategies for Animal Pregnancy in Non-clinical Reproductive Toxicity Testing of Drugs[J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 449-456. DOI: 10.12300/j.issn.1674-5817.2024.177.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2024.177
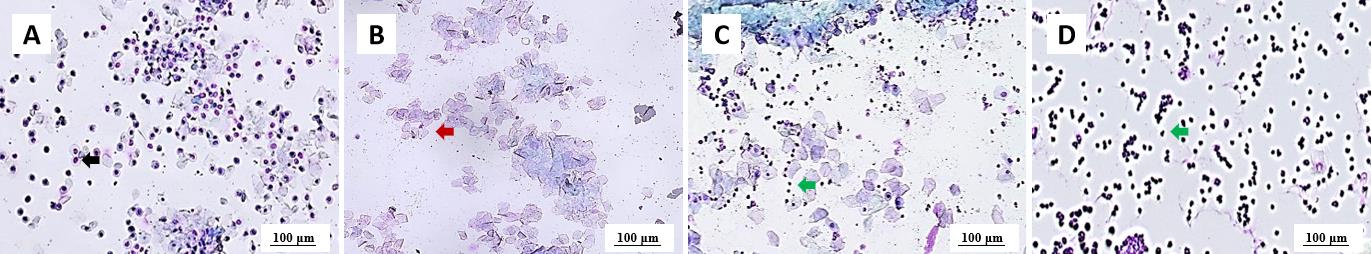
Figure 1 Typical vaginal smear characteristics from adult female rats at each stage of estrous cycleNote:A, proestrus; B, estrus; C, metestrus; D, diestrus. The black arrow indicates nucleated epithelial cells; the red arrow indicates anucleated epithelial cells; the green arrows indicate leukocytes. The staining method of the smear was hematoxylin and eosin (HE) staining. The magnification of the images is 40 and the scale bars are 100 μm.
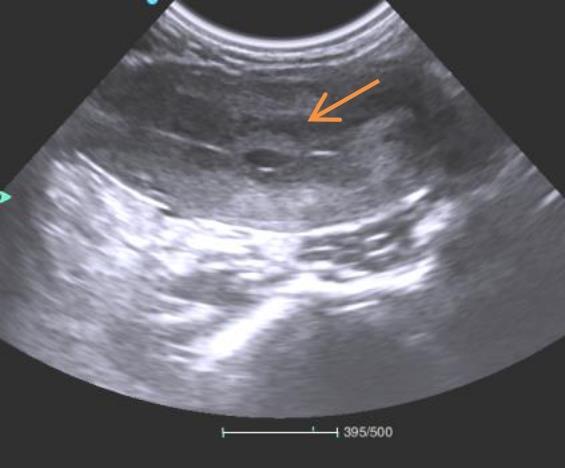
Figure 2 Ultrasound cross-sectional view of gestational sac in a female monkey during early pregnancyNote:The area indicated by the arrow in the figure shows a well-formed gestational sac structure.
| [1] | 黄冬妍, 吴建辉. 生殖毒理学研究动物模型的建立方法及应用评价[J]. 实验动物与比较医学, 2024, 44(5):550-559. DOI:10.12300/j.issn.1674-5817.2024.028 . |
| HUANG D Y, WU J H. Establishment methods and application evaluation of animal models in reproductive toxicology research[J]. Lab Anim Comp Med, 2024, 44(5):550-559. DOI:10.12300/j.issn.1674-5817.2024.028 . | |
| [2] | 林蔚, 张荣标, 陈润. SD大鼠毒性试验生殖指标参考值建立及不同体重大鼠孕后生殖指标分析[J]. 海峡预防医学杂志, 2023, 29(4):60-62. |
| LIN W, ZHANG R B, CHEN R. Establishment of reference value for reproductive index in SD rats toxicity test and analysis of post-pregnancy reproductive index in rats of different body weights[J]. Strait J Prev Med, 2023, 29(4):60-62. | |
| [3] | 刘晓宇, 王娇, 钱欣, 等. 围绝经期大鼠动情周期变化的研究[J]. 哈尔滨医科大学学报, 2014, 48(5):358-360. DOI:CNKI:SUN:HYDX.0.2014-05-003 . |
| LIU X Y, WANG J, QIAN X, et al. Changes of estrous cycle of perimenopausal rats[J]. J Harbin Med Univ, 2014, 48(5):358-360. DOI:CNKI:SUN:HYDX.0.2014-05-003 . | |
| [4] | 陈思充, 陈子豪, 高青华, 等. 不同阴道涂片方法的比较及对大鼠动情周期的判断[J]. 解剖科学进展, 2023, 29(1):93-95. DOI: 10.16695/j.cnki.1006-2947.2023.01.025 . |
| CHEN S C, CHEN Z H, GAO Q H, et al. Comparison of different vaginal smears and judgment of estrous cycle in rats[J]. Prog Anat Sci, 2023, 29(1):93-95. DOI: 10.16695/j.cnki.1006-2947.2023.01.025 . | |
| [5] | 闫婧, 赵季宇, 马梦娜, 等. 大鼠阴道细胞涂片技术及其HE染色方法的研究[J]. 山西中医药大学学报, 2023, 24(1):45-51. DOI: 10.19763/j.cnki.2096-7403.2023.01.07 . |
| YAN J, ZHAO J Y, MA M N, et al. Study on smear technique and HE staining of rat vaginal cells[J]. J Shanxi Univ Chin Med, 2023, 24(1):45-51. DOI: 10.19763/j.cnki.2096-7403.2023.01.07 . | |
| [6] | 于农淇, 梁莎莎, 黄健, 等. 阴道分泌物在动物发情鉴定中的研究进展[J]. 中国牛业科学, 2022, 48(5):66-70. DOI:10.3969/j.issn.1001-9111.2022.05.015 . |
| YU N Q, LIANG S S, HUANG J, et al. Research progress of vaginal secretions in animal estrus identification[J]. China Cattle Sci, 2022, 48(5):66-70. DOI:10.3969/j.issn.1001-9111.2022.05.015 . | |
| [7] | 罗成龙, 程皓洋, 张琪, 等. 雄性大鼠性动机的检测方法与评价指标研究进展[J]. 中华男科学杂志, 2024, 30(6):569-573. DOI: 10.13263/j.cnki.nja.2024.06.012 . |
| LUO C L, CHENG H Y, ZHANG Q, et al. Detection methods and evaluation indexes of sexual motivation in male rats: an update[J]. Natl J Androl, 2024, 30(6):569-573. DOI: 10.13263/j.cnki.nja.2024.06.012 . | |
| [8] | 王洁, 吴素慧, 尚海霞. 性成熟雌性SD大鼠动情周期的观察[J]. 当代医学, 2013, 19(28):25-26. DOI:10.3969/j.issn.1009-4393.2013.28.015 . |
| WANG J, WU S H, SHANG H X. Observation of the estrous cycle in sexually mature female sd rats[J]. Contemp Med, 2013, 19(28):25-26. DOI:10.3969/j.issn.1009-4393.2013.28.015 . | |
| [9] | 张森, 王新, 韦旭斌, 等. 大鼠发情周期各阶段的阴道细胞变化观察[J]. 动物医学进展, 2006, 27(2):69-72. DOI: 10.16437/j.cnki.1007-5038.2006.02.018 . |
| ZHANG S, WANG X, WEI X B, et al. Observation on the vaginal smear photographs for each stage of estrus cycle in rats[J]. Prog Vet Med, 2006, 27(2):69-72. DOI: 10.16437/j.cnki.1007-5038.2006.02.018 . | |
| [10] | 谢晓婕, 易兵, 杨刚坤. 兔生殖毒性评价试验中动物的选择及饲养管理要求[C]. 第九届药物毒理学年会——新时代·新技术·新策略·新健康论文集. 武汉: 2019: 2. DOI: 10.26914/c.cnkihy.2019.072236 . |
| XIE X J, YI B, YANG G K. Animal selection and husbandry requirements in rabbit reproductive toxicity evaluation tests [C]. Proceedings of the 9th Annual Conference on Drug Toxicology - New Era, New Technologies, New Strategies, New Health. Wuhan: 2019: 2. DOI:10.26914/c.cnkihy.2019.072236 . | |
| [11] | 黄雪如, 黄博定, 付丽萍, 等. 家兔生殖生理特点与提高繁殖力措施[J]. 中国养兔, 2020(3):42-43, 36. DOI:10.3969/j.issn.1005-6327.2020.03.015 . |
| HUANG X R, HUANG B D, FU L P, et al. Reproductive physiological characteristics of rabbits and measures to improve their fertility[J]. Chin J Rabbit Farming, 2020(3):42-43, 36. DOI:10.3969/j.issn.1005-6327.2020.03.015 . | |
| [12] | 李召英. 临床促进杂交母兔发情受孕实用技巧[J]. 中国养兔杂志, 2023(2):30-32, 35. DOI: 10.3969/j.issn.1005-6327.2021.06.009 . |
| LI Z Y. Practical skills of promoting estrus pregnancy of hybrid female rabbits in clinic[J]. Chin J Rabbit Farming, 2023(2):30-32, 35. DOI: 10.3969/j.issn.1005-6327.2021.06.009 . | |
| [13] | 孙世坤, 桑雷, 王锦祥, 等. 不同光源对闽西南黑母兔发情及激素GnRH水平影响[J]. 中国畜禽种业, 2021, 17(1):42-43. DOI: 10.3969/j.issn.1673-4556.2021.01.022 . |
| SUN S K, SANG L, WANG J X, et al. Effect of different light sources on estrus and GnRH level of female black rabbit in southwest Fujian[J]. Chin Livest Poult Breed, 2021, 17(1):42-43. DOI: 10.3969/j.issn.1673-4556.2021.01.022 . | |
| [14] | 王玉军, 张晓茜, 方有贵, 等. 高原鼠兔发情周期性激素与性器官变化特征[J]. 兽类学报, 2024, 44(3):351-359. DOI: 10.16829/j.slxb.150872 . |
| WANG Y J, ZHANG X Q, FANG Y G, et al. Characteristics of sex hormone level and sex organ histology in estrus cycle of plateau pika(Ochotona curzoniae)[J]. Acta Theriol Sin, 2024, 44(3):351-359. DOI: 10.16829/j.slxb.150872 . | |
| [15] | 潘孝青, 王杏龙, 杨杰, 等. LED单色光对兔行为及同期发情影响的机理研究[J]. 浙江农业学报, 2020, 32(12):2128-2137. DOI:10.3969/j.issn.1004-1524.2020.12.03 . |
| PAN X Q, WANG X L, YANG J, et al. Effects of different LED light colors on estrus synchronization of rabbits and their molecular regulation mechanism through retina-pineal gland pathway[J]. Acta Agric Zhejiangensis, 2020, 32(12):2128-2137. DOI: 10.3969/j.issn.1004-1524.2020.12.03 . | |
| [16] | LIN H H, KUANG M C, HOSSAIN I, et al. A nutrient-specific gut hormone arbitrates between courtship and feeding[J]. Nature, 2022, 602(7898):632-638. DOI:10.1038/s41586-022-04408-7 . |
| [17] | 刘鹍, 卫澜欣, 管晓琳, 等. 实验用新西兰兔压力水平的探究[J]. 现代畜牧科技, 2022(12):14-17. DOI: 10.19369/j.cnki.2095-9737.2022.12.005 . |
| LIU K, WEI L X, GUAN X L, et al. Preliminary exploration of pressure level in experimental New Zealand rabbits[J]. Mod Anim Husb Sci Technol, 2022(12):14-17. DOI: 10.19369/j.cnki.2095-9737.2022.12.005 . | |
| [18] | 周墨. 秋季种母兔管理要点[J]. 农家致富, 2024(19):39. |
| ZHOU M. Key management points for breeding rabbits in autumn[J]. Farmhouse Fortune, 2024(19):39. | |
| [19] | 孙祖越, 周莉, 闫晗, 等. 如何成功开展药物非临床生殖毒性试验[J]. 中国新药杂志, 2011, 20(22):2195-2204. DOI: CNKI:SUN:ZXYZ.0.2011-22-006 . |
| SUN Z Y, ZHOU L, YAN H, et al. How to successfully carry out non-clinical reproductive toxicity study on new drugs[J]. Chin J New Drugs, 2011, 20(22):2195-2204. DOI: CNKI:SUN:ZXYZ.0.2011-22-006 . | |
| [20] | 伶俐. 人工授精对母兔繁殖性能的影响[J]. 吉林畜牧兽医, 2022, 43(4):89-90. DOI: 10.3969/j.issn.1004-4264.2012.08.026 . |
| LING L. Effect of artificial insemination on reproductive performance of female rabbits[J]. Jilin Anim Husb Vet Med, 2022, 43(4):89-90. DOI: 10.3969/j.issn.1004-4264.2012.08.026 . | |
| [21] | 王家洁, 杨祺颖, 石宏. 非人灵长类动物模型在女性不孕症研究中的运用[J]. 生命科学, 2022, 34(11):1456-1464. DOI: 10.13376/j.cbls/2022159 . |
| WANG J J, YANG Q Y, SHI H. The use of non-human primate models in female infertility research[J]. Chin Bull Life Sci, 2022, 34(11):1456-1464. DOI: 10.13376/j.cbls/2022159 . | |
| [22] | 杜永洪, 赖国旗, 邹建中, 等. 重庆地区雌性恒河猴生殖生理的季节性变化[J]. 四川动物, 2008, 27(2):197-200. DOI: CNKI:SUN:SCDW.0.2008-02-010 . |
| DU Y H, LAI G Q, ZOU J Z, et al. Seasonal changes of reproductive physiology in female Rhesus monkey in Chongqing[J]. Sichuan J Zool, 2008, 27(2):197-200. DOI: CNKI:SUN:SCDW.0.2008-02-010 . | |
| [23] | LUETJENS C M, WEINBAUER G F. Functional assessment of sexual maturity in male macaques (Macaca fascicularis)[J]. Regul Toxicol Pharmacol, 2012, 63(3):391-400. DOI:10.1016/j.yrtph.2012.05.003 . |
| [24] | KU W W, PAGLIUSI F, FOLEY G, et al. A simple orchidometric method for the preliminary assessment of maturity status in male Cynomolgus monkeys (Macaca fascicularis) used for nonclinical safety studies[J]. J Pharmacol Toxicol Methods, 2010, 61(1):32-37. DOI:10.1016/j.vascn.2009.10.006 . |
| [25] | PLANT T M. Neurobiological bases underlying the control of the onset of puberty in the Rhesus monkey: a representative higher primate[J]. Front Neuroendocrinol, 2001, 22(2):107-139. DOI:10.1006/frne.2001.0211 . |
| [26] | PLANT T M, RAMASWAMY S. Kisspeptin and the regulation of the hypothalamic–pituitary–gonadal axis in the Rhesus monkey (Macaca mulatta)[J]. Peptides, 2009, 30(1):67-75. DOI:10.1016/j.peptides.2008.06.029 . |
| [27] | 袁芳, 潘晓靓, 林海霞, 等. 非人灵长类模型在生殖发育毒性评价中的应用[J]. 中国新药杂志, 2012, 21(9):989-993, 1006. DOI: CNKI:SUN:ZXYZ.0.2012-09-012 . |
| YUAN F, PAN X L, LIN H X, et al. Nonhuman Primates as animal models for developmental and reproductive toxicity study[J]. Chin J New Drugs, 2012, 21(9):989-993, 1006. DOI: CNKI:SUN:ZXYZ.0.2012-09-012 . | |
| [28] | NIEHOFF M O, BERGMANN M, WEINBAUER G F. Effects of social housing of sexually mature male Cynomolgus monkeys during general and reproductive toxicity evaluation[J]. Reprod Toxicol, 2010, 29(1):57-67. DOI:10.1016/j.reprotox. 2009.09.007 . |
| [29] | LIU X, LIU X Y, WANG X Q, et al. Multi-omics analysis reveals changes in tryptophan and cholesterol metabolism before and after sexual maturation in captive macaques[J]. BMC Genomics, 2023, 24(1):308. DOI:10.1186/s12864-023-09404-3 . |
| [30] | ANTJE F, EBERHARD B, F G W. Embryo fetal development studies in nonhuman primates[J]. Methods Mol Biol, 2013, (947)169-183.DOI: 10.1007/978-1-62703-131-8_14 . |
| [31] | BUSE E, HABERMANN G, OSTERBURG I, et al. Reproductive/developmental toxicity and immunotoxicity assessment in the nonhuman primate model[J]. Toxicology, 2003, 185(3):221-227. DOI:10.1016/s0300-483x(02)00614-5 . |
| [32] | 花秀春, 时彦胜, 孙兆增, 等. 人工饲养恒河猴、食蟹猴的繁殖性能初报[J]. 中国实验动物学报, 2009, 17(3):219-221. DOI: 10.3969/j.issn.1005-4847.2009.03.015 . |
| HUA X C, SHI Y S, SUN Z Z, et al. Preliminary study on the reproductive characteristics of Rhesus and Cynomolgus monkeys bred in captivity in Beijing area[J]. Acta Lab Animalis Sci Sin, 2009, 17(3):219-221. DOI: 10.3969/j.issn.1005-4847.2009.03.015 . | |
| [33] | 王双棋, 韦秋奖, 江海天, 等. 昆明地区食蟹猴种群繁殖规律与繁殖率的分析[J]. 现代畜牧兽医, 2024(3):64-67. DOI: 10.20154/j.cnki.issn1672-9692.2024.03.015 . |
| WANG S Q, WEI Q J, JIANG H T, et al. Analysis of reproductive rule and reproductive rate of Cynomolgus monkey population in Kunming area[J]. Mod J Anim Husb Vet Med, 2024(3):64-67. DOI: 10.20154/j.cnki.issn1672-9692.2024.03.015 . |
| [1] | WANG Hanyue, CHEN Jiawei, GAO Xiangbin, LUO Wei, LIU Suning. Research Overview on Corpora Cardiaca Function of Model Animal Drosophila melanogaster [J]. Laboratory Animal and Comparative Medicine, 2025, (): 1-14. |
| [2] | . [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 508-514. |
| [3] | LIU Yueqin, XUE Weiguo, WANG Shuyou, SHEN Yaohua, JIA Shuyong, WANG Guangjun, SONG Xiaojing. Observation of Digestive Tract Tissue Morphology in Mice Using Probe-Based Confocal Laser Endomicroscopy [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 457-465. |
| [4] | ZHENG Qingyong, YANG Donghua, MA Zhichao, ZHOU Ziyu, LU Yang, WANG Jingyu, XING Lina, KANG Yingying, DU Li, ZHAO Chunxiang, DI Baoshan, TIAN Jinhui. Recommendations for Standardized Reporting of Systematic Reviews and Meta-Analysis of Animal Experiments [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 496-507. |
| [5] | WANG Tingjun, LUO Hao, CHEN Qi. Discussion on AI-Based Digital Upgrade and Application Practice of Laboratory Animal Centers [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 473-482. |
| [6] | WANG Jiaoxiang, ZHANG Lu, CHEN Shuhan, JIAO Deling, ZHAO Heng, WEI Taiyun, GUO Jianxiong, XU Kaixiang, WEI Hongjiang. Construction and Functional Validation of GTKO/hCD55 Gene-Edited Xenotransplant Donor Pigs [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 379-392. |
| [7] | QIN Chao, LI Shuangxing, ZHAO Tingting, JIANG Chenchen, ZHAO Jing, YANG Yanwei, LIN Zhi, WANG Sanlong, WEN Hairuo. Study on the 90-day Feeding Experimental Background Data of SD Rats for Drug Safety Evaluation [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 439-448. |
| [8] | JIAO Qingzhen, WU Guihua, TANG Wen, FAN Fan, FENG Kai, YANG Chunxiang, QIAO Jian, DENG Sufang. Dynamic Monitoring and Analysis of Ammonia Concentration in Laboratory Animal Facilities Under Suspension of Heating Ventilation and Air Conditioning System [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 490-495. |
| [9] | LIU Wentao, LUO Yanhong, LONG Yongxia, LUO Qihui, CHEN Zhengli, LIU Lida. Common Environmental Problems and Testing Experiences in Laboratory Animal Facilities in Sichuan Province [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 483-489. |
| [10] | ZHAO Xin, WANG Chenxi, SHI Wenqing, LOU Yuefen. Advances in the Application of Zebrafish in the Research of Inflammatory Bowel Disease Mechanisms and Drug Development [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 422-431. |
| [11] | GONG Leilei, WANG Xiaoxia, FENG Xuewei, LI Xinlei, ZHAO Han, ZHANG Xueyan, FENG Xin. A Mouse Model and Mechanism Study of Premature Ovarian Insufficiency Induced by Different Concentrations of Cyclophosphamide [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 403-410. |
| [12] | LIN Zhenhua, CHU Xiangyu, WEI Zhenxi, DONG Chuanjun, ZHAO Zenglin, SUN Xiaoxia, LI Qingyu, ZHANG Qi. Evaluation of the Safety and Efficacy of Bone Cement in Experimental Pigs Using Vertebroplasty [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 466-472. |
| [13] | JIANG Juan, SONG Ning, LIAN Wenbo, SHAO Congcong, GU Wenwen, SHI Yan. Comparison of Histopathological and Molecular Pathological Phenotypes in Mouse Models of Intrauterine Adhesions Induced by Two Concentrations of Ethanol Perfusion [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 393-402. |
| [14] | CHEN Ziyi, SUN Hongyan, KANG Pinfang, WU Wenjuan. Research Advances in Animal Experimental Models of Pulmonary Hypertension [J]. Laboratory Animal and Comparative Medicine, 2025, (): 1-12. |
| [15] | XU Yingtao, WANG Mengmeng, LIN Ping, CHI Haitao, WANG Yi, BAI Ying. Exosomes Improve Ischemic Stroke by Regulation of Ferroptosis Through the NRF2/SLC7A11/GPX4 Pathway in Mice [J]. Laboratory Animal and Comparative Medicine, 2025, (): 1-11. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||