
Laboratory Animal and Comparative Medicine
• XXXX XXXX •
LIU Kun, LAN Qing, Yi Bing, XIE Xiaojie( )(
)( )
)
Online:2025-05-21
Contact:
XIE Xiaojie
CLC Number:
LIU Kun,LAN Qing,Yi Bing,et al. Key Challenges and Mitigation Strategies for Animal Pregnancy in Nonclinical Reproductive Toxicity Studies[J]. Laboratory Animal and Comparative Medicine. DOI: 10.12300/j.issn.1674-5817.2024.177.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2024.177
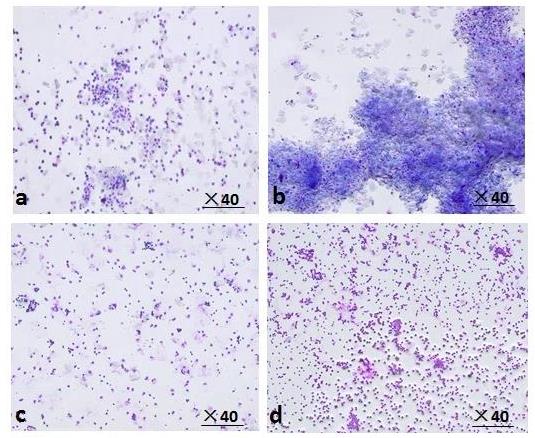
Figure 1 Typical vaginal smear characteristics across estrous cycle stages in adult female rats Note:1-a represents the Proestrus stage (P), 1-b represents the Estrus stage (E), 1-c represents the Metestrus stage (M), and 1-d represents the Diestrus stage (D).

Figure 2 Testicular volume measurement device Note:The volume indicated by the arrow in Figure represents an approximate unilateral testicular volume of 10 mL.

Figure 3 B-Ultrasound cross-sectional view of the gestational sac in female monkeys during early pregnancy Note:The area indicated by the arrow in Figure shows a well-formed gestational sac structure.
| 1 | 黄冬妍,吴建辉 .生殖毒理学研究动物模型的建立方法及应用评价[J].实验动物与比较医学,2024,44(5):550-559.DOI:10.12300/j.issn.1674-5817.2024.028 . |
| HUANG D Y , WU J H . Establishment methods and application evaluation of animal models for reproductive toxicology research [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 550-559. DOI: 10.12300/j.issn.1674-5817.2024.028 . | |
| 2 | 林蔚,张荣标,陈润 .SD大鼠毒性试验生殖指标参考值建立及不同体重大鼠孕后生殖指标分析[J].海峡预防医学杂志,2023,29(4):60-62. |
| LIN W , ZHANG R B , CHEN R . Establishment of reference values for reproductive indices in SD rat toxicity tests and analysis of reproductive indices in pregnant rats with different body weights [J]. Strait Journal of Preventive Medicine, 2023, 29(4): 60-62. | |
| 3 | 闫婧,赵季宇,马梦娜,文坛,王红阳,金晓飞 .大鼠阴道细胞涂片技术及其HE染色方法的研究[J].山西中医药大学学报,2023,24(1):45-51.DOI:10.19763/j.cnki.2096-7403.2023.01.07 . |
| YAN J , ZHAO J Y , MA M N , et al . Study on vaginal smear technique and HE staining method in rats [J]. Journal of Shanxi University of Chinese Medicine, 2023, 24(1): 45-51. DOI:10.19763/j.cnki.2096-7403.2023.01.07 . | |
| 4 | 陈思充,陈子豪,高青华,郝丽英 .不同阴道涂片方法的比较及对大鼠动情周期的判断[J].解剖科学进展,2023,29(1):93-95102.DOI:10.16695/j.cnki.1006-2947.2023.01.025 . |
| CHEN S C , CHEN Z H , GAO Q H , et al . Comparison of different vaginal smear methods and determination of estrous cycle in rats [J]. Progress of Anatomical Sciences, 2023, 29(1): 93-95, 102. DOI:10.16695/j.cnki.1006-2947.2023.01.025 . | |
| 5 | 徐海瑾,孔斌,刘晓兵,宋辉,李卉 .热应激对雌性小鼠发情周期及生殖激素分泌的影响[J].农业科学研究,2019,40(4):17-2026. |
| XU H J , KONG B , LIU X B , et al . Effects of heat stress on estrous cycle and reproductive hormone secretion in female mice [J]. Agricultural Science Research, 2019, 40(4): 17-20, 26. | |
| 6 | 于农淇,梁莎莎,黄健,等 .阴道分泌物在动物发情鉴定中的研究进展[J].中国牛业科学,2022,48(05):66-70. |
| YU N Q , LIANG S S , HUANG J , et al . Research progress on vaginal secretions in animal estrus identification [J]. China Cattle Science, 2022, 48(5): 66-70. | |
| 7 | 罗成龙,程皓洋,张琪(综述,焦拥政(审校 .雄性大鼠性动机的检测方法与评价指标研究进展[J].中华男科学杂志,2024,30(6):569-573. DOI:10.13263/j.cnki.nja.2024.06.012 . |
| LUO C L , CHENG H Y , ZHANG Q review) , et al . Research progress on detection methods and evaluation indicators of sexual motivation in male rats [J]. Chinese Journal of Andrology, 2024, 30(6): 569-573. DOI:10.13263/j.cnki.nja.2024.06.012 . | |
| 8 | 张森,王新,韦旭斌,谭建华 .大鼠发情周期各阶段的阴道细胞变化观察[J].动物医学进展,2006,27(2):69-72. |
| ZHANG S , WANG X , WEI X B , et al . Observation of vaginal cell changes during different stages of estrous cycle in rats [J]. Progress in Veterinary Medicine, 2006, 27(2): 69-72. | |
| 9 | 谢晓婕, 易兵, 杨刚坤 . 兔生殖毒性评价试验中动物的选择及饲养管理要求[C]//中国药理学会安全药理学专业委员会, 中华中医药学会中药毒理学与安全性研究分会, 中国毒理学会中药与天然药物毒理专业委员会, 中国药理学会药物毒理专业委员会, 中国药理学会药物毒理与安全性评价专业委员会. 第九届药物毒理学年会——新时代·新技术·新策略·新健康论文集. 成都华西海圻医药科技有限公司(国家成都新药安全性评价中心), 2019:2. DOI:10.26914/c.cnkihy.2019.072236 . |
| XIE X J , YI B , YANG G K . Animal Selection and Husbandry Requirements in Rabbit Reproductive Toxicity Evaluation Tests [C]// Professional Committee of Safety Pharmacology, Chinese Pharmacological Society; Branch of Toxicological and Safety Research on Chinese Materia Medica, China Association of Chinese Medicine; Professional Committee of Toxicology of Chinese Medicine and Natural Products, Chinese Society of Toxicology; Professional Committee of Drug Toxicology, Chinese Pharmacological Society; Professional Committee of Drug Toxicology and Safety Evaluation, Chinese Pharmacological Society. Proceedings of the 9th Annual Conference on Drug Toxicology - New Era • New Technologies • New Strategies • New Health. Chengdu: Chengdu West China Frontier Pharmatech Co., Ltd. (National Chengdu Center for New Drug Safety Evaluation), 2019: 2. DOI:10.26914/c.cnkihy.2019.072236 . | |
| 10 | 李召英 .临床促进杂交母兔发情受孕实用技巧[J]. 中国养兔, 2023(2): 30-32, 35. |
| LI Z Y . Practical techniques for promoting estrus and pregnancy in hybrid female rabbits [J]. Chinese Rabbit Farming, 2023(2): 30-32, 35. | |
| 11 | 王玉军,张晓茜,方有贵,杨其恩,贾功雪 .高原鼠兔发情周期性激素与性器官变化特征[J].兽类学报,2024,44(3):351-359.DOI:10.16829/j.slxb.150872 . |
| WANG Y J , ZHANG X Q , FANG Y G , et al . Characteristics of periodic hormone and sexual organ changes during estrus in plateau pikas [J]. Acta Theriologica Sinica, 2024, 44(3): 351-359. DOI:10.16829/j.slxb.150872 . | |
| 12 | 黄雪如, 黄博定, 付丽萍, 等 . 家兔生殖生理特点与提高繁殖力措施[J]. 中国养兔, 2020(3): 42-43, 36. |
| HUANG X R , HUANG B D , FU L P , et al . Reproductive physiological characteristics of rabbits and measures to improve fertility [J]. Chinese Rabbit Farming, 2020(3): 42-43, 36. | |
| 13 | 潘孝青, 王杏龙, 杨杰, 等 . LED单色光对兔行为及同期发情影响的机理研究[J]. 浙江农业学报, 2020, 32(12): 2128-2137. DOI:10.3969/j.issn.1004-1524.2020.12.03 . |
| PAN X Q , WANG X L , YANG J , et al . Mechanism of LED monochromatic light on rabbit behavior and estrus synchronization[J]. Acta Agriculturae Zhejiangensis, 2020, 32(12): 2128-2137. DOI:10.3969/j.issn.1004-1524.2020.12.03 . | |
| 14 | LIN H. H. , KUANG M. C. , HOSSAIN I. , et al . A nutrient-specific gut hormone arbitrates between courtship and feeding. Nature 602, 632–638 (2022). DOI:10.1038/s41586-022-04408-7 . |
| 15 | 周墨 . 秋季种母兔管理要点[J]. 农家致富, 2024(19):39-39. |
| ZHOU M . Key points for managing breeding female rabbits in autumn[J]. Peasant Family Getting Rich, 2024(19): 39. | |
| 16 | 孙祖越,周莉,闫晗,何桂林 .如何成功开展药物非临床生殖毒性试验[J].中国新药杂志,2011,20(22):2195-2204. |
| SUN Z Y , ZHOU L , YAN H , et al . How to successfully conduct non-clinical reproductive toxicity tests for drugs[J]. Chinese Journal of New Drugs, 2011, 20(22): 2195-2204. | |
| 17 | 伶俐 . 人工授精对母兔繁殖性能的影响[J]. 吉林畜牧兽医, 2022, 43(4):89-90. |
| LING L . Effects of artificial insemination on reproductive performance of female rabbits[J]. Jilin Animal Husbandry and Veterinary Medicine, 2022, 43(4): 89-90. | |
| 18 | 王家洁, 杨祺颖, 石宏 . 非人灵长类动物模型在女性不孕症研究中的运用[J]. 生命科学, 2022, 34(11):1456-1464. |
| WANG J J , YANG Q Y , SHI H . Application of non-human primate models in female infertility research[J]. Chinese Bulletin of Life Sciences, 2022, 34(11): 1456-1464. | |
| 19 | 杜永洪, 赖国旗, 邹建中, 等 . 重庆地区雌性恒河猴生殖生理的季节性变化[J]. 四川动物, 2008, 27(2):197-200. |
| DU Y H , LAI G Q , ZOU J Z , et al . Seasonal variations in reproductive physiology of female rhesus monkeys in Chongqing area[J]. Sichuan Journal of Zoology, 2008, 27(2): 197-200. | |
| 20 | Luetjens C.M. , Weinbauer G.F. , Functional assessment of sexual maturity in male macaques (Macaca fascicularis), Regulatory Toxicology and Pharmacology, Volume 63, Issue 3, 2012, Pages 391-400. DOI:10.1016/j.yrtph.2012.05.003 . |
| 21 | TONY M. Plant, Neurobiological Bases Underlying the Control of the Onset of Puberty in the Rhesus Monkey: A Representative Higher Primate, Frontiers in Neuroendocrinology, Volume 22, Issue 2, 2001, Pages 107-139.DOI:10.1006/frne.2001.0211 . |
| 22 | PLANT M T , RAMASWAMY S . Kisspeptin and the regulation of the hypothalamic–pituitary–gonadal axis in the rhesus monkey (Macaca mulatta) [J]. Peptides, 2008, 30(1): 67-75. DOI:10.1016/j.peptides.2008.06.029 . |
| 23 | LUETJENS C , WEINBAUER G . Functional assessment of sexual maturity in male macaques (Macaca fascicularis)[J]. Regulatory Toxicology and Pharmacology, 2012, 63(3): 391-400. DOI:10.1016/j.yrtph.2012.05.003 . |
| 24 | 袁芳, 潘晓靓, 林海霞, 等 . 非人灵长类模型在生殖发育毒性评价中的应用[J]. 中国新药杂志, 2012, 21(9): 989-993+1006. |
| YUAN F , PAN X J , LIN H X , et al . Application of non-human primate models in reproductive and developmental toxicity evaluation[J]. Chinese Journal of New Drugs, 2012, 21(9): 989-993+1006. | |
| 25 | 花秀春, 时彦胜, 孙兆增, 等 . 人工饲养恒河猴、食蟹猴的繁殖性能初报[J]. 中国实验动物学报, 2009, 17(3):219-221. |
| HUA X C , SHI Y S , SUN Z Z , et al . Preliminary report on reproductive performance of artificially bred rhesus monkeys and cynomolgus monkeys[J]. Acta Laboratorium Animalis Scientia Sinica, 2009, 17(3): 219-221. | |
| 26 | 王双棋, 韦秋奖, 江海天, 等 . 昆明地区食蟹猴种群繁殖规律与繁殖率的分析[J]. 现代畜牧兽医, 2024(3):64-67. DOI:10.20154 /j.cnki.issn.1672-9692.2024.03.015 . |
| WANG S Q , WEI Q J , JIANG H T , et al . Analysis of reproductive patterns and fertility rates in cynomolgus monkey populations in Kunming region[J]. Modern Journal of Animal Husbandry and Veterinary Medicine, 2024(3): 64-67. DOI:10.20154/j.cnki.issn.1672-9692.2024.03.015 . | |
| 27 | 孙世坤, 桑雷, 王锦祥, 等 . 不同光源对闽西南黑母兔发情及激素GnRH水平影响[J]. 中国畜禽种业, 2021, 17(1):42-43. |
| SUN S K , SANG L , WANG J X , et al . Effects of different light sources on estrus and GnRH hormone levels in Minxi black female rabbits[J]. China Livestock and Poultry Breeding, 2021, 17(1): 42-43. | |
| 28 | 刘鹍, 卫澜欣, 管晓琳, 等 . 实验用新西兰兔压力水平的探究[J]. 现代畜牧科技, 2022(12):14-17. DOI:10.19369/j.cnki.2095-9737.2022.12.005 . |
| LIU K , WEI L X , GUAN X L , et al . Investigation on stress levels in experimental New Zealand rabbits[J]. Modern Animal Husbandry Science and Technology, 2022(12): 14-17. DOI:10.19369/j.cnki.2095-9737.2022.12.005 . | |
| 29 | W W K , FP, Foley . A simple orchidometric method for the preliminary assessment of maturity status in male cynomolgus monkeys (Macaca fascicularis) used for nonclinical safety studies. [J]. Journal of Pharmacological and Toxicological Methods, 2010, 61(1): 32-37. DOI:10.1016/j.vascn.2009.10.006 . |
| 30 | NIEHOFF O M , BERGMANN M , WEINBAUER F G . Effects of social housing of sexually mature male cynomolgus monkeys during general and reproductive toxicity evaluation[J]. Reproductive Toxicology, 2009, 29(1):57-67. DOI:10.1016/j.reprotox.2009.09.007 . |
| 31 | XU L , XU Y L , XIN Q W , et al .Multi-omics analysis reveals changes in tryptophan and cholesterol metabolism before and after sexual maturation in captive macaques[J].BMC genomics,2023,24(1):308-308. DOI:10.1186/s12864-023-09404-3 . |
| 32 | ANTJE F , EBERHARD B , F G W . Embryo fetal development studies in nonhuman primates[J]. Methods in molecular biology (Clifton, N.J.), 2013, 947169-83.DOI: 10.1007/978-1-62703-131-8_14 . |
| 33 | BUSE E , HABERMANN G , OSTERBURG I , et al .Reproductive/developmental toxicity and immunotoxicity assessment in the nonhuman primate model[J].Toxicology,2003,185(3):221-227. DOI:10.1016/s0300-483x(02)00614-5 . |
| [1] | Jiaqi CHEN, Longbao LÜ, Feiyan ZHANG, Rui LI, Yijiang LI, Lihong LI, Xiaodi ZHANG. Application of Blood Microsampling and Its Implementation of the 3Rs in Non-clinical Studies of Drugs [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 306-312. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||