
Laboratory Animal and Comparative Medicine
• XXXX XXXX •
XU Yingtao( ), WANG Mengmeng, LIN Ping, CHI Haitao, WANG Yi, BAI Ying(
), WANG Mengmeng, LIN Ping, CHI Haitao, WANG Yi, BAI Ying( )
)
Online:2025-08-04
Contact:
BAI Ying
CLC Number:
XU Yingtao,WANG Mengmeng,LIN Ping,et al. Exosomes Improve Ischemic Stroke by Regulation of Ferroptosis Through the NRF2/SLC7A11/GPX4 Pathway in Mice[J]. Laboratory Animal and Comparative Medicine. DOI: 10.12300/j.issn.1674-5817.2025.034.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2025.034
引物名称 Primer name | 引物序列 Sequence(5’→3’) |
|---|---|
| GAPDH-F | AGGTCGGTGTGAACGGATTTG |
| GAPDH-R | GGGGTCGTTGATGGCAACA |
| NRF2-F | CTTTAGTCAGCGACAGAAGGAC |
| NRF2-R | AGGCATCTTGTTTGGGAATGTG |
| SLC7A11-F | CACCGGGGTCGGTTTTCTTA |
| SLC7A11-R | GGCAGATGGCCAAGCTTTTG |
| GPX4-F | CTATGGTCCCATGGAGGAGC |
| GPX4-R | AGGCAGACCTTCATGAGTGC |
Table1 Sequence of primers
引物名称 Primer name | 引物序列 Sequence(5’→3’) |
|---|---|
| GAPDH-F | AGGTCGGTGTGAACGGATTTG |
| GAPDH-R | GGGGTCGTTGATGGCAACA |
| NRF2-F | CTTTAGTCAGCGACAGAAGGAC |
| NRF2-R | AGGCATCTTGTTTGGGAATGTG |
| SLC7A11-F | CACCGGGGTCGGTTTTCTTA |
| SLC7A11-R | GGCAGATGGCCAAGCTTTTG |
| GPX4-F | CTATGGTCCCATGGAGGAGC |
| GPX4-R | AGGCAGACCTTCATGAGTGC |
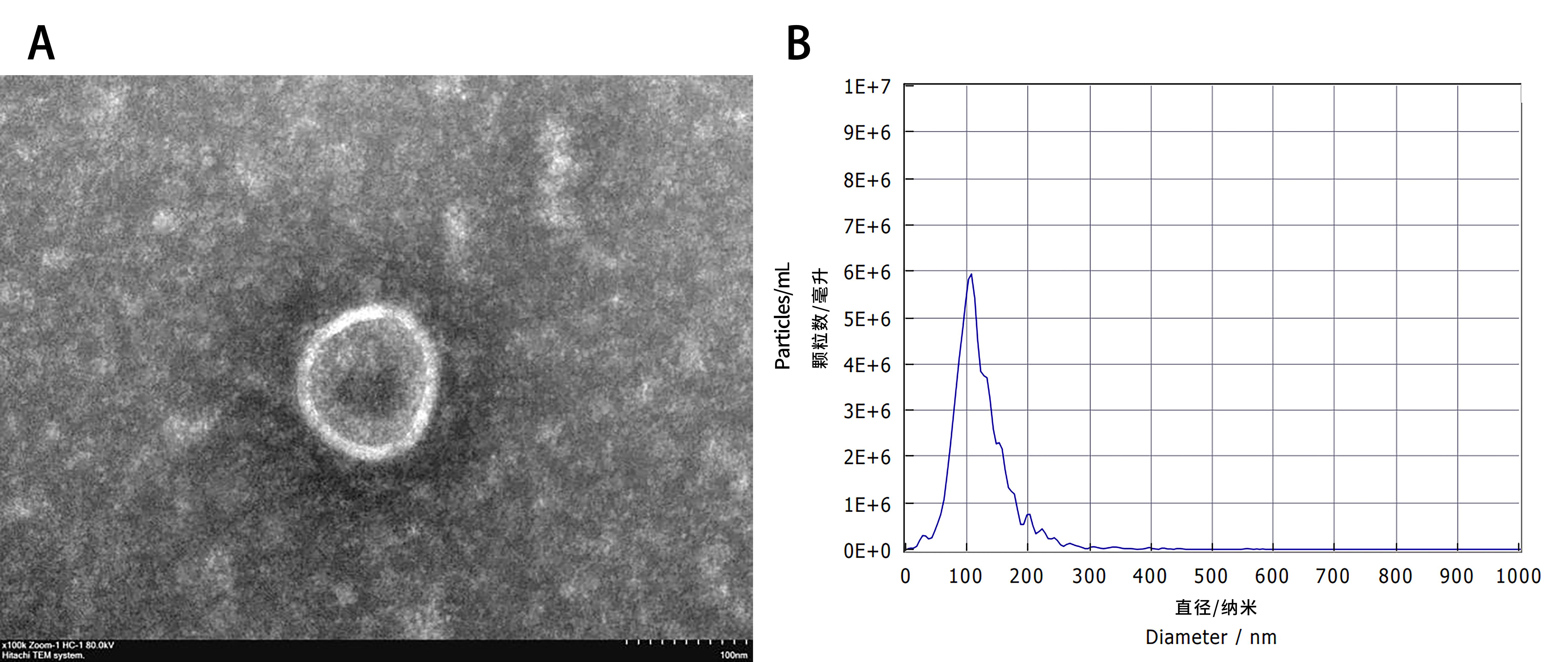
Figure 1 Electron microscopy observation of extracted exosome and analysis of particle sizeNote:A, the exosomes observed under the electron microscope have a diameter of approximately 100 nm and are in the shape of a tea tray; B, the exosome suspension extracted by particle size analysis (NTA), with the abscissa representing the diameter in nanometers (diameter/nm) and the ordinate representing the number of particles per milliliter (particle/mL).
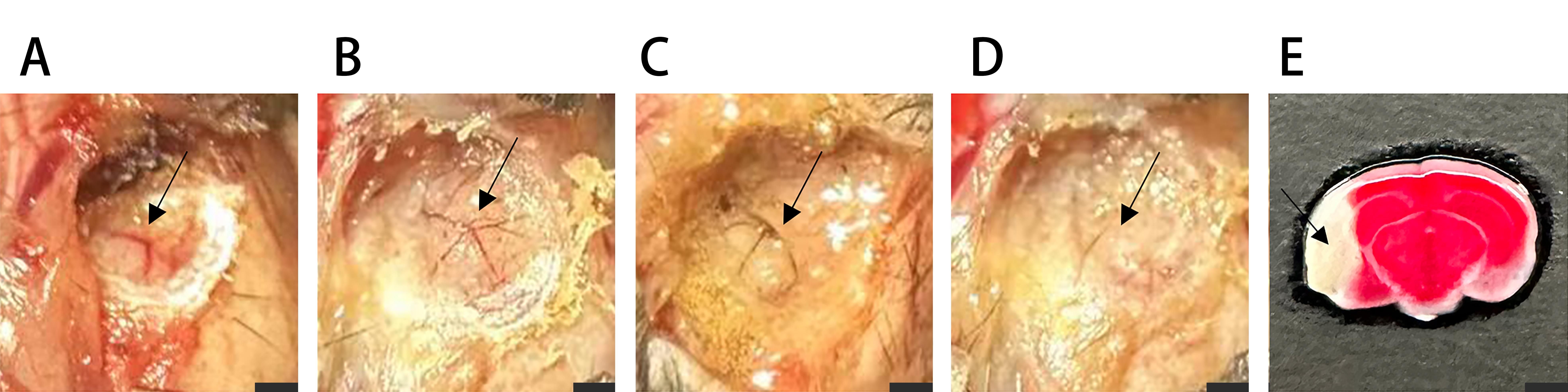
Figure 2 The process of establishing the MCAO model, changes in MCA blood flow and TTC staining manifestations of the infarction areaNote:A, The black arrow indicates MCA, which is in a Y-shaped configuration; B-D, Electrocoagulation gradually blocks MCA, and blood supply gradually disappears; E, In the MCAO model, the brain is subjected to TTC staining treatment. The white area indicated by the black arrow is the infarction area; The scale of A-D is 3 mm, and that of E is 5 mm.

Figure 3 The neurological deficit score of mice and the percentage of cerebral infarction volume were detected by TTC staining of brain slices after brain removalNote:A, Longa scores of each group of mice after modeling and exosome intervention; B, after extracting the brains of the mice, the slices were stained with TTC. The results showed that each column contained 6 slices representing a complete brain. Normal brain tissue was red, while brain tissue in the infarcted area was white; C, the percentage of brain infarction volume was calculated; there were 8 mice in each group. **P<0.01, ***P<0.001.
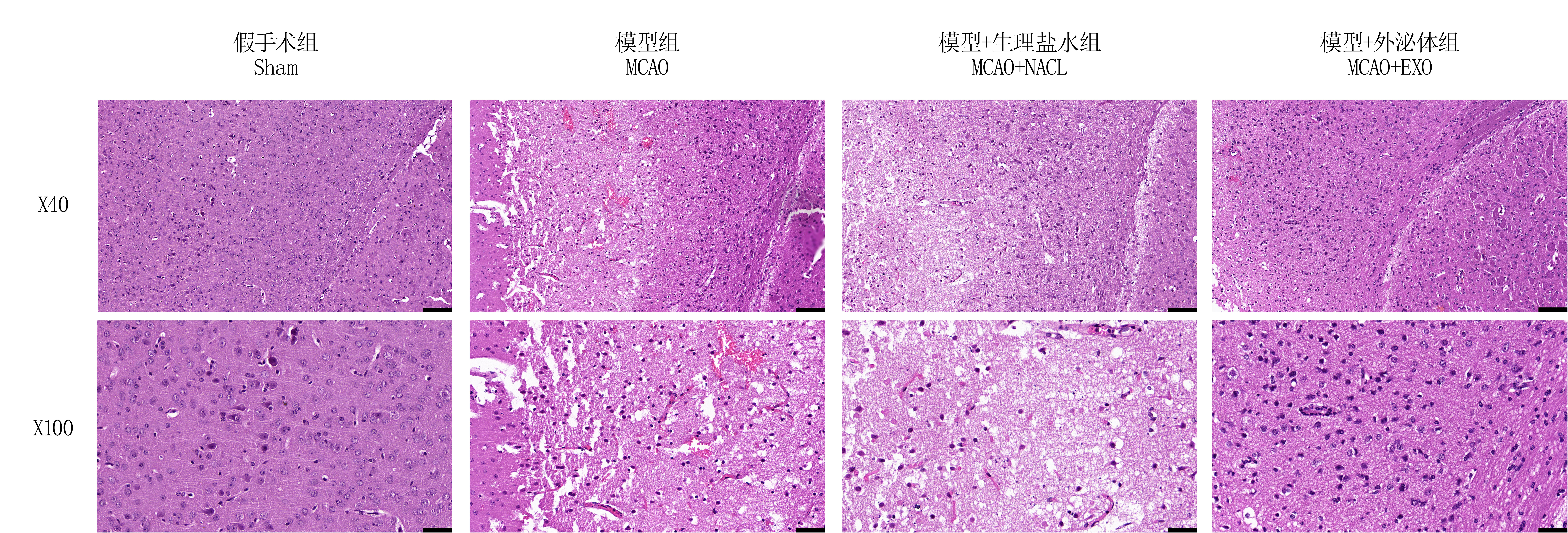
Figure 4 After removing the brain of the mice, the sections were stained with HE to observe the morphology of nerve cells(×40、×100)Note:The scale of the ×40 field of view in the first row of the figure is 100 μm, and the scale of the ×100 field of view in the second row is 40 μm; there were 8 mice in each group.The scale of the ×40 field of view in the first row is 100 μm, and the scale of the ×100 field of view in the second row is 40 μm; the area within the red frame in the first row ×40 field of view corresponds to the overall field of view in the second row ×100; there are 8 mice in each group.
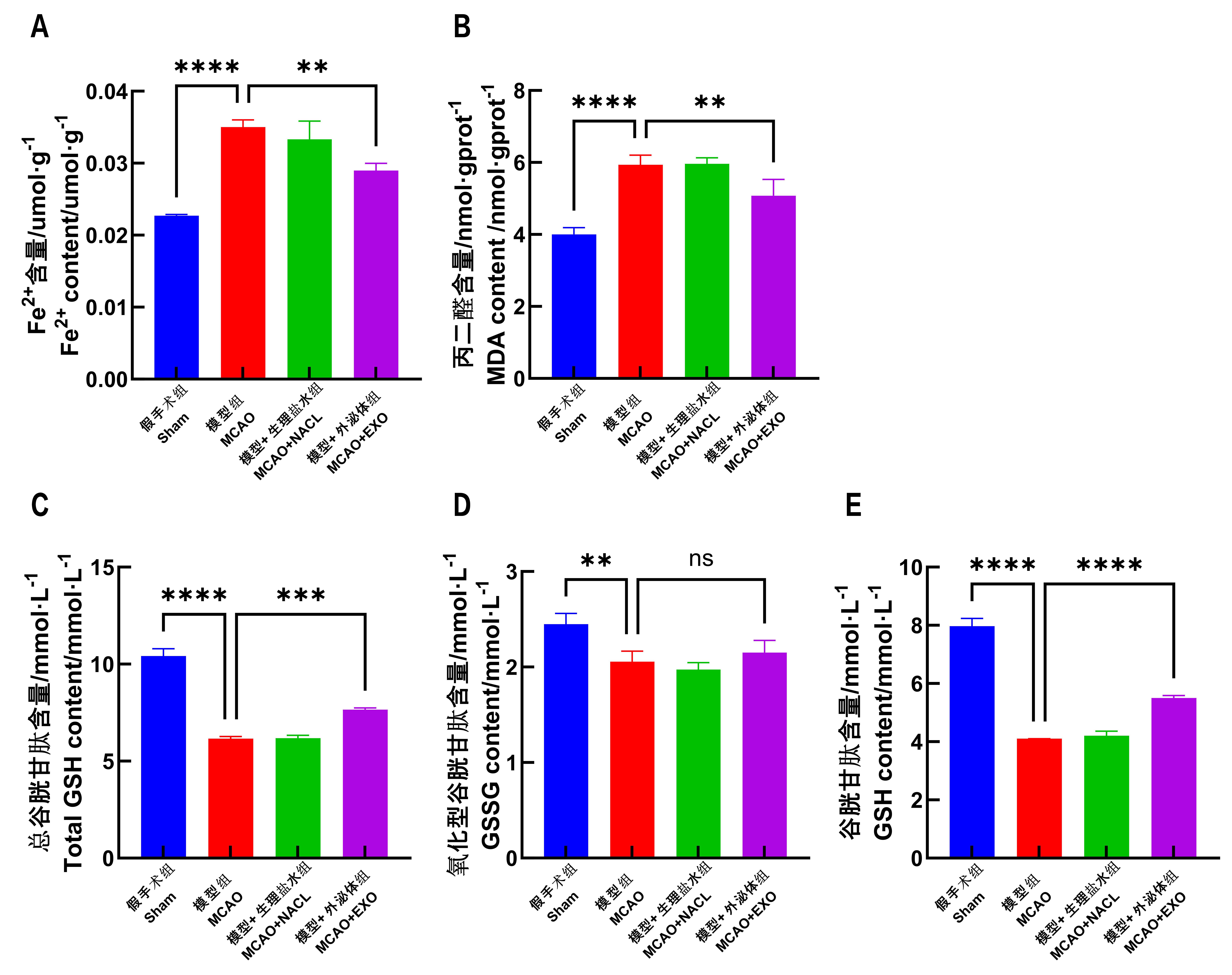
Figure 5 After extracting the brain of the mice, the content of Fe2+, MDA, Total GSH, GSSG and GSH in the brain infarction area and the surrounding brain tissues was detected using the micro-methodNote:A, detect Fe2+ content; B, detect MDA content; C, measure the total GSH content; D, measure the GSSG content; E, calculate the GSH content, which is the difference between the total GSH and GSSG; There were 8 mice in each group. **P<0.01,***P<0.001,****P<0.0001.
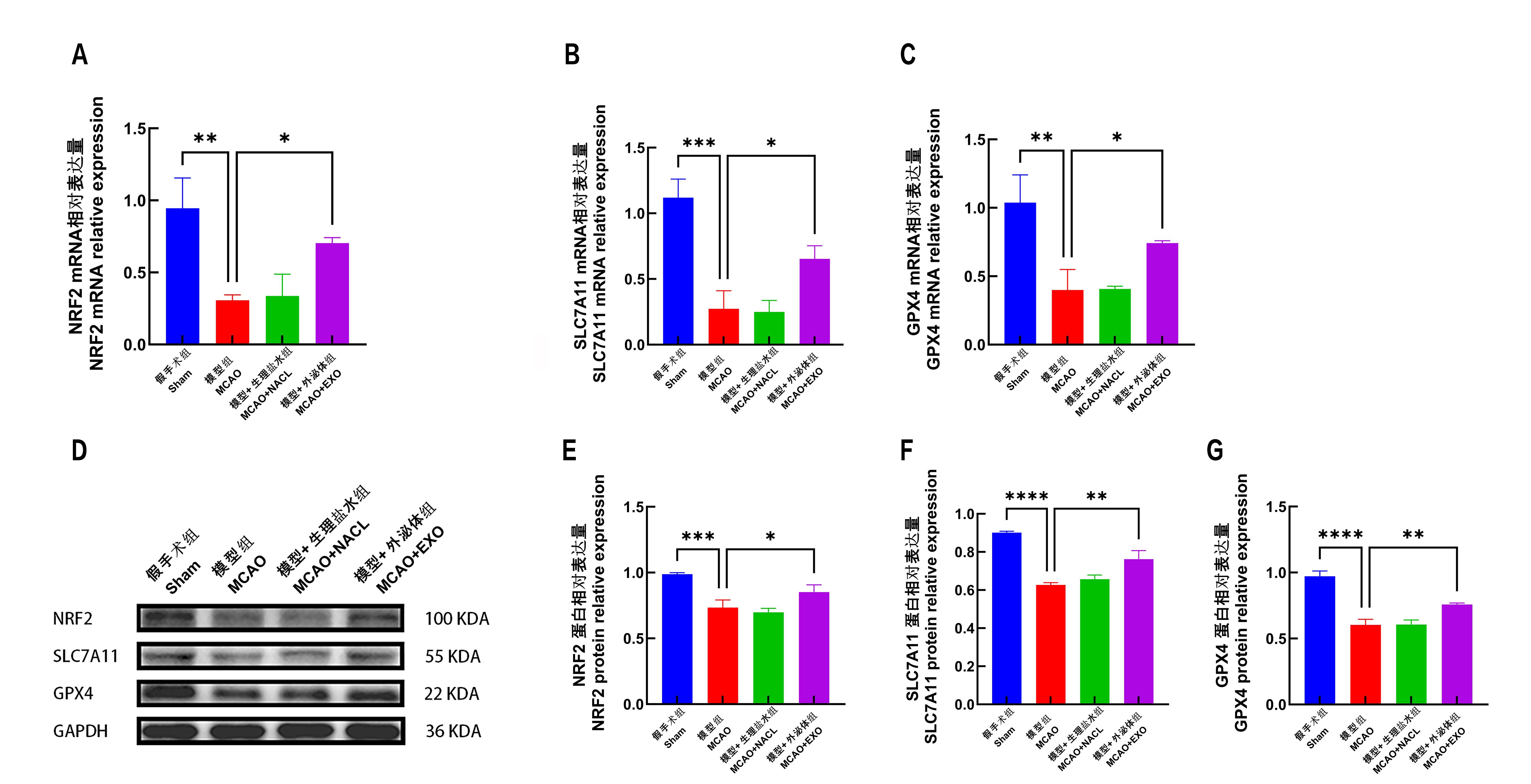
Figure 6 After the brain of mice was removed, the mRNA and protein expressions of NRF2, SLC7A11 and GPX4 in the infarct area and the surrounding brain tissue were detected.Note:A, analysis of NRF2 mRNA expression and its relative quantity; B, analysis of SLC7A11 mRNA expression and its relative quantity; C, analysis of GPX4 mRNA expression and its relative quantity; D, protein bands of NRF2, SLC7A11, GPX4 and GAPDH (reference protein) in each group of Western Blot analysis of mice; on the right side, the corresponding molecular weights of the proteins are indicated; E, protein expression and relative quantification analysis of NRF2; F, protein expression and relative quantification analysis of SLC7A11; G, protein expression and relative quantification analysis of GPX4; 8 mice in each group. *P<0.05,**P<0.01,***P<0.001,****P<0.000 1.
| [1] | MENDELSON S J, PRABHAKARAN S. Diagnosis and management of transient ischemic attack and acute ischemic stroke: a review[J]. JAMA, 2021, 325: 1088–1098. DOI: 0.1001/jama.2020.26867 . |
| [2] | GREEN T L, MCNAIR N D, HINKLE J L, et al. Care of the patient with acute ischemic stroke (posthyperacute and prehospital discharge): Update to 2009 comprehensive nursing care scientific statement: a scientific statement from the American Heart Association[J]. Stroke, 2021, 52(5):e179-e197. DOI: 10.1161/STR.0000000000000357 . |
| [3] | QIN C, YANG S, CHU Y H, ZHANG H, et al. Signaling pathways involved in ischemic stroke: molecular mechanisms and therapeutic interventions[J]. Signal Transduct Target Ther, 2022, 7(1):215. DOI: 10.1038/s41392-022-01064-1 . |
| [4] | TUO Q Z, LEI P. Ferroptosis in ischemic stroke: Animal models and mechanisms[J]. Zool Res, 2024, 45(6):1235-1248. DOI: 10.24272/j.issn.2095-8137.2024.239 . |
| [5] | JIANG X, STOCKWELL B R, CONRAD M. Ferroptosis: mechanisms, biology and role in disease[J]. Nat Rev Mol Cell Biol, 2021, 22(4):266-282. DOI: 10.1038/s41580-020-00324-8 . |
| [6] | LIANG Y, DUAN L, LU J, et al. Engineering exosomes for targeted drug delivery[J]. Theranostics, 2021, 11(7):3183-3195. DOI: 10.7150/thno.52570 . |
| [7] | CHEN Y, LI B, QUAN J, et al. Inhibition of ferroptosis by mesenchymal stem cell-derived exosomes in acute spinal cord injury: Role of Nrf2/GCH1/BH4 axis[J]. Neurospine, 2024, 21(2):642-655. DOI: 10.14245/ns.2448038.019 . |
| [8] | LI X, ZHANG X, LIU Y, et al. Exosomes derived from mesenchyml stem cells ameliorate oxygen-glucose deprivation/reoxygenation-induced neuronal injury via transferring MicroRNA-194 and targeting Bach1[J]. Tissue Cell, 2021, 73:101651. DOI: 10.1016/j.tice.2021.101651 . |
| [9] | WANG M, BAI Y, CHI H, et al. miR-451 protects against ischemic stroke by targeting Phd3[J]. Exp Neurol, 2021, 343:113777. DOI: 10.1016/j.expneurol.2021.113777 . |
| [10] | TANG D, CHEN X, KANG R, et al. Ferroptosis: molecular mechanisms and health implications[J]. Cell Res, 2021, 31(2):107-125. DOI: 10.1038/s41422-020-00441-1 . |
| [11] | SHEN K, WANG X, WANG Y, et al. miR-125b-5p in adipose derived stem cells exosome alleviates pulmonary microvascular endothelial cells ferroptosis via Keap1/Nrf2/GPX4 in sepsis lung injury[J]. Redox Biol, 2023, 62:102655. DOI: 10.1016/j.redox.2023.102655 . |
| [12] | LIN F, CHEN W, ZHOU J, et al. Mesenchymal stem cells protect against ferroptosis via exosome-mediated stabilization of SLC7A11 in acute liver injury[J]. Cell Death Dis, 2022, 13(3):271. DOI: 10.1038/s41419-022-04708-w . |
| [13] | LIU H, ZHANG T A, ZHANG W Y, et al. Rhein attenuates cerebral ischemia-reperfusion injury via inhibition of ferroptosis through NRF2/SLC7A11/GPX4 pathway[J]. Exp Neurol, 2023, 369: 114541. DOI: 10.1016/j.expneurol.2023.114541 |
| [14] | FU C, WU Y, LIU S, et al. Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia[J]. J Ethnopharmacol, 2022, 289: 115021. doi: 10.1016/j.jep.2022.115021 . |
| [1] | LIU Yayi, JIA Yunfeng, ZUO Yiming, ZHANG Junping, LÜ Shichao. Progress and Evaluation of Animal Model of Heart Qi-Yin Deficiency Syndrome [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 411-421. |
| [2] | ZHAO Xin, WANG Chenxi, SHI Wenqing, LOU Yuefen. Advances in the Application of Zebrafish in the Research of Inflammatory Bowel Disease Mechanisms and Drug Development [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 422-431. |
| [3] | GONG Leilei, WANG Xiaoxia, FENG Xuewei, LI Xinlei, ZHAO Han, ZHANG Xueyan, FENG Xin. A Mouse Model and Mechanism Study of Premature Ovarian Insufficiency Induced by Different Concentrations of Cyclophosphamide [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 403-410. |
| [4] | JIANG Juan, SONG Ning, LIAN Wenbo, SHAO Congcong, GU Wenwen, SHI Yan. Comparison of Histopathological and Molecular Pathological Phenotypes in Mouse Models of Intrauterine Adhesions Induced by Two Concentrations of Ethanol Perfusion [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 393-402. |
| [5] | LUO Lianlian, YUAN Yanchun, WANG Junling, SHI Guangsen. Advances in Mouse Models of Amyotrophic Lateral Sclerosis [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 290-299. |
| [6] | PAN Yicong, JIANG Wenhong, HU Ming, QIN Xiao. Optimization of Surgical Procedure and Efficacy Evaluation of Aortic Calcification Model in Rats with Chronic Kidney Disease [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 279-289. |
| [7] | CHEN Yuhan, CHEN Jinling, LI Xin, OU Yanhua, WANG Si, CHEN Jingyi, WANG Xingyi, YUAN Jiali, DUAN Yuanyuan, YANG Zhongshan, NIU Haitao. Analysis of Animal Models of Myasthenia Gravis Based on Its Clinical Characteristics in Chinese and Western Medicine [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 176-186. |
| [8] | LIAN Hui, JIANG Yanling, LIU Jia, ZHANG Yuli, XIE Wei, XUE Xiaoou, LI Jian. Construction and Evaluation of a Rat Model of Abnormal Uterine Bleeding [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 130-146. |
| [9] | LUO Shixiong, ZHANG Sai, CHEN Hui. Research Progress in Establishment and Evaluation of Common Asthma Animal Models [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 167-175. |
| [10] | WANG Biying, LU Jiashuo, ZAN Guiying, CHEN Ruosong, CHAI Jingrui, LIU Jinggen, WANG Yujun. Establishment Methods and Application Progress of Rodent Models for Drug Addiction [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 158-166. |
| [11] | PAN Qianjia, GE Junyi, HU Nan, HUA Fei, GU Min. Differential Analysis of Oral Microbiota in db/db Mouse Model of Type 2 Diabetes Utilizing 16S rRNA Sequencing [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 147-157. |
| [12] | WANG Qianqian, TAO Sijue, WEI Zhen, JIN Huihui, LIU Ping, WANG Lie. A Case Study of Using Assisted Reproductive Technology to Rescue Genetically Modified Mice with Reproductive Disorder Phenotypes [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 79-86. |
| [13] | FEI Bin, GUO Wenke, GUO Jianping. Research Progress on Animal Models for Hernia Diseases and New Hernia Repair Materials [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 55-66. |
| [14] | ZHAO He, ZHANG Fan, XIAO Yuzhou, AN Xuefang, ZHANG Tao, LI Li. Preliminary Diagnosis and Characterization of a Spontaneous Immature Testicular Teratoma in an Interferon Receptor-Deficient Mouse Model [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 691-694. |
| [15] | YANG Jiahao, DING Chunlei, QIAN Fenghua, SUN Qi, JIANG Xusheng, CHEN Wen, SHEN Mengwen. Research Progress on Animal Models of Sepsis-Related Organ Injury [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 636-644. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||