
Laboratory Animal and Comparative Medicine ›› 2023, Vol. 43 ›› Issue (3): 243-252.DOI: 10.12300/j.issn.1674-5817.2022.187
• Experimental Animal and Comparative Pharmacology • Previous Articles Next Articles
Wenwen GUO1,2( ), Ya ZHAO2, Yinghua WANG2, Ke LIU2, Xu GE2, Yanying ZHANG1,3, Yongfeng WANG1(
), Ya ZHAO2, Yinghua WANG2, Ke LIU2, Xu GE2, Yanying ZHANG1,3, Yongfeng WANG1( )(
)( ), Changhong SHI2(
), Changhong SHI2( )(
)( )
)
Received:2022-12-06
Revised:2023-04-13
Online:2023-06-25
Published:2023-06-25
Contact:
Yongfeng WANG, Changhong SHI
CLC Number:
Wenwen GUO,Ya ZHAO,Yinghua WANG,et al. Repairing Effects of Ginsenoside Rg1 on Traumatic Brain Injury in Mice[J]. Laboratory Animal and Comparative Medicine, 2023, 43(3): 243-252. DOI: 10.12300/j.issn.1674-5817.2022.187.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2022.187
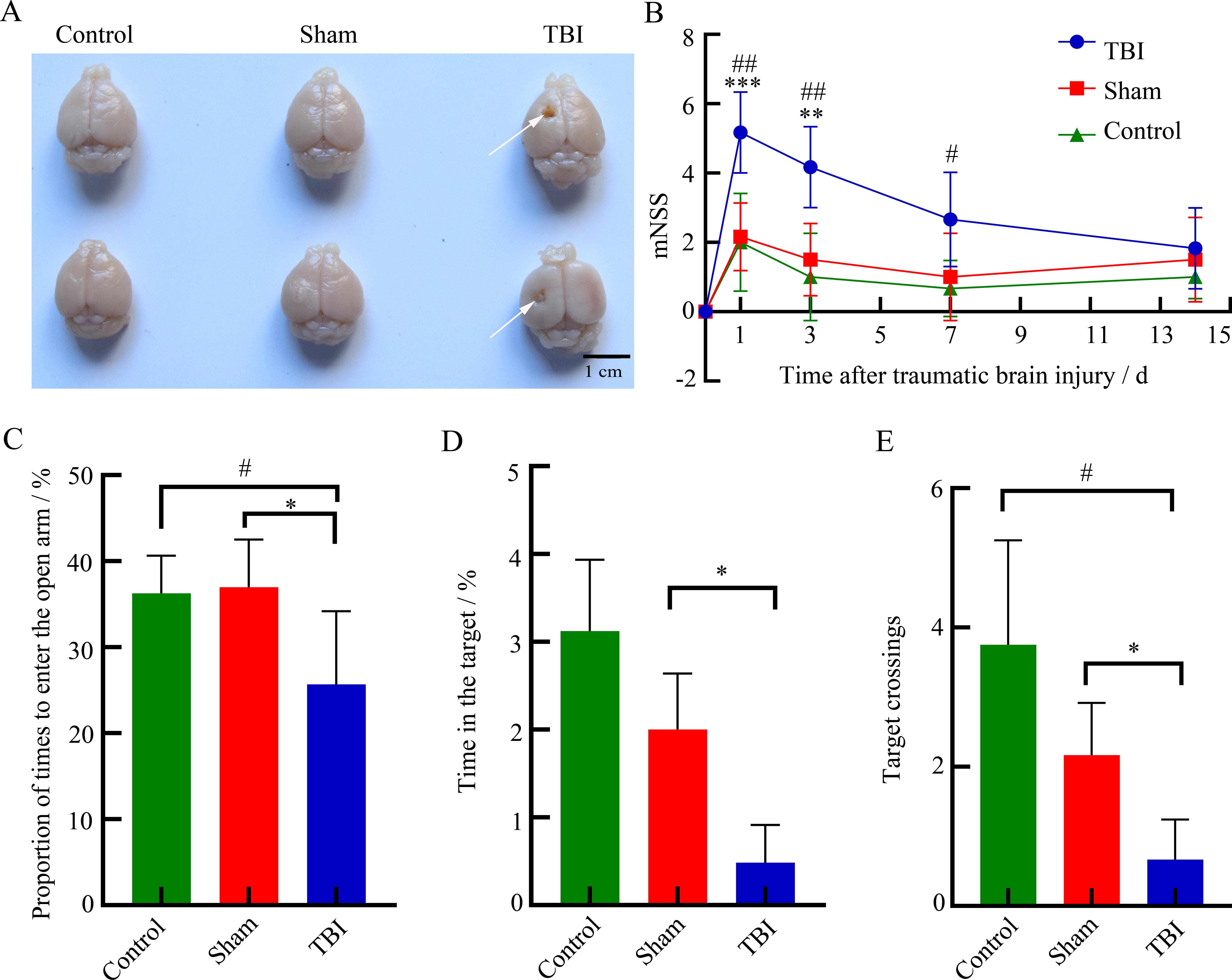
Figure 1 Establishment and evaluation of traumatic brain injury (TBI) model in miceNote: A, Observation of brain tissue injury (the white arrow refers to damaged brain region by TBI); B, Neurological function score (mNSS is the improved nerve injury severity score); C, Elevated cross maze experiment; D-E, Water maze experiment. Control is the blank group without any treatment; Sham is the sham operation group; TBI is TBI modeling group by controlled cortical impact method, with 9 mice in each group. Compared with the sham group, ?P<0.05, ??P<0.01, ???P<0.001; Compared with control group, #P<0.05, ##P<0.01.
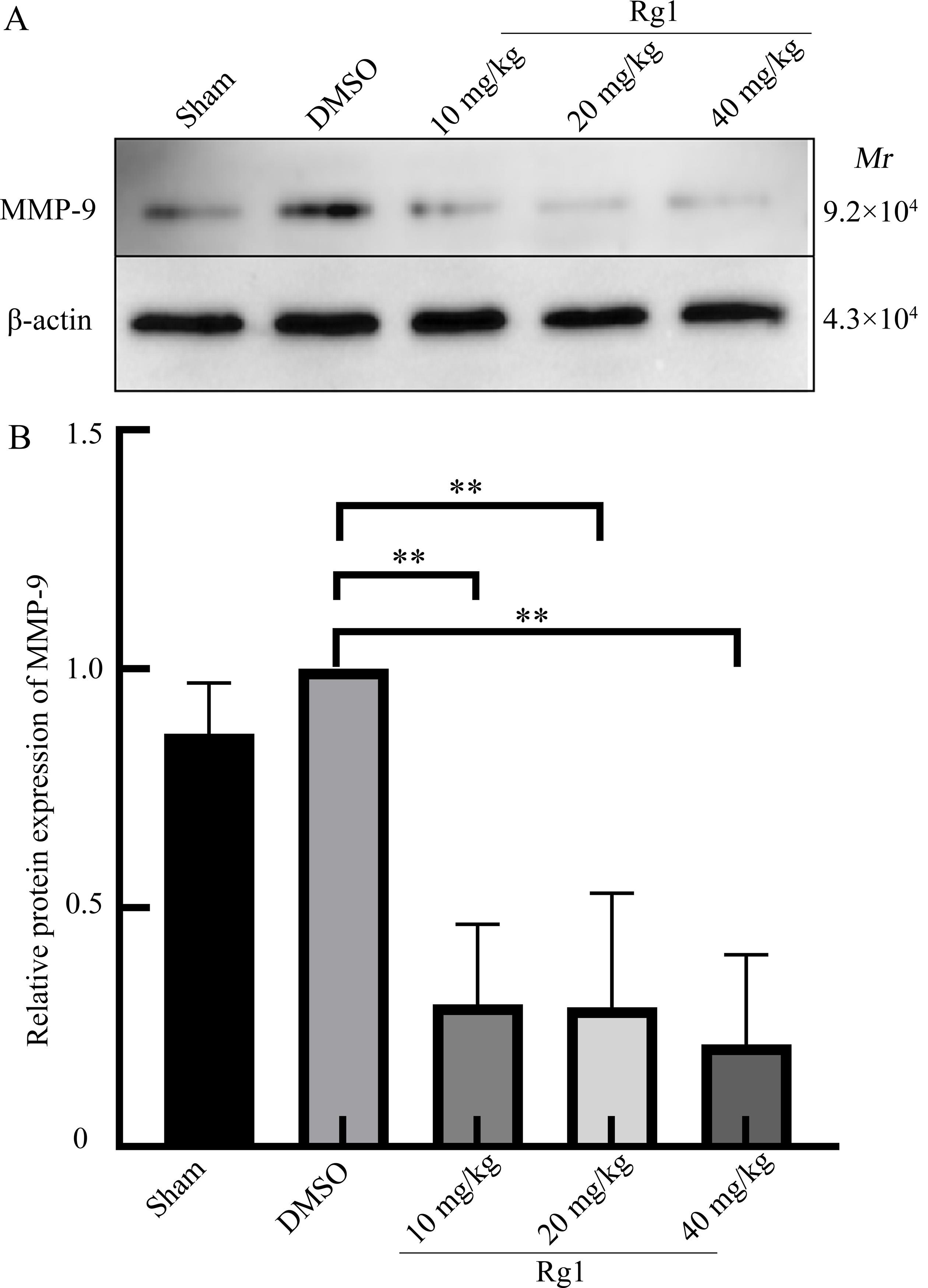
Figure 2 Effect of ginsenoside Rg1 on blood-brain barrier after traumatic brain injury (TBI) in miceNote: MMP-9, matrix metalloprotein-9. Sham is the sham operation group; DMSO is the solvent control group after TBI modeling by controlled cortical impact method; 10 mg/kg, 20 mg/kg, and 40 mg/kg Rg1 are 10, 20, 40 mg/kg of ginsenoside Rg1 treatment groups after TBI modeling by controlled cortical impact method, with 8 mice in each group. Compared with DMSO group, ??P<0.01.
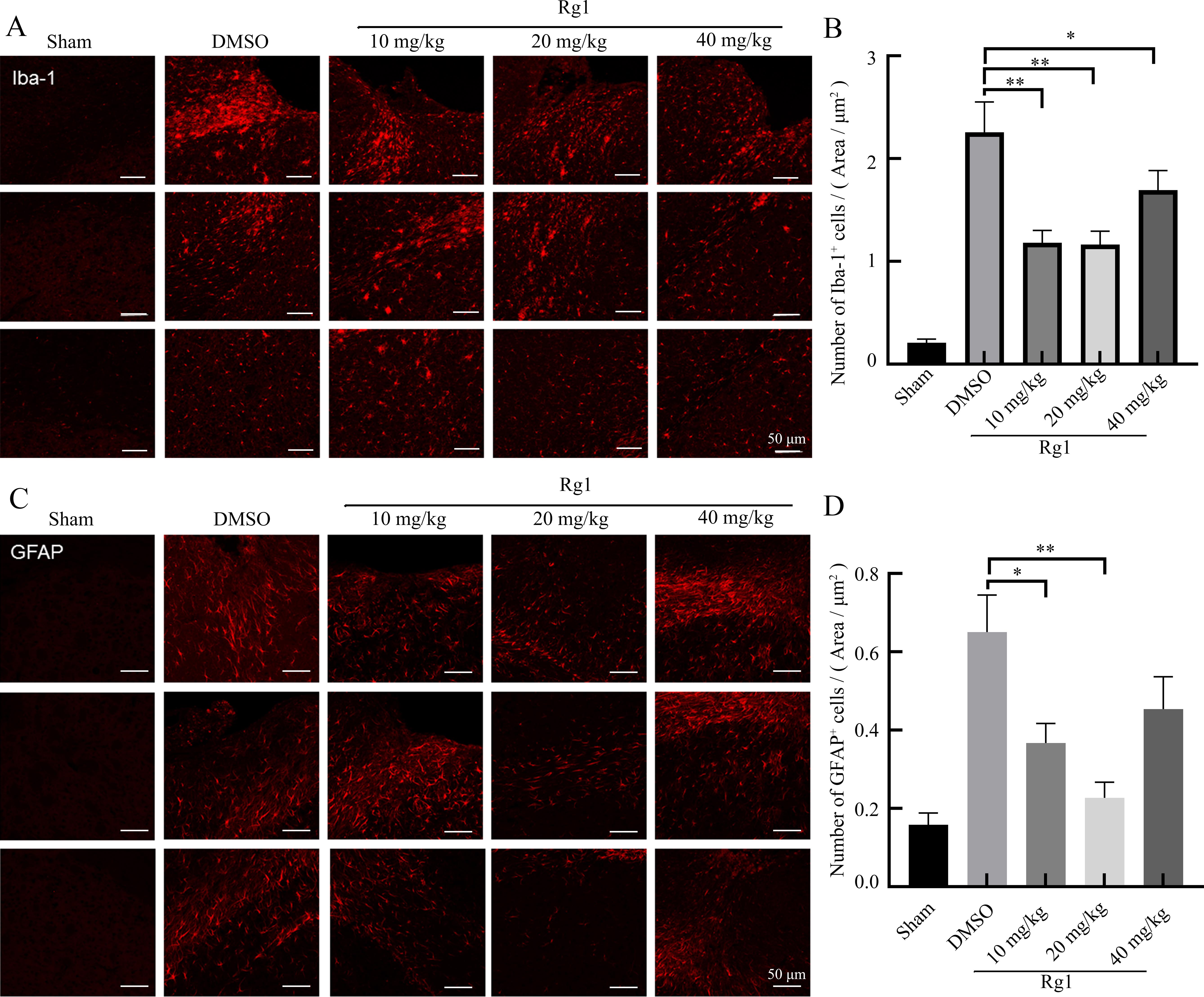
Figure 3 Effect of ginsenoside Rg1 on microglia and astrocytes 28 d after traumatic brain injury (TBI) in miceNote: A-B show ionized calcium binding adapter molecule 1 (Iba-1) protein expression (reflecting microglia activity) and the number of Iba-1 positive cells in brain tissues of mice in each group detected by immunofluorescence staining. C-D show glial fibrillary acidic protein (GFAP) expression (reflecting the activity of astrocytes) and the number of GFAP positive cells in the brain tissue of mice in each group detected by immunofluorescence staining. In Figures A and C, the top, middle, and bottom three photos in each group show three slices of similar layers in the brain tissue of three mice. Sham is the sham operation group; DMSO is the solvent control group after TBI modeling by controlled cortical impact method; 10 mg/kg, 20 mg/kg, 40 mg/kg Rg1 are 10, 20, 40 mg/kg of ginsenoside Rg1 treatment groups after TBI modeling by controlled cortical impact method, with 8 mice in each group. Compared with DMSO group, ?P<0.05, ??P<0.01.
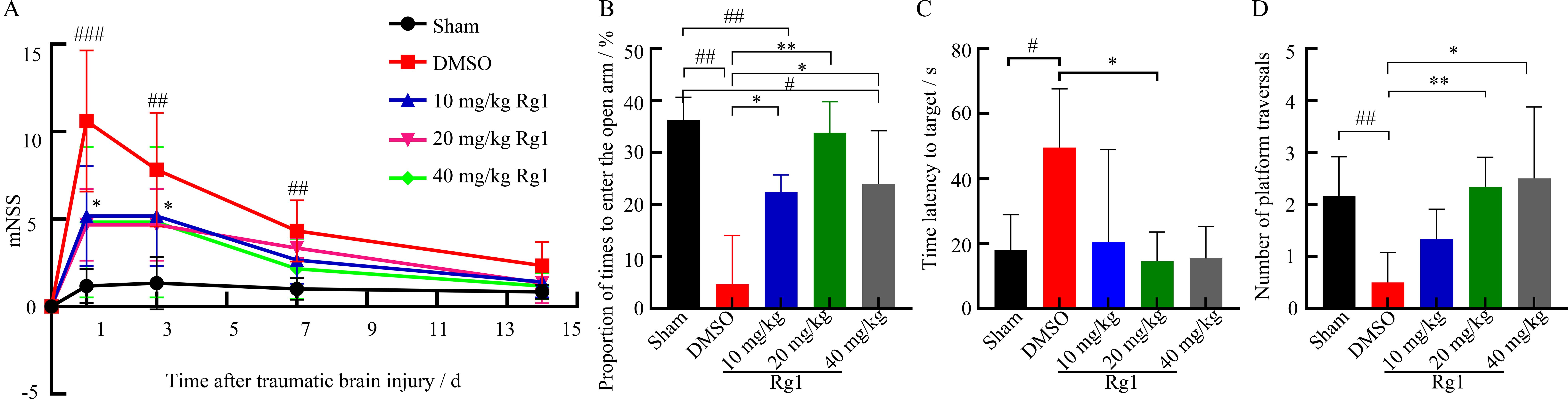
Figure 4 Effect of ginsenoside Rg1 on behavioral performance in mice after traumatic brain injury (TBI)Note: A, mNSS neurological function score; B, Elevated cross maze experiment; C-D, water maze experiment. Sham is the sham operation group; DMSO is the solvent control group after TBI modeling by controlled cortical impact method; 10 mg/kg, 20 mg/kg, 40 mg/kg Rg1 are 10, 20, 40 mg/kg of ginsenoside Rg1 treatment groups after TBI modeling by controlled cortical impact method, with 8 mice in each group. Compared with Sham group, #P<0.05, ##P<0.01; compared with DMSO group, ?P<0.05, ??P<0.01.
| 1 | LANGLOIS J A, RUTLAND-BROWN W, WALD M M. The epidemiology and impact of traumatic brain injury: a brief overview[J]. J Head Trauma Rehabil, 2006, 21(5):375-378. DOI: 10.1097/00001199-200609000-00001 . |
| 2 | KATZ D I, BERNICK C, DODICK D W, et al. National institute of neurological disorders and stroke consensus diagnostic criteria for traumatic encephalopathy syndrome[J]. Neurology, 2021, 96(18):848-863. DOI: 10.1212/WNL. 0000000000011850 . |
| 3 | SILVERBERG N D, IACCARINO M A, PANENKA W J, et al. Management of concussion and mild traumatic brain injury: a synthesis of practice guidelines[J]. Arch Phys Med Rehabil, 2020, 101(2):382-393. DOI: 10.1016/j.apmr.2019.10.179 . |
| 4 | GALGANO M, TOSHKEZI G, QIU X C, et al. Traumatic brain injury: current treatment strategies and future endeavors[J]. Cell Transplant, 2017, 26(7):1118-1130. DOI: 10.1177/0963689717714102 . |
| 5 | AHMED T, RAZA S H, MARYAM A, et al. Ginsenoside Rb1 as a neuroprotective agent: a review[J]. Brain Res Bull, 2016, 125:30-43. DOI: 10.1016/j.brainresbull.2016.04.002 . |
| 6 | GAO J, BAI H J, LI Q, et al. In vitro investigation of the mechanism underlying the effect of ginsenoside on the proliferation and differentiation of neural stem cells subjected to oxygen-glucose deprivation/reperfusion[J]. Int J Mol Med, 2018, 41(1):353-363. DOI: 10.3892/ijmm.2017.3253 . |
| 7 | WU Y X, WU H J, ZENG J X, et al. Mild traumatic brain injury induces microvascular injury and accelerates Alzheimer-like pathogenesis in mice[J]. Acta Neuropathol Commun, 2021, 9(1):74. DOI: 10.1186/s40478-021-01178-7 . |
| 8 | CUI W X, WU X, FENG D Y, et al. Acrolein induces systemic coagulopathy via autophagy-dependent secretion of von willebrand factor in mice after traumatic brain injury[J]. Neurosci Bull, 2021, 37(8):1160-1175. DOI: 10.1007/s12264-021-00681-0 . |
| 9 | CHEN Y F, LI J, MA B T, et al. MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat[J]. Aging (Albany NY), 2020, 12(18):18274-18296. DOI: 10.18632/aging.103692 . |
| 10 | LONG X B, YAO X L, JIANG Q, et al. Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury[J]. J Neuroinflammation, 2020, 17(1):89. DOI: 10.1186/s12974-020-01761-0 . |
| 11 | ZHANG Y L, ZHANG Y, CHOPP M, et al. Mesenchymal stem cell-derived exosomes improve functional recovery in rats after traumatic brain injury: a dose-response and therapeutic window study[J]. Neurorehabil Neural Repair, 2020, 34(7):616-626. DOI: 10.1177/1545968320926164 . |
| 12 | ARAKI T, YOKOTA H, MORITA A. Pediatric traumatic brain injury: characteristic features, diagnosis, and management[J]. Neurol Med Chir (Tokyo), 2017, 57(2):82-93. DOI: 10.2176/nmc.ra.2016-0191 . |
| 13 | 雷勋明. 人参皂苷Rg1对新生鼠缺氧缺血性脑损伤海马神经元凋亡及学习记忆能力的影响[J]. 中国中西医结合儿科学, 2018, 10(4): 277-279. DOI: 10.3969/j.issn.1674-3865.2018.04.001 . |
| LEI X M. Effects of ginseng Rg1 on hippocampal neuronal apoptosis and learning ability of neonatal rats with hypoxic-ischemic brain damage[J]. Chin Pediatr Integr Tradit West Med, 2018, 10(4): 277-279. DOI: 10.3969/j.issn.1674-3865.2018.04.001 . | |
| 14 | SONG X Y, HU J F, CHU S F, et al. Ginsenoside Rg1 attenuates okadaic acid induced spatial memory impairment by the GSK3β/tau signaling pathway and the Aβ formation prevention in rats[J]. Eur J Pharmacol, 2013, 710(1-3):29-38. DOI: 10.1016/j.ejphar.2013.03.051 . |
| 15 | ZOU S F, CHEN W, DING H, et al. Involvement of autophagy in the protective effects of ginsenoside Rb1 in a rat model of traumatic brain injury[J]. Eur J Drug Metab Pharmacokinet, 2022, 47(6):869-877. DOI: 10.1007/s13318-022-00799-0 . |
| 16 | ZHANG Z R, SONG Z J, SHEN F M, et al. Ginsenoside Rg1 prevents PTSD-like behaviors in mice through promoting synaptic proteins, reducing Kir4.1 and TNF-α in the Hippocampus[J]. Mol Neurobiol, 2021, 58(4):1550-1563. DOI: 10.1007/s12035-020-02213-9 . |
| 17 | HU B Y, LIU X J, QIANG R, et al. Treatment with ginseng total saponins improves the neurorestoration of rat after traumatic brain injury[J]. J Ethnopharmacol, 2014, 155(2):1243-1255. DOI: 10.1016/j.jep.2014.07.009 . |
| 18 | ZHAI K F, DUAN H, WANG W, et al. Ginsenoside Rg1 ameliorates blood-brain barrier disruption and traumatic brain injury via attenuating macrophages derived exosomes miR-21 release[J]. Acta Pharm Sin B, 2021, 11(11):3493-3507. DOI: 10.1016/j.apsb.2021.03.032 . |
| 19 | DHANDA S, SANDHIR R. Blood-brain barrier permeability is exacerbated in experimental model of hepatic encephalopathy via MMP-9 activation and downregulation of tight junction proteins[J]. Mol Neurobiol, 2018, 55(5):3642-3659. DOI: 10.1007/s12035-017-0521-7 . |
| 20 | REMPE R G, HARTZ A M S, BAUER B. Matrix metallo-proteinases in the brain and blood-brain barrier: versatile breakers and makers[J]. J Cereb Blood Flow Metab, 2016, 36(9):1481-1507. DOI: 10.1177/0271678X16655551 . |
| 21 | LI Y H, MENG Q, YANG M B, et al. Current trends in drug metabolism and pharmacokinetics[J]. Acta Pharm Sin B, 2019, 9(6):1113-1144. DOI: 10.1016/j.apsb.2019.10.001 . |
| 22 | XIONG Y, MAHMOOD A, CHOPP M. Animal models of traumatic brain injury[J]. Nat Rev Neurosci, 2013, 14(2):128-142. DOI: 10.1038/nrn3407 . |
| 23 | KARVE I P, TAYLOR J M, CRACK P J. The contribution of astrocytes and microglia to traumatic brain injury[J]. Br J Pharmacol, 2016, 173(4):692-702. DOI: 10.1111/bph.13125 . |
| 24 | SOFRONIEW M V, VINTERS H V. Astrocytes: biology and pathology[J]. Acta Neuropathol, 2010, 119(1):7-35. DOI: 10.1007/s00401-009-0619-8 . |
| 25 | VILLAPOL S, BYRNES K R, SYMES A J. Temporal dynamics of cerebral blood flow, cortical damage, apoptosis, astrocyte-vasculature interaction and astrogliosis in the pericontusional region after traumatic brain injury[J]. Front Neurol, 2014, 5:82. DOI: 10.3389/fneur.2014.00082 . |
| 26 | KOLIATSOS V E, RAO V. The behavioral neuroscience of traumatic brain injury[J]. Psychiatr Clin North Am, 2020, 43(2):305-330. DOI: 10.1016/j.psc.2020.02.009 . |
| [1] | LIU Yueqin, XUE Weiguo, WANG Shuyou, SHEN Yaohua, JIA Shuyong, WANG Guangjun, SONG Xiaojing. Observation of Digestive Tract Tissue Morphology in Mice Using Probe-Based Confocal Laser Endomicroscopy [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 457-465. |
| [2] | KONG Zhihao, WEI Xiaofeng, YU Lingzhi, FENG Liping, ZHU Qi, SHI Guojun, WANG Chen. Isolation and Identification of Staphylococcus xylosus in Nude Mice with Squamous Skin Scurfs [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 368-375. |
| [3] | XU Qiuyu, YAN Guofeng, FU Li, FAN Wenhua, ZHOU Jing, ZHU Lian, QIU Shuwen, ZHANG Jie, WU Ling. A Mouse Model of Polycystic Ovary Syndrome Established Through Subcutaneous Administration of Letrozole Sustained-Release Pellets and Hepatic Transcriptome Analysis [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 119-129. |
| [4] | LIU Rongle, CHENG Hao, SHANG Fusheng, CHANG Shufu, XU Ping. Study on Cardiac Aging Phenotypes of SHJH hr Mice [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 13-20. |
| [5] | WU Zhihao, CAO Shuyang, ZHOU Zhengyu. Establishment of an Intestinal Fibrosis Model Associated with Inflammatory Bowel Disease in VDR-/- Mice Induced by Helicobacter hepaticus Infection and Mechanism Exploration [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 37-46. |
| [6] | ZHANG Nan, LI Huaiyin, LIAN Xiaodi, WEI Juanpeng, GAO Ming. Effects of Different Durations of Light Exposure on Body Weight and Learning and Memory Abilities of NIH Mice [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 73-78. |
| [7] | ZHAO Xiaona, WANG Peng, YE Maoqing, QU Xinkai. Establishment of a New Hyperglycemic Obesity Cardiac Dysfunction Mouse Model with Triacsin C [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 605-612. |
| [8] | TAN He, YANG Xiaohui, ZHANG Daxiu, WANG Guicheng. Optimal Adaptation Period for Metabolic Cage Experiments in Mice at Different Developmental Stages [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 502-510. |
| [9] | MENG Yu, LIANG Dongli, ZHENG Linlin, ZHOU Yuanyuan, WANG Zhaoxia. Optimization and Evaluation of Conditions for Orthotopic Nude Mouse Models of Human Liver Tumor Cells [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 511-522. |
| [10] | Jing QIN, Yong ZHAO, Caiqin ZHANG, Bing BAI, Changhong SHI. Construction and Evaluation of Theranostic Near-infrared Fluorescent Probe for Targeting Inflammatory Brain Edema [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 243-250. |
| [11] | Yisu ZHANG, Xinru LIU, Ruojie WU, Rui LIU, Hong OUYANG, Xiaohong LI. Establishment and Evaluation of Mouse Model of Pregnancy Pain-depression Comorbidity Induced by Chronic Unpredictable Stress, Complete Freund's Adjuvant and Formalin [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 259-269. |
| [12] | Dong WU, Rui SHI, Peishan LUO, Ling'en LI, Xijing SHENG, Mengyang WANG, Lu NI, Sujuan WANG, Huixin YANG, Jing ZHAO. Effects of Different Pellet Feed Hardness on Growth and Reproduction, Feed Utilization Rate, and Environmental Dust in Laboratory Mice [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 313-320. |
| [13] | Yun LIU, Tingting FENG, Wei TONG, Zhi GUO, Xia LI, Qi KONG, Zhiguang XIANG. Glycyrrhizic Acid Showed Therapeutic Effects on Severe Pulmonary Damages in Mice Induced by Pneumonia Virus of Mice Infection [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 251-258. |
| [14] | Jinhua HU, Jingjie HAN, Min JIN, Bin HU, Yuefen LOU. Effects of Puerarin on Bone Density in Rats and Mice: A Meta-analysis [J]. Laboratory Animal and Comparative Medicine, 2024, 44(2): 149-161. |
| [15] | Min LIANG, Yang GUO, Jinjin WANG, Mengyan ZHU, Jun CHI, Yanjuan CHEN, Chengji WANG, Zhilan YU, Ruling SHEN. Construction of Dmd Gene Mutant Mice and Phenotype Verification in Muscle and Immune Systems [J]. Laboratory Animal and Comparative Medicine, 2024, 44(1): 42-51. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||