
Laboratory Animal and Comparative Medicine ›› 2023, Vol. 43 ›› Issue (3): 282-287.DOI: 10.12300/j.issn.1674-5817.2022.166
• Model Animals and Animal Models • Previous Articles Next Articles
Jinxian YANG1,2( ), Shujuan WANG1, Jinyun ZHAI1, Shunxing ZHU1,2(
), Shujuan WANG1, Jinyun ZHAI1, Shunxing ZHU1,2( )(
)( )
)
Received:2022-10-31
Revised:2023-04-17
Online:2023-06-25
Published:2023-06-25
Contact:
Shunxing ZHU
CLC Number:
Jinxian YANG,Shujuan WANG,Jinyun ZHAI,et al. Downregulation of Micall2a Gene Expression Inhibited Vascular Development in Zebrafish[J]. Laboratory Animal and Comparative Medicine, 2023, 43(3): 282-287. DOI: 10.12300/j.issn.1674-5817.2022.166.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2022.166
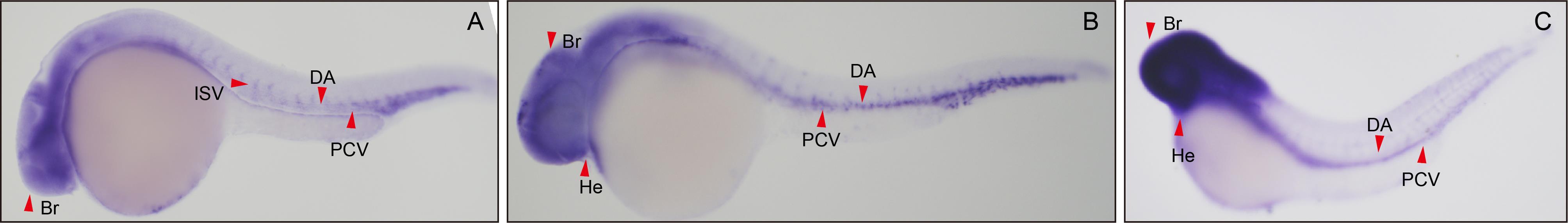
Figure 1 Expression of the Micall2a gene in the vascular system of zebrafish embryosNote: A-C, Micall2a expression levels at the 24th, 36th, and 48th hours post fertilization in the zebrafish. ISV, intersegmental vessel; DA, dorsal aorta; PCV, posterior cardinal vein; Br, brain; He, heart. Red arrow indicates the location.
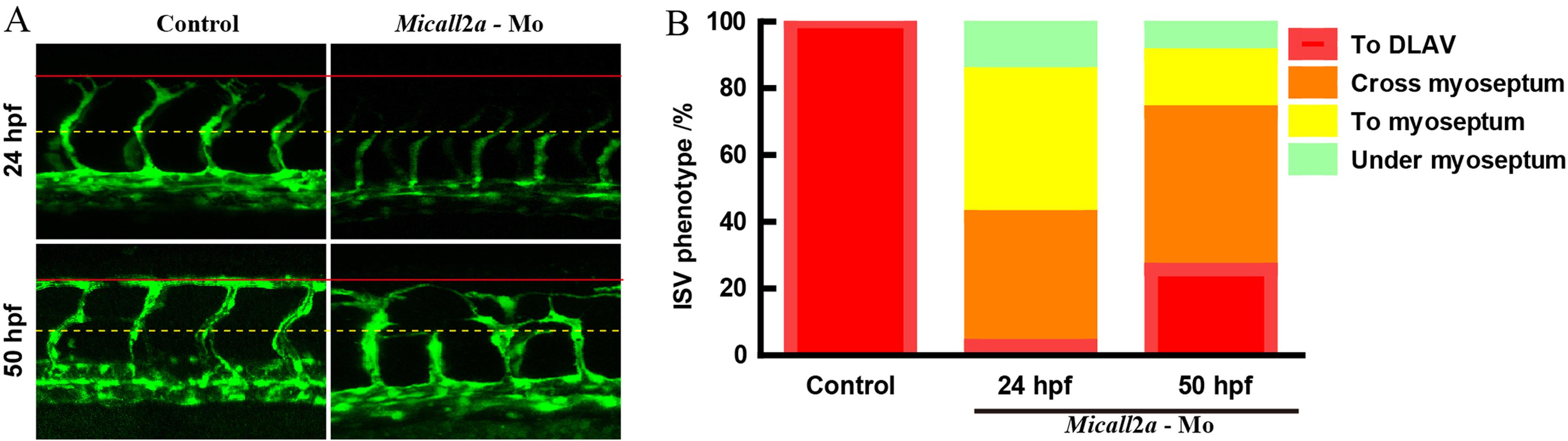
Figure 2 Morphology of zebrafish embryos larval internodal vascular after the knock-down of Micall2a gene at 24 and 50 hpfNote:(A) Laser confocal microscope image of internode vascular morphology in zebrafish embryos with microinjection of morpholine antisense oligonucleotide knockdown of the expression of Micall2a in Tg (fli :GFP) transgenic lines (Micall2a-Mo group) and injection of the same volume of PBS in transgenic zebrafish (control group) cultured to 24 and 50 hpf. The dorsal longitudinal anastomosis vessel (DLAV) is indicated by a solid red line and the horizontal septum by a yellow dashed line. (B) Phenotypic proportion of internodal vascular (ISV) growth in zebrafish embryos (red indicates blood vessel growth to the dorsal longitudinal anastomotic blood vessel, orange indicates growth through the myseptum, yellow indicates growth to the transverse myodiam, and green indicates submyspheric growth). 10 embryos per group and 7 ISVs per embryo were counted.
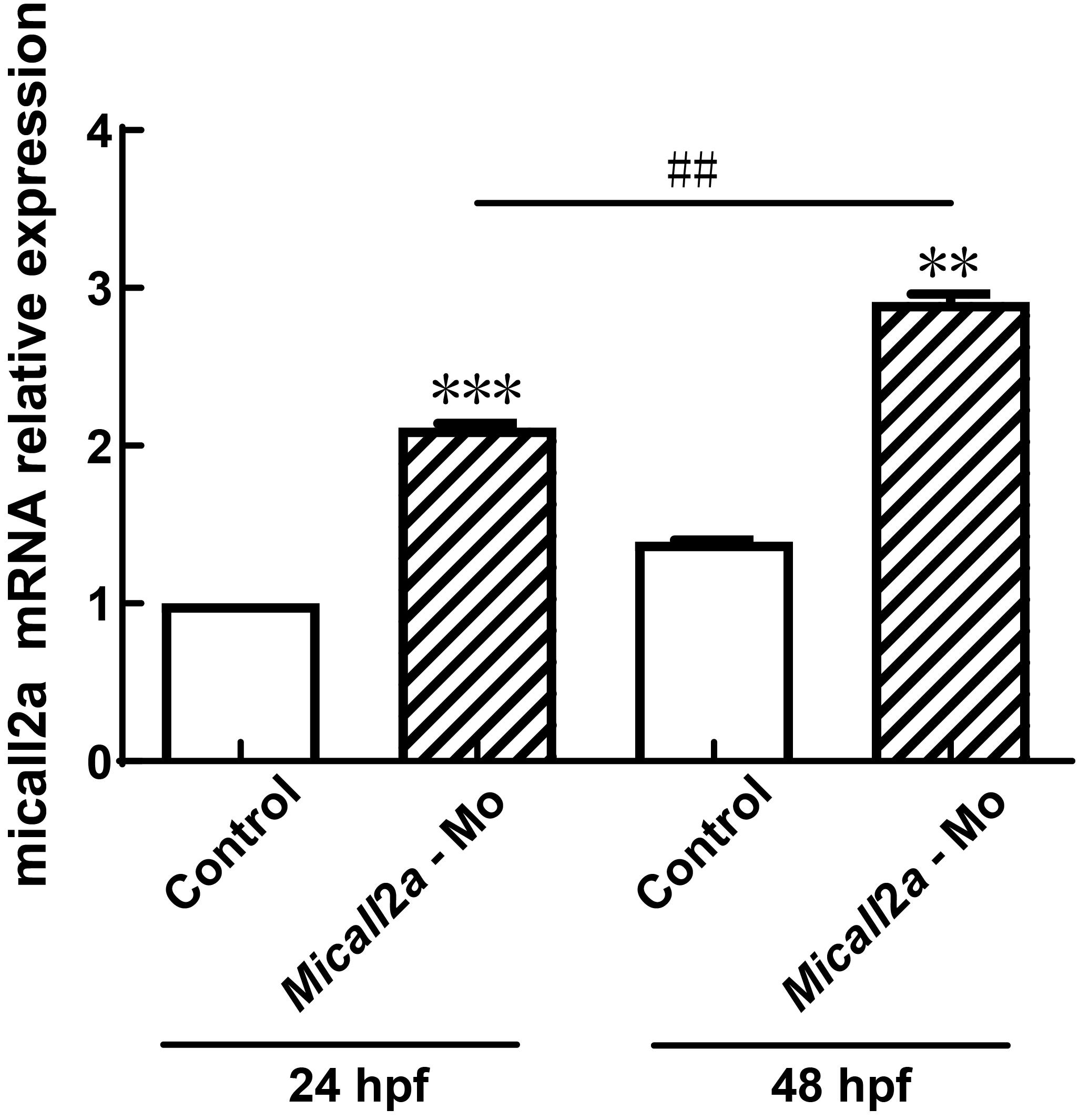
Figure 3 Real-time PCR analysis of Micall2a gene expression changes in zebrafish embryos after injection of Micall2a-MoNote:The Micall2a-Mo group was microinjected with morpholine antisense oligonucleotide and the expression of Micall2a in Tg (fli:GFP) transgenic lines and cultured to 24 and 50 hpf. The control group was injected with an equal volume of PBS. Compared with the control group, ??P<0.01, ???P<0.001. Compared with 24 hpf, ##P<0.01. The experiment was repeated three times for each group of 15 embryos.
| 1 | HUNG R J, YAZDANI U, YOON J, et al. Mical links semaphorins to F-actin disassembly[J]. Nature, 2010, 463(7282):823-827. DOI: 10.1038/nature08724 . |
| 2 | BEUCHLE D, SCHWARZ H, LANGEGGER M, et al. Drosophila MICAL regulates myofilament organization and synaptic structure[J]. Mech Dev, 2007, 124(5):390-406. DOI: 10.1016/j.mod.2007.01.006 . |
| 3 | WEIDE T, TEUBER J, BAYER M, et al. MICAL-1 isoforms, novel rab1 interacting proteins[J]. Biochem Biophys Res Commun, 2003, 306(1):79-86. DOI: 10.1016/s0006-291x(03)00918-5 . |
| 4 | XUE Y L, KUOK C XIAO A,et al. Identification and expression analysis of mical family genes in zebrafish[J]. J Genet Genom, 2010, 37(10):685-693. DOI: 10.1016/S1673-8527(09)60086-2 . |
| 5 | YANG Y X, YE F W, XIA T X, et al. High MICAL-L2 expression and its role in the prognosis of colon adenocarcinoma[J]. BMC Cancer, 2022, 22(1):487. DOI: 10.1186/s12885-022-09614-0 . |
| 6 | WU H, YESILYURT H G, YOON J, et al. The MICALs are a family of F-actin dismantling oxidoreductases conserved from drosophila to humans[J]. Sci Rep, 2018, 8(1):937. DOI: 10.1038/s41598-017-17943-5 . |
| 7 | WANG Y, DENG W, ZHANG Y, et al. MICAL2 promotes breast cancer cell migration by maintaining epidermal growth factor receptor (EGFR) stability and EGFR/P38 signalling activation[J]. Acta Physiol (Oxf), 2018, 222(2):10.1111/apha.12920. DOI: 10.1111/apha.12920 . |
| 8 | BAKKERS J. Zebrafish as a model to study cardiac development and human cardiac disease[J]. Cardiovasc Res, 2011, 91(2):279-288. DOI: 10.1093/cvr/cvr098 . |
| 9 | HOGAN B M, SCHULTE-MERKER S. How to plumb a Pisces: understanding vascular development and disease using zebrafish embryos[J]. Dev Cell, 2017, 42(6):567-583. DOI: 10.1016/j.devcel.2017.08.015 . |
| 10 | 江霞, 钱豪杰, 魏迅, 等. 斑马鱼糖尿病模型的构建及应用进展[J]. 实验动物与比较医学, 2020, 40(6):547-552. DOI: 10.3969/j.issn.1674-5817.2020.06.016 . |
| JIANG X, QIAN H J, WEI X, et al. Research progress in construction and application of diabetes model in zebrafish[J]. Lab Anim Comp Med, 2020, 40(6):547-552. DOI: 10.3969/j.issn.1674-5817.2020.06.016 . | |
| 11 | Folkman J. Angiogenesis:an organizing principle for drug discovery[J].Nat Rev Drug Discov, 2007,6(4):273-286.DOI:10.1038/nrd2115 . |
| 12 | WANG J L, ZHANG X H, XU X Y, et al. Pro-angiogenic activity of Tongnao Decoction on HUVECs in vitro and zebrafish in vivo [J]. J Ethnopharmacol, 2020, 254:112737. DOI: 10.1016/j.jep.2020.112737 . |
| 13 | CASEY M J, STEWART R A. Zebrafish as a model to study neuroblastoma development[J]. Cell Tissue Res, 2018, 372(2):223-232. DOI: 10.1007/s00441-017-2702-0 . |
| 14 | CHITRAMUTHU B P, BENNETT H P J. High resolution whole mount in situ hybridization within zebrafish embryos to study gene expression and function[J]. J Vis Exp, 2013(80): e50644. DOI: 10.3791/50644 . |
| 15 | 张晶晶, 王新, 刘东. C型利钠肽基因调控斑马鱼胚胎血管发育[J]. 生理学报, 2017, 69(1): 11-16. DOI: 10.13294/j.aps.2016.0098 . |
| ZHANG J J, WANG X, LIU D. C-type natriuretic peptide gene regulates the vascular development of zebrafish embryos[J]. Acta Physiol Sin, 2017, 69(1): 11-16. DOI: 10.13294/j.aps.2016.0098 . | |
| 16 | SUMMERTON J E. Morpholino, siRNA, and S-DNA compared: impact of structure and mechanism of action on off-target effects and sequence specificity[J]. Curr Top Med Chem, 2007, 7(7):651-660. DOI: 10.2174/156802607780487740 . |
| 17 | ZHOU Y P, GUNPUT R A F, ADOLFS Y, et al. MICALs in control of the cytoskeleton, exocytosis, and cell death[J]. Cell Mol Life Sci, 2011, 68(24):4033-4044. DOI: 10.1007/s00018-011-0787-2 . |
| 18 | RAHAJENG J, GIRIDHARAN S S P, CAI B S, et al. Important relationships between Rab and MICAL proteins in endocytic trafficking[J]. World J Biol Chem, 2010, 1(8):254-264. DOI: 10.4331/wjbc.v1.i8.254 . |
| 19 | TERAI T, NISHIMURA N, KANDA I, et al. JRAB/MICAL-L2 is a junctional Rab13-binding protein mediating the endocytic recycling of occludin[J]. Mol Biol Cell, 2006, 17(5):2465-2475. DOI: 10.1091/mbc.e05-09-0826 . |
| 20 | 杨晋娴, 王新, 刘东, 等. Clec14a基因调控斑马鱼胚胎血管发育[J]. 交通医学, 2018, 32(6):535-539. DOI: 10.1016/j.bbrc.2016.12.118 . |
| YANG J X, WANG X, LIU D, et al. Clec14a gene contributes to zebrafish angiogenesis[J]. Med J Commun, 2018, 32(6): 535-539. DOI: 10.1016/j.bbrc.2016.12.118 . | |
| 21 | DIOMEDE F, DILETTA MARCONI G, FONTICOLI L, et al. Functional relationship between osteogenesis and angiogenesis in tissue regeneration[J]. Int J Mol Sci, 2020, 21(9):3242. DOI: 10.3390/ijms21093242 . |
| 22 | 王晓航, 陈洋, 戚中田, 等. 血脑屏障动物模型的研究进展[J]. 海军军医大学学报, 2022(3):314-319. |
| WANG X H, CHEN Y, QI Z T, et al. Animal models of blood-brain barrier: research progress[J]. Acad J Nav Med Univ, 2022(3):314-319. |
| [1] | ZHAO Xin, WANG Chenxi, SHI Wenqing, LOU Yuefen. Advances in the Application of Zebrafish in the Research of Inflammatory Bowel Disease Mechanisms and Drug Development [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 422-431. |
| [2] | Jinxing LIN, Xindong WANG, Xuebing BAI, Liping FENG, Shuwu XIE, Qiusheng CHEN. Fine Structure of the Trunk Kidney and Distribution of Its Secreted Exosomes in the Adult Zebrafish [J]. Laboratory Animal and Comparative Medicine, 2023, 43(5): 531-540. |
| [3] | Zhigang TAN, Jinxin LIU, Chuya ZHENG, Wenfeng LIAO, Luping FENG, Hongli PENG, Xiu YAN, Zhenjian ZHUO. Advances and Applications in Animal Models of Neuroblastoma [J]. Laboratory Animal and Comparative Medicine, 2023, 43(3): 288-296. |
| [4] | Shirong JIN, Ye HUA, Huaxing ZI, Xufei DU, Jiwen BU. An Optimized Experimental Zebrafish Breeding Scheme for Significantly Enhancing Reproductive Efficiency and Service Life [J]. Laboratory Animal and Comparative Medicine, 2023, 43(3): 297-306. |
| [5] | JIANG Xia, QIAN Haojie, WEI Xun, ZHENG Yuxuan, ZHOU Zhengyu. Research Progress in Construction and Application of Diabetes Model in Zebrafish [J]. Laboratory Animal and Comparative Medicine, 2020, 40(6): 547-552. |
| [6] | DI Yanan, ZHU Liying, QIAN Wen, PAN Wei. Application of Zebrafish as A Model Animal in Research of Human Eye Diseases [J]. Laboratory Animal and Comparative Medicine, 2020, 40(5): 440-. |
| [7] | WANG Xue, LIU Kechun, YANG Xueliang, MA Yukui, ZHANG Yun. Impacts of Okadaic Acid on Neurological Function and Behavior of Zebrafish Larvae#br# [J]. Laboratory Animal and Comparative Medicine, 2020, 40(3): 190-. |
| [8] | Li Ying-niang, Dai Wei, Sheng Jian. Analysis of Goiter in Zebrafish [J]. Laboratory Animal and Comparative Medicine, 2019, 39(6): 462-466. |
| [9] | WANG Li-mei, ZHANG Jian, LIU Zhong-hua, YANG Wei. Research Progress on Zebrafish Model of Retinoblastoma and rb1 Gene [J]. Laboratory Animal and Comparative Medicine, 2019, 39(3): 244-248. |
| [10] | ZHOU Bin, SHENG Zhe-jin, FENG Chen-zhuo, LI Li-mei. A Melanoma Zebrafish Model for Real-time Imaging in vivo [J]. Laboratory Animal and Comparative Medicine, 2018, 38(1): 22-28. |
| [11] | TIAN Fang, WANG Yu-zhu, XIA Min-jie, SUN Bing, DING Xun-cheng, LI Wei-hua, XU Hui-hui, HU Jing-ying. Optimization on Methodology of Histopathological Examination in Adult Zebrafish Visceral Tissues [J]. Laboratory Animal and Comparative Medicine, 2017, 37(6): 465-469. |
| [12] | JIN Lu, LI Yan, LI Zhi-cao, WU Xi-jun, ZHOU Yan-hua, HE Zhi-xu, SHU Li-ping. Effects of Irx5a Gene Overexpression on Early Hematopoietic in Zebrafish Embryos [J]. Laboratory Animal and Comparative Medicine, 2017, 37(4): 309-314. |
| [13] | LIN Jin-xing, FENG Li-ping, HU Jian-hua, GAO Cheng. Microscopical Observation on Pigment Cells in Fins and Scales of Zebrafish [J]. Laboratory Animal and Comparative Medicine, 2017, 37(2): 94-101. |
| [14] | ZHANG Cheng-da, ZHANG Li-li, ZHANG Li-jiang, CHEN Yun-xiang. Estabilishment of Cardiotoxicity Model in Zebrafish Induced by Doxorubicin [J]. Laboratory Animal and Comparative Medicine, 2015, 35(5): 362-366. |
| [15] | ZHANG Yong, ZHU Xiao-yu, GUO Sheng-ya, LI Chun-qi. Zebrafish Thrombosis Model Induced by Phenyl Hydrazine [J]. Laboratory Animal and Comparative Medicine, 2015, 35(1): 27-31. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||