
Laboratory Animal and Comparative Medicine ›› 2023, Vol. 43 ›› Issue (3): 288-296.DOI: 10.12300/j.issn.1674-5817.2022.194
• Model Animals and Animal Models • Previous Articles Next Articles
Zhigang TAN1,2( ), Jinxin LIU1,2, Chuya ZHENG1,2, Wenfeng LIAO1,2, Luping FENG1,2, Hongli PENG1,2, Xiu YAN3, Zhenjian ZHUO1,2(
), Jinxin LIU1,2, Chuya ZHENG1,2, Wenfeng LIAO1,2, Luping FENG1,2, Hongli PENG1,2, Xiu YAN3, Zhenjian ZHUO1,2( )(
)( )
)
Received:2022-12-29
Revised:2023-04-11
Online:2023-06-25
Published:2023-06-25
Contact:
Zhenjian ZHUO
CLC Number:
Zhigang TAN,Jinxin LIU,Chuya ZHENG,et al. Advances and Applications in Animal Models of Neuroblastoma[J]. Laboratory Animal and Comparative Medicine, 2023, 43(3): 288-296. DOI: 10.12300/j.issn.1674-5817.2022.194.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2022.194
细胞系 Cell line | 小鼠品系 Mouse stain | MYCN基因状态 MYCN status | ALK基因突变 ALK mutation | P53基因突变 P53 mutation | 参考文献 Reference |
|---|---|---|---|---|---|
| KELLY | BALB/c-nude小鼠 | 扩增 | 野生型 | 野生型 | [ |
| CHP-212 | NSG小鼠 | 扩增 | 野生型 | 野生型 | [ |
| SKNAS | BALB/c-nude小鼠 | 非扩增 | 野生型 | H168R | [ |
| SH-SY-5Y | Foxn1nu/Nju小鼠、ICR小鼠、BALB/c-nude小鼠 | 非扩增 | F1174L | 野生型 | [ |
| IMR-32 | NSG小鼠 | 扩增 | 野生型 | 野生型 | [ |
| IMR-05 | SHC小鼠 | 扩增 | 野生型 | 野生型 | [ |
| LA-N-5 | BALB/c-nude小鼠 | 扩增 | R1275Q | 野生型 | [ |
| NB-1 | BALB/c-nude小鼠 | 扩增 | 野生型扩增 | 野生型 | [ |
| SK-N-BE(2) | SCID-Beige小鼠 | 扩增 | 野生型 | C135F | [ |
| SK-N-BE(2)-C | BALB/c- nude小鼠、Foxn1nu/Nju小鼠 | 扩增 | 野生型 | C135F | [ |
| CHP-134 | NOD-SCID小鼠 | 扩增 | 野生型 | 野生型 | [ |
| SK-N-DZ | BALB/c-nude小鼠 | 扩增 | 野生型 | R110L | [ |
Table 1 Frequently used preclinical laboratory mouse models derived from NB cell lines
细胞系 Cell line | 小鼠品系 Mouse stain | MYCN基因状态 MYCN status | ALK基因突变 ALK mutation | P53基因突变 P53 mutation | 参考文献 Reference |
|---|---|---|---|---|---|
| KELLY | BALB/c-nude小鼠 | 扩增 | 野生型 | 野生型 | [ |
| CHP-212 | NSG小鼠 | 扩增 | 野生型 | 野生型 | [ |
| SKNAS | BALB/c-nude小鼠 | 非扩增 | 野生型 | H168R | [ |
| SH-SY-5Y | Foxn1nu/Nju小鼠、ICR小鼠、BALB/c-nude小鼠 | 非扩增 | F1174L | 野生型 | [ |
| IMR-32 | NSG小鼠 | 扩增 | 野生型 | 野生型 | [ |
| IMR-05 | SHC小鼠 | 扩增 | 野生型 | 野生型 | [ |
| LA-N-5 | BALB/c-nude小鼠 | 扩增 | R1275Q | 野生型 | [ |
| NB-1 | BALB/c-nude小鼠 | 扩增 | 野生型扩增 | 野生型 | [ |
| SK-N-BE(2) | SCID-Beige小鼠 | 扩增 | 野生型 | C135F | [ |
| SK-N-BE(2)-C | BALB/c- nude小鼠、Foxn1nu/Nju小鼠 | 扩增 | 野生型 | C135F | [ |
| CHP-134 | NOD-SCID小鼠 | 扩增 | 野生型 | 野生型 | [ |
| SK-N-DZ | BALB/c-nude小鼠 | 扩增 | 野生型 | R110L | [ |
小鼠模型 Mouse model | 优势 Advantage | 局限性 Limitation | 参考文献 Reference |
|---|---|---|---|
| Th-MYCN | 代表高危NB型,成瘤率高 | 成瘤时间长,转移少 | [ |
| LSL-MYCN;dβh-iCre | 比Th-MYCN更明确的转基因插入,发病率更高 | 转移率低 | [ |
| Th-MYCN/CASP8(KO) | 存在转移,成瘤率高 | 引起原发肿瘤细胞外基质结构的改变 | [ |
| Th-MYCN/Trp53(KI) | 诱导性P53丢失 | P53突变多发于复发肿瘤中,小鼠的存活率低 | [ |
| ALK(F1174) | 符合NB表型 | 临床常见率低 | [ |
| Th-MYCN/ALK(F1174) | 成瘤率高,肿瘤生长快 | 相关性低 | [ |
| SV40 Tag | 与NB表型一致,肿瘤发病率高,存在转移 | 所有小鼠在28周龄前死亡 | [ |
Table 2 The common genetically engineered mouse models
小鼠模型 Mouse model | 优势 Advantage | 局限性 Limitation | 参考文献 Reference |
|---|---|---|---|
| Th-MYCN | 代表高危NB型,成瘤率高 | 成瘤时间长,转移少 | [ |
| LSL-MYCN;dβh-iCre | 比Th-MYCN更明确的转基因插入,发病率更高 | 转移率低 | [ |
| Th-MYCN/CASP8(KO) | 存在转移,成瘤率高 | 引起原发肿瘤细胞外基质结构的改变 | [ |
| Th-MYCN/Trp53(KI) | 诱导性P53丢失 | P53突变多发于复发肿瘤中,小鼠的存活率低 | [ |
| ALK(F1174) | 符合NB表型 | 临床常见率低 | [ |
| Th-MYCN/ALK(F1174) | 成瘤率高,肿瘤生长快 | 相关性低 | [ |
| SV40 Tag | 与NB表型一致,肿瘤发病率高,存在转移 | 所有小鼠在28周龄前死亡 | [ |
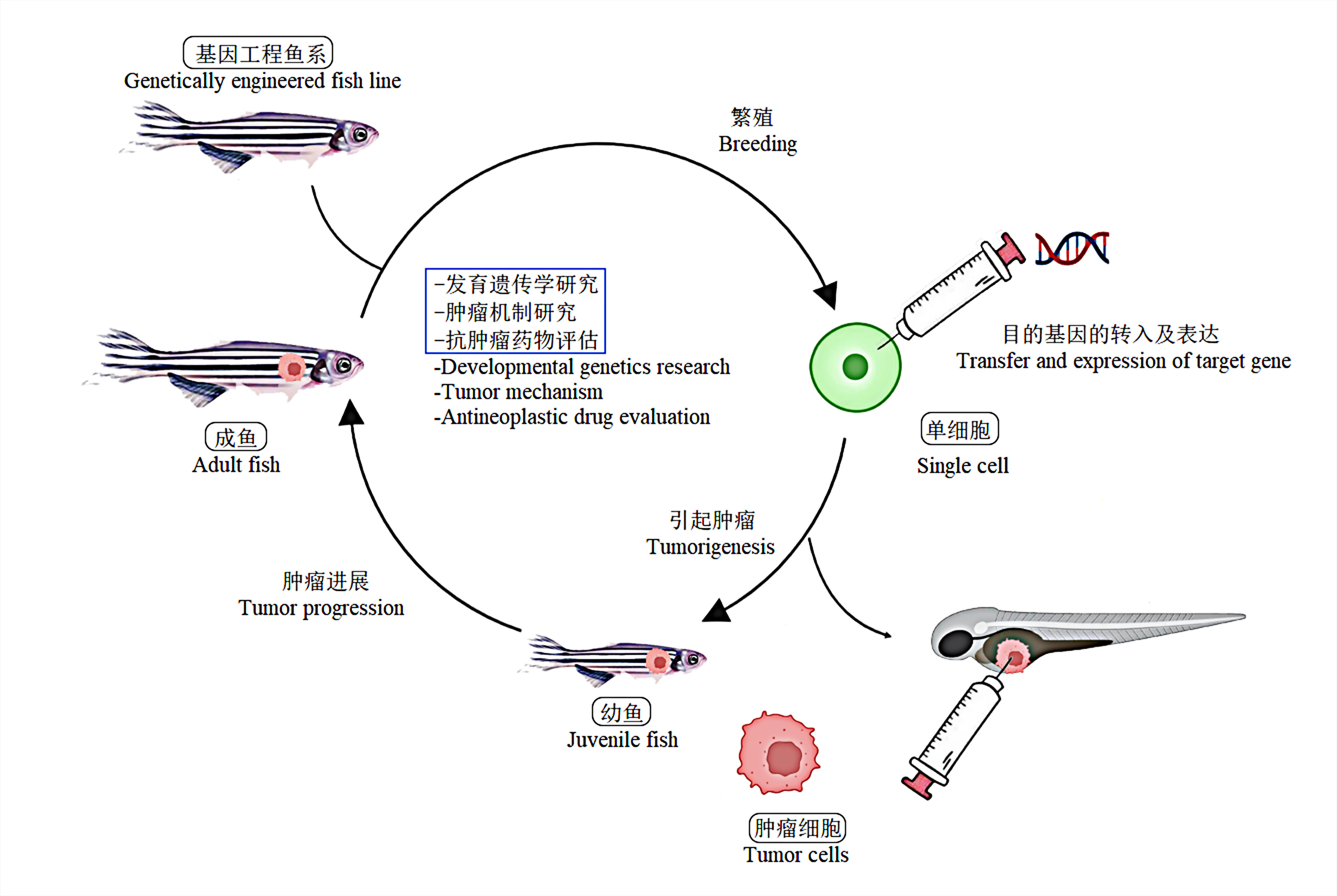
Figure 1 Process diagram of zebrafish model for NB researchNote:Zebrafish tumor model can be mainly used for the developmental genetics research, studies on tumor mechanisms, and antineoplastic drug evaluation.
| 1 | ZAFAR A, WANG W, LIU G, et al. Molecular targeting therapies for neuroblastoma: progress and challenges[J]. Med Res Rev, 2021, 41(2):961-1021. DOI: 10.1002/med.21750 . |
| 2 | CHUNG C, BOTERBERG T, LUCAS J, et al. Neuroblastoma[J]. Pediatr Blood Cancer, 2021, 68:e28473. DOI: 10.1002/pbc. 28473 . |
| 3 | SUN X F, ZHEN Z J, GUO Y, et al. Oral metronomic maintenance therapy can improve survival in high-risk neuroblastoma patients not treated with ASCT or anti-GD2 antibodies[J]. Cancers, 2021, 13(14):3494. DOI: 10.3390/cancers13143494 . |
| 4 | HELSON L, DAS S K, HAJDU S I. Human neuroblastoma in nude mice[J]. Cancer Res, 1975, 35(9): 2594-2599. |
| 5 | BOGDEN A E, COBB W R, LEPAGE D J, et al. Chemotherapy responsiveness of human tumors as first transplant generation xenografts in the normal mouse: six-day subrenal capsule assay[J]. Cancer, 1981, 48(1):10-20. DOI: 10.1002/1097-0142(19810701)48:1<10: AID-CNCR2820480105>3.0.CO;2-I . |
| 6 | KHANNA C, JABOIN J J, DRAKOS E, et al. Biologically relevant orthotopic neuroblastoma xenograft models: primary adrenal tumor growth and spontaneous distant metastasis[J]. In Vivo, 2002, 16(2):77-85. |
| 7 | ROWE D H, HUANG J Z, LI J, et al. Suppression of primary tumor growth in a mouse model of human neuroblastoma[J]. J Pediatr Surg, 2000, 35(6):977-981. DOI: 10.1053/jpsu. 2000.6946 . |
| 8 | FLICKINGER K S, JUDWARE R, LECHNER R, et al. Integrin expression in human neuroblastoma cells with or without N-myc amplification and in ectopic/orthotopic nude mouse tumors[J]. Exp Cell Res, 1994, 213(1):156-163. DOI: 10.1006/excr.1994.1185 . |
| 9 | KANG J, ISHOLA T A, BAREGAMIAN N, et al. Bombesin induces angiogenesis and neuroblastoma growth[J]. Cancer Lett, 2007, 253(2):273-281. DOI: 10.1016/j.canlet.2007.02.007 . |
| 10 | 刘波, 苗佳宁, 张斯萌, 等. 神经母细胞瘤肾上腺原位移植瘤动物模型的建立[J]. 中国比较医学杂志, 2021, 31(12):1-6. DOI: 10.3969/j.issn.1671-7856.2021.12.001 . |
| LIU B, MIAO J N, ZHANG S M, et al. Establishment of an orthotopic xenografted animal model of neuroblastoma[J]. Chin J Comp Med, 2021, 31(12):1-6. DOI: 10.3969/j.issn.1671-7856.2021.12.001 . | |
| 11 | SENEVIRATNE J A, CARTER D R, MITTRA R, et al. Inhibition of mitochondrial translocase SLC25A5 and histone deacetylation is an effective combination therapy in neuroblastoma[J]. Int J Cancer, 2023, 152(7):1399-1413. DOI: 10.1002/ijc.34349 . |
| 12 | MISSIOS P, ROCHA E L DA, PEARSON D S, et al. LIN28B alters ribosomal dynamics to promote metastasis in MYCN-driven malignancy[J]. J Clin Invest, 2021, 131(22):e145142. DOI: 10.1172/JCI145142 . |
| 13 | CANDIDO M F, MEDEIROS M, VERONEZ L C, et al. Drugging hijacked kinase pathways in pediatric oncology: opportunities and current scenario[J]. Pharmaceutics, 2023, 15(2):664. DOI: 10.3390/pharmaceutics15020664 . |
| 14 | GU Y Y, ZHONG K, PENG L Z, et al. TRAF4 silencing induces cell apoptosis and improves retinoic acid sensitivity in human neuroblastoma[J]. Neurochem Res, 2023, 48(7):2116-2128. DOI: 10.1007/s11064-023-03882-3 . |
| 15 | CONDURAT A L, AMINZADEH-GOHARI S, MALNAR M, et al. Verteporfin-induced proteotoxicity impairs cell homeostasis and survival in neuroblastoma subtypes independent of YAP/TAZ expression[J]. Sci Rep, 2023, 13(1):3760. DOI: 10.1038/s41598-023-29796-2 . |
| 16 | HE Y, LUO M H, LEI S, et al. Luteoloside induces G0/G1 phase arrest of neuroblastoma cells by targeting p38 MAPK[J]. Molecules, 2023, 28(4):1748. DOI: 10.3390/molecules28041748 . |
| 17 | GAO Y, VOLEGOVA M, NASHOLM N, et al. Synergistic anti-tumor effect of combining selective CDK7 and BRD4 inhibition in neuroblastoma[J]. Front Oncol, 2022, 11:773186. DOI: 10.3389/fonc.2021.773186 . |
| 18 | MAKVANDI M, SAMANTA M, MARTORANO P, et al. Pre-clinical investigation of astatine-211-parthanatine for high-risk neuroblastoma[J]. Commun Biol, 2022, 5(1):1260. DOI: 10.1038/s42003-022-04209-8 . |
| 19 | ZHU Q Q, FENG C, LIAO W W, et al. Target delivery of MYCN siRNA by folate-nanoliposomes delivery system in a metastatic neuroblastoma model[J]. Cancer Cell Int, 2013, 13(1):65. DOI: 10.1186/1475-2867-13-65 . |
| 20 | RYU S, HAYASHI M, AIKAWA H, et al. Heterogeneous distribution of alectinib in neuroblastoma xenografts revealed by matrix-assisted laser desorption ionization mass spectrometry imaging: a pilot study[J]. Br J Pharmacol, 2018, 175(1):29-37. DOI: 10.1111/bph.14067 . |
| 21 | NOMURA M, SHIMBO T, MIYAMOTO Y, et al. 13-Cis retinoic acid can enhance the antitumor activity of non-replicating Sendai virus particle against neuroblastoma[J]. Cancer Sci, 2013, 104(2):238-244. DOI: 10.1111/cas.12063 . |
| 22 | SEPPORTA M V, PRAZ V, BALMAS BOURLOUD K, et al. TWIST1 expression is associated with high-risk neuroblastoma and promotes primary and metastatic tumor growth[J]. Commun Biol, 2022, 5(1):42. DOI: 10.1038/s42003-021-02958-6 . |
| 23 | LAMPIS S, RAIELI S, MONTEMURRO L, et al. The MYCN inhibitor BGA002 restores the retinoic acid response leading to differentiation or apoptosis by the mTOR block in MYCN-amplified neuroblastoma[J]. J Exp Clin Cancer Res, 2022, 41(1):160. DOI: 10.1186/s13046-022-02367-5 . |
| 24 | XIAO H L, LI Y H, ZHANG Y, et al. Long noncoding RNA LINC01296 regulates the cell proliferation, migration and invasion in neuroblastoma[J]. Metab Brain Dis, 2022, 37(4):1247-1258. DOI: 10.1007/s11011-022-00935-4 . |
| 25 | KAMILI A, GIFFORD A J, LI N, et al. Accelerating development of high-risk neuroblastoma patient-derived xenograft models for preclinical testing and personalised therapy[J]. Br J Cancer, 2020, 122(5):680-691. DOI: 10.1038/s41416-019-0682-4 . |
| 26 | BYRNE F L, MCCARROLL J A, KAVALLARIS M. Analyses of tumor burden in vivo and metastasis ex vivo using luciferase-expressing cancer cells in an orthotopic mouse model of neuroblastoma[J]. Methods Mol Biol, 2016, 1372:61-77. DOI: 10.1007/978-1-4939-3148-4_5 . |
| 27 | GRANT C N, WILLS C A, LIU X M, et al. Thoracic neuroblastoma: a novel surgical model for the study of extra-adrenal neuroblastoma[J]. In Vivo, 2022, 36(1):49-56. DOI: 10.21873/invivo.12675 . |
| 28 | WEISS W A, ALDAPE K, MOHAPATRA G, et al. Targeted expression of MYCN causes neuroblastoma in transgenic mice[J]. EMBO J, 1997, 16(11):2985-2995. DOI: 10.1093/emboj/16.11.2985 . |
| 29 | MARSHALL G M, CARTER D R, CHEUNG B B, et al. The prenatal origins of cancer[J]. Nat Rev Cancer, 2014, 14(4):277-289. DOI: 10.1038/nrc3679 . |
| 30 | Rasmuson A, Segerström L, Nethander M, et al. Tumor development, growth characteristics and spectrum of genetic aberrations in the TH-MYCN mouse model of neuroblastoma[J]. PLoS One, 2012, 7(12):e51297. |
| 31 | ALTHOFF K, BECKERS A, BELL E, et al. A Cre-conditional MYCN-driven neuroblastoma mouse model as an improved tool for preclinical studies[J]. Oncogene, 2015, 34(26):3357-3368. DOI: 10.1038/onc.2014.269 . |
| 32 | ROSSWOG C, FASSUNKE J, ERNST A, et al. Genomic ALK alterations in primary and relapsed neuroblastoma[J]. Br J Cancer, 2023, 128(8):1559-1571. DOI: 10.1038/s41416-023-02208-y . |
| 33 | BRESLER S C, WEISER D A, HUWE P J, et al. ALK mutations confer differential oncogenic activation and sensitivity to ALK inhibition therapy in neuroblastoma[J]. Cancer Cell, 2014, 26(5): 682-694. DOI: 10.1016/j.ccell.2014.09.019 . |
| 34 | HEUKAMP L C, THOR T, SCHRAMM A, et al. Targeted expression of mutated ALK induces neuroblastoma in transgenic mice[J]. Sci Transl Med, 2012, 4(141):141ra91. DOI: 10.1126/scitranslmed.3003967 . |
| 35 | BERRY T, LUTHER W, BHATNAGAR N, et al. The ALK(F1174L) mutation potentiates the oncogenic activity of MYCN in neuroblastoma[J]. Cancer Cell, 2012, 22(1):117-130. DOI: 10.1016/j.ccr.2012.06.001 . |
| 36 | UEDA T, NAKATA Y, YAMASAKI N, et al. ALK(R1275Q) perturbs extracellular matrix, enhances cell invasion and leads to the development of neuroblastoma in cooperation with MYCN[J]. Oncogene, 2016, 35(34):4447-4458. DOI: 10.1038/onc.2015.519 . |
| 37 | LIN Z H, RADAEVA M, CHERKASOV A, et al. Lin28 regulates cancer cell stemness for tumour progression[J]. Cancers, 2022, 14(19):4640. DOI: 10.3390/cancers14194640 . |
| 38 | MOLENAAR J J, DOMINGO-FERNÁNDEZ R, EBUS M E, et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression[J]. Nat Genet, 2012, 44(11):1199-1206. DOI: 10.1038/ng.2436 . |
| 39 | MASSUDI H, LUO J S, HOLIEN J K, et al. Inhibitors of the oncogenic PA2G4-MYCN protein-protein interface[J]. Cancers, 2023, 15(6):1822. DOI: 10.3390/cancers15061822 . |
| 40 | KAMBE K, IGUCHI M, HIGASHI M, et al. Development of minimally invasive cancer immunotherapy using anti-disialoganglioside GD2 antibody-producing mesenchymal stem cells for a neuroblastoma mouse model[J]. Pediatr Surg Int, 2022, 39(1):43. DOI: 10.1007/s00383-022-05310-z . |
| 41 | TEITZ T, INOUE M, VALENTINE M B, et al. Th-MYCN mice with caspase-8 deficiency develop advanced neuroblastoma with bone marrow metastasis[J]. Cancer Res, 2013, 73(13):4086-4097. DOI: 10.1158/0008-5472.CAN-12-2681 . |
| 42 | YOGEV O, BARKER K, SIKKA A, et al. p53 loss in MYC-driven neuroblastoma leads to metabolic adaptations supporting radioresistance[J]. Cancer Res, 2016, 76(10):3025-3035. DOI: 10.1158/0008-5472.CAN-15-1939 . |
| 43 | EIBL R H, SCHNEEMANN M. Medulloblastoma: from TP53 mutations to molecular classification and liquid biopsy[J]. Biology, 2023, 12(2):267. DOI: 10.3390/biology12020267 . |
| 44 | MITREVSKA K, MERLOS RODRIGO M A, CERNEI N, et al. Chick chorioallantoic membrane (CAM) assay for the evaluation of the antitumor and antimetastatic activity of platinum-based drugs in association with the impact on the amino acid metabolism[J]. Mater Today Bio, 2023, 19:100570. DOI: 10.1016/j.mtbio.2023.100570 . |
| 45 | RIBATTI D, ALESSANDRI G, VACCA A, et al. Human neuroblastoma cells produce extracellular matrix-degrading enzymes, induce endothelial cell proliferation and are angiogenic in vivo [J]. Int J Cancer, 1998, 77(3):449-454. DOI: 10.1002/(sici)1097-0215(19980729)77:3449: aid-ijc22>3.0.co;2-1 . |
| 46 | MANGIERI D, NICO B, COLUCCIA A M L, al at. An alternative in vivo system for testing angiogenic potential of human neuroblastoma cells[J]. Cancer Lett, 2009, 277(2):199-204. DOI: 10.1016/j.canlet.2008.12.014 . |
| 47 | HERRMANN A, RICE M, LÉVY R, et al. Cellular memory of hypoxia elicits neuroblastoma metastasis and enables invasion by non-aggressive neighbouring cells[J]. Oncogenesis, 2015, 4(2): e138. DOI: 10.1038/oncsis.2014.52 . |
| 48 | SWADI R, MATHER G, PIZER B L, et al. Optimising the chick chorioallantoic membrane xenograft model of neuroblastoma for drug delivery[J]. BMC Cancer, 2018, 18(1):28. DOI: 10.1186/s12885-017-3978-x . |
| 49 | LI S, YEO K S, LEVEE T M, et al. Zebrafish as a neuroblastoma model: progress made, promise for the future[J]. Cells, 2021, 10(3):580. DOI: 10.3390/cells10030580 . |
| 50 | STANTON M F. Diethylnitrosamine-induced hepatic degeneration and neoplasia in the aquarium fish, brachydanio rerio[J]. J Natl Cancer Inst, 1965, 34:117-130. DOI: 10.1093/jnci/34.1.117 . |
| 51 | LANGENAU D M, TRAVER D, FERRANDO A A, et al. Myc-induced T cell leukemia in transgenic zebrafish[J]. Science, 2003, 299(5608):887-890. DOI: 10.1126/science.1080280 . |
| 52 | ETCHIN J, KANKI J P, LOOK A T. Zebrafish as a model for the study of human cancer[J]. Methods Cell Biol, 2011, 105:309-337. DOI: 10.1016/B978-0-12-381320-6.00013-8 . |
| 53 | FEITSMA H, CUPPEN E. Zebrafish as a cancer model[J]. Mol Cancer Res, 2008, 6(5):685-694. DOI: 10.1158/1541-7786.MCR-07-2167 . |
| 54 | BENJAMIN D C, HYNES R O. Intravital imaging of metastasis in adult Zebrafish[J]. BMC Cancer, 2017, 17(1):660. DOI: 10.1186/s12885-017-3647-0 . |
| 55 | TAO T, SONDALLE S B, SHI H, et al. The pre-rRNA processing factor DEF is rate limiting for the pathogenesis of MYCN-driven neuroblastoma[J]. Oncogene, 2017, 36(27):3852-3867. DOI: 10.1038/onc.2016.527 . |
| 56 | ZHU S Z, LEE J S, GUO F, et al. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis[J]. Cancer Cell, 2012, 21(3):362-373. DOI: 10.1016/j.ccr.2012.02.010 . |
| 57 | ZHANG X L, DONG Z W, ZHANG C, et al. Critical role for GAB2 in neuroblastoma pathogenesis through the promotion of SHP2/MYCN cooperation[J]. Cell Rep, 2017, 18(12):2932-2942. DOI: 10.1016/j.celrep.2017.02.065 . |
| 58 | COSTA B, ESTRADA M F, MENDES R V, et al. Zebrafish avatars towards personalized medicine-a comparative review between avatar models[J]. Cells, 2020, 9(2):293. DOI: 10.3390/cells9020293 . |
| 59 | FIOR R, PÓVOA V, MENDES R V, et al. Single-cell functional and chemosensitive profiling of combinatorial colorectal therapy in zebrafish xenografts[J]. Proc Natl Acad Sci USA, 2017, 114(39):E8234-E8243. DOI: 10.1073/pnas.1618389114 . |
| 60 | ALMSTEDT E, ELGENDY R, HEKMATI N, et al. Integrative discovery of treatments for high-risk neuroblastoma[J]. Nat Commun, 2020, 11(1):71. DOI: 10.1038/s41467-019-13817-8 . |
| 61 | VEINOTTE C J, DELLAIRE G, BERMAN J N. Hooking the big one: the potential of zebrafish xenotransplantation to reform cancer drug screening in the genomic era[J]. Dis Model Mech, 2014, 7(7):745-754. DOI: 10.1242/dmm.015784 . |
| 62 | HANEY M G, MOORE L H, BLACKBURN J S. Drug screening of primary patient derived tumor xenografts in zebrafish[J]. J Vis Exp, 2020(158):10.3791/60996. DOI: 10.3791/60996 . |
| 63 | CABEZAS-SÁINZ P, PENSADO-LÓPEZ A, SÁINZ B Jr, et al. Modeling cancer using zebrafish xenografts: drawbacks for mimicking the human microenvironment[J]. Cells, 2020, 9(9):1978. DOI: 10.3390/cells9091978 . |
| 64 | IBARRA B A, JIANG X H, TREFFY R W, et al. Injection of human neuroblastoma cells into neural crest streams in live zebrafish embryos[J]. STAR Protoc, 2022, 3(2):101380. DOI: 10.1016/j.xpro.2022.101380 . |
| 65 | DELLOYE-BOURGEOIS C, BERTIN L, THOINET K,et al. Microenvironment-driven shift of cohesion/detachment balance within tumors induces a switch toward metastasis in neuroblastoma[J]. Cancer Cell, 2017, 32(4):427-443.e8. DOI: 10.1016/j.ccell.2017.09.006 . |
| 66 | ZHU S Z, ZHANG X L, WEICHERT-LEAHEY N, et al. LMO1 synergizes with MYCN to promote neuroblastoma initiation and metastasis[J]. Cancer Cell, 2017, 32(3):310-323.e5. DOI: 10.1016/j.ccell.2017.08.002 . |
| 67 | DONG Z W, YEO K S, LOPEZ G, et al. GAS7 deficiency promotes metastasis in MYCN-Driven neuroblastoma[J]. Cancer Res, 2021, 81(11):2995-3007. DOI: 10.1158/0008-5472.CAN-20-1890 . |
| 68 | YANG T Y, LI J H, ZHUO Z J, et al. TTF1 suppresses neuroblastoma growth and induces neuroblastoma differentiation by targeting TrkA and the miR-204/TrkB axis[J]. iScience, 2022, 25(7):104655. DOI: 10.1016/j.isci. 2022. 104655 . |
| 69 | MIAO L, ZHUO Z J, TANG J, et al. FABP4 deactivates NF-κB-IL1α pathway by ubiquitinating ATPB in tumor-associated macrophages and promotes neuroblastoma progression[J]. Clin Transl Med, 2021, 11(4): e395. DOI: 10.1002/ctm2.395 . |
| 70 | GARBATI P, BARBIERI R, CALDERONI M, et al. Efficacy of a three drug-based therapy for neuroblastoma in mice[J]. Int J Mol Sci, 2021, 22(13):6753. DOI: 10.3390/ijms22136753 . |
| 71 | HARUKI H, PEDERSEN M G, GORSKA K I, et al. Tetrahydrobiopterin biosynthesis as an off-target of sulfa drugs[J]. Science, 2013, 340(6135):987-991. DOI: 10.1126/science.1232972 . |
| 72 | SHANG T S, KOTAMRAJU S, ZHAO H T, et al. Sepiapterin attenuates 1-methyl-4-phenylpyridinium-induced apoptosis in neuroblastoma cells transfected with neuronal NOS: role of tetrahydrobiopterin, nitric oxide, and proteasome activation[J]. Free Radic Biol Med, 2005, 39(8):1059-1074. DOI: 10.1016/j.freeradbiomed.2005.05.022 . |
| 73 | MOONEY M R, GEERTS D, KORT E J, et al. Anti-tumor effect of sulfasalazine in neuroblastoma[J]. Biochem Pharmacol, 2019, 162:237-249. DOI: 10.1016/j.bcp.2019.01.007 . |
| 74 | MAÑAS A, AALTONEN K, ANDERSSON N, et al. Clinically relevant treatment of PDX models reveals patterns of neuroblastoma chemoresistance[J]. Sci Adv, 2022, 8(43): eabq4617. DOI: 10.1126/sciadv.abq4617 . |
| 75 | ZHANG H M, XIA H F, CHEN H, et al. The inhibition of GHR enhanced cytotoxic effects of etoposide on neuroblastoma[J]. Cell Signal, 2021, 86:110081. DOI: 10.1016/j.cellsig. 2021.110081 . |
| 76 | NUNES C, DEPESTEL L, MUS L, et al. RRM2 enhances MYCN- driven neuroblastoma formation and acts as a synergistic target with CHK1 inhibition[J]. Sci Adv, 2022, 8(28): eabn1382. DOI: 10.1126/sciadv.abn1382 . |
| 77 | COSTA A, THIRANT C, KRAMDI A, et al. Single-cell transcriptomics reveals shared immunosuppressive landscapes of mouse and human neuroblastoma[J]. J Immunother Cancer, 2022, 10(8): e004807. DOI: 10.1136/jitc-2022-004807 . |
| 78 | LI N, TORRES M B, SPETZ M R, et al. CAR T cells targeting tumor-associated exons of glypican 2 regress neuroblastoma in mice[J]. Cell Rep Med, 2021, 2(6):100297. DOI: 10.1016/j.xcrm.2021.100297 . |
| 79 | THERUVATH J, MENARD M, SMITH B A H, et al. Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication[J]. Nat Med, 2022, 28(2):333-344. DOI: 10.1038/s41591-021-01625-x . |
| 80 | SWADI R R, SAMPAT K, HERRMANN A, et al. CDK inhibitors reduce cell proliferation and reverse hypoxia-induced metastasis of neuroblastoma tumours in a chick embryo model[J]. Sci Rep, 2019, 9(1):9136. DOI: 10.1038/s41598-019-45571-8 . |
| 81 | LEIBA J, ÖZBILGIÇ R, HERNÁNDEZ L, et al. Molecular actors of inflammation and their signaling pathways: mechanistic insights from zebrafish[J]. Biology, 2023, 12(2):153. DOI: 10.3390/biology12020153 . |
| [1] | LIU Yayi, JIA Yunfeng, ZUO Yiming, ZHANG Junping, LÜ Shichao. Progress and Evaluation of Animal Model of Heart Qi-Yin Deficiency Syndrome [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 411-421. |
| [2] | ZHAO Xin, WANG Chenxi, SHI Wenqing, LOU Yuefen. Advances in the Application of Zebrafish in the Research of Inflammatory Bowel Disease Mechanisms and Drug Development [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 422-431. |
| [3] | LIU Yueqin, XUE Weiguo, WANG Shuyou, SHEN Yaohua, JIA Shuyong, WANG Guangjun, SONG Xiaojing. Observation of Digestive Tract Tissue Morphology in Mice Using Probe-Based Confocal Laser Endomicroscopy [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 457-465. |
| [4] | KONG Zhihao, WEI Xiaofeng, YU Lingzhi, FENG Liping, ZHU Qi, SHI Guojun, WANG Chen. Isolation and Identification of Staphylococcus xylosus in Nude Mice with Squamous Skin Scurfs [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 368-375. |
| [5] | PAN Yicong, JIANG Wenhong, HU Ming, QIN Xiao. Optimization of Surgical Procedure and Efficacy Evaluation of Aortic Calcification Model in Rats with Chronic Kidney Disease [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 279-289. |
| [6] | CHEN Yuhan, CHEN Jinling, LI Xin, OU Yanhua, WANG Si, CHEN Jingyi, WANG Xingyi, YUAN Jiali, DUAN Yuanyuan, YANG Zhongshan, NIU Haitao. Analysis of Animal Models of Myasthenia Gravis Based on Its Clinical Characteristics in Chinese and Western Medicine [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 176-186. |
| [7] | LIAN Hui, JIANG Yanling, LIU Jia, ZHANG Yuli, XIE Wei, XUE Xiaoou, LI Jian. Construction and Evaluation of a Rat Model of Abnormal Uterine Bleeding [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 130-146. |
| [8] | LUO Shixiong, ZHANG Sai, CHEN Hui. Research Progress in Establishment and Evaluation of Common Asthma Animal Models [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 167-175. |
| [9] | XU Qiuyu, YAN Guofeng, FU Li, FAN Wenhua, ZHOU Jing, ZHU Lian, QIU Shuwen, ZHANG Jie, WU Ling. A Mouse Model of Polycystic Ovary Syndrome Established Through Subcutaneous Administration of Letrozole Sustained-Release Pellets and Hepatic Transcriptome Analysis [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 119-129. |
| [10] | WANG Biying, LU Jiashuo, ZAN Guiying, CHEN Ruosong, CHAI Jingrui, LIU Jinggen, WANG Yujun. Establishment Methods and Application Progress of Rodent Models for Drug Addiction [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 158-166. |
| [11] | WU Zhihao, CAO Shuyang, ZHOU Zhengyu. Establishment of an Intestinal Fibrosis Model Associated with Inflammatory Bowel Disease in VDR-/- Mice Induced by Helicobacter hepaticus Infection and Mechanism Exploration [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 37-46. |
| [12] | ZHANG Nan, LI Huaiyin, LIAN Xiaodi, WEI Juanpeng, GAO Ming. Effects of Different Durations of Light Exposure on Body Weight and Learning and Memory Abilities of NIH Mice [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 73-78. |
| [13] | FEI Bin, GUO Wenke, GUO Jianping. Research Progress on Animal Models for Hernia Diseases and New Hernia Repair Materials [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 55-66. |
| [14] | LIU Rongle, CHENG Hao, SHANG Fusheng, CHANG Shufu, XU Ping. Study on Cardiac Aging Phenotypes of SHJH hr Mice [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 13-20. |
| [15] | YANG Jiahao, DING Chunlei, QIAN Fenghua, SUN Qi, JIANG Xusheng, CHEN Wen, SHEN Mengwen. Research Progress on Animal Models of Sepsis-Related Organ Injury [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 636-644. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||