
Laboratory Animal and Comparative Medicine ›› 2023, Vol. 43 ›› Issue (5): 531-540.DOI: 10.12300/j.issn.1674-5817.2023.070
• Research Reports • Previous Articles Next Articles
Jinxing LIN1( )(
)( ), Xindong WANG2,3, Xuebing BAI2, Liping FENG1, Shuwu XIE1, Qiusheng CHEN2(
), Xindong WANG2,3, Xuebing BAI2, Liping FENG1, Shuwu XIE1, Qiusheng CHEN2( )(
)( )
)
Received:2023-06-05
Revised:2023-10-12
Online:2023-10-25
Published:2023-10-25
Contact:
Jinxing LIN, Qiusheng CHEN
CLC Number:
Jinxing LIN,Xindong WANG,Xuebing BAI,et al. Fine Structure of the Trunk Kidney and Distribution of Its Secreted Exosomes in the Adult Zebrafish[J]. Laboratory Animal and Comparative Medicine, 2023, 43(5): 531-540. DOI: 10.12300/j.issn.1674-5817.2023.070.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2023.070
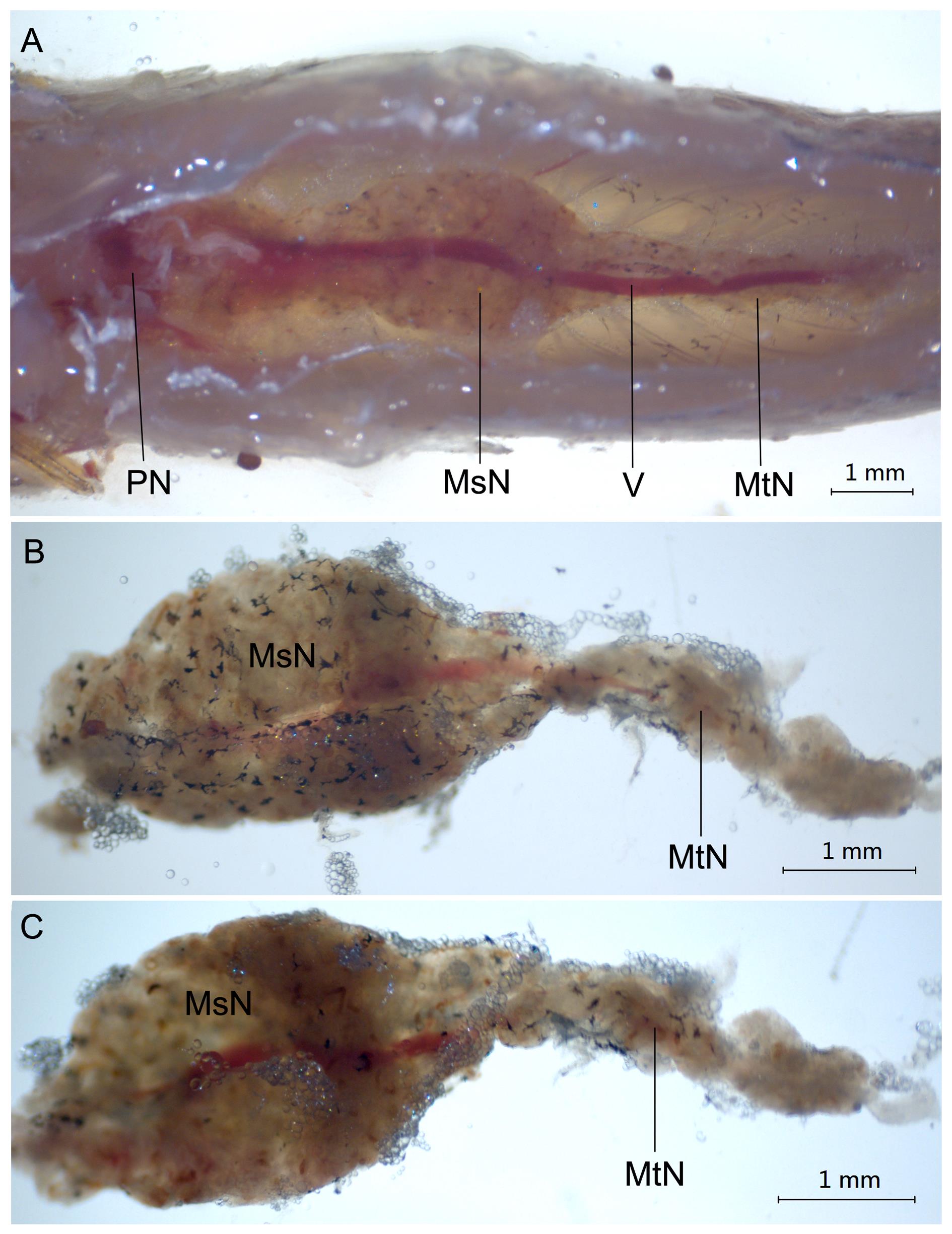
Figure 1 Anatomical structure of the kidney in adult zebrafish by stereomicroscopeNote:A, The position of kidney in zebrafish; B, Dorsal view of the trunk kidney in zebrafish; C, Ventral view of the trunk kidney in zebrafish. The scale is 1 mm. PN, Pronephros; MsN, Mesonephros; MtN, Metanephros; V, Vein.
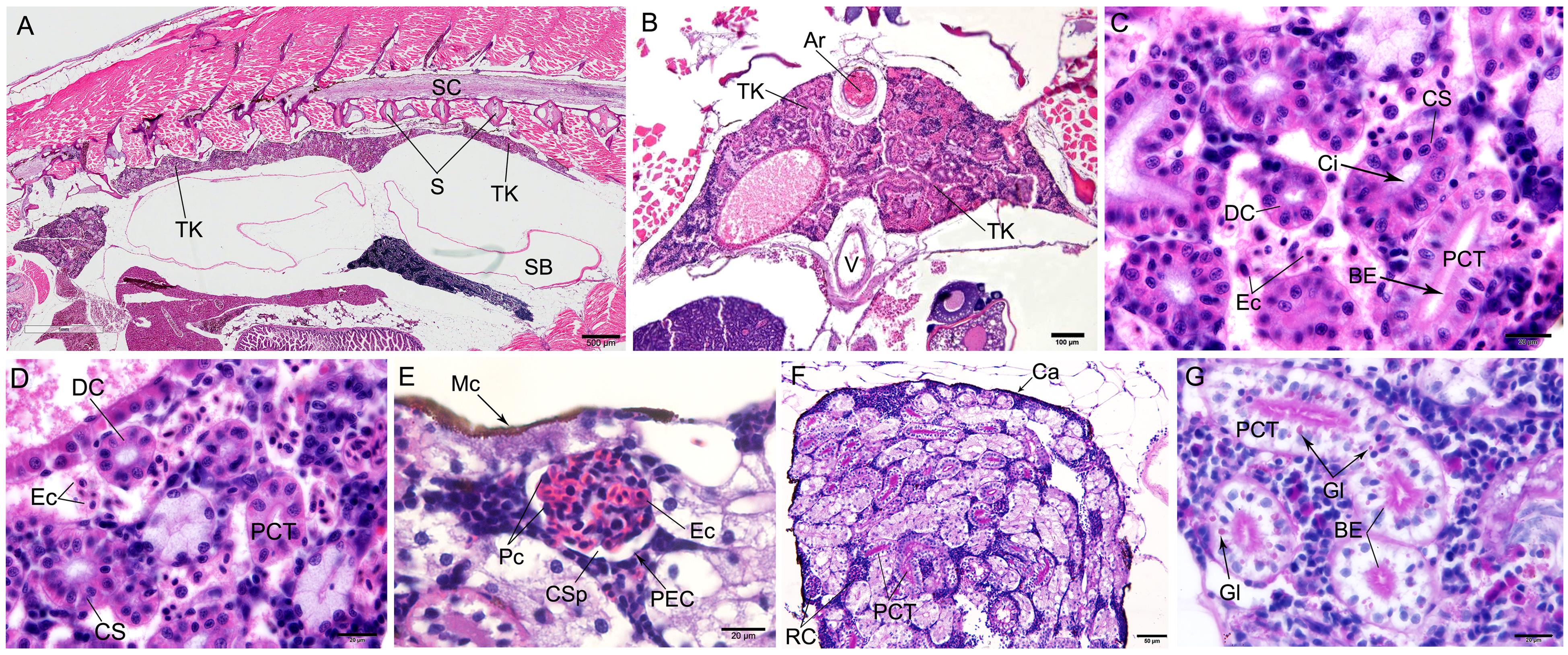
Figure 2 Microstructure of trunk kidney in the zebrafish detected by HE staining (A-E) and periodic acid-Schiff staining(F-G)Note:A, Morphological structure of zebrafish kidney in longitudinal section, the scale is 500 μm; B, Morphological structure of zebrafish kidney in cross section, the scale is 100 μm; C,Morphological structure of renal tubules of zebrafish, the scale is 20 μm; D, The renal tubules of zebrafish were divided into proximal convoluted tubules, distal convoluted tubules and cervical segments, the scale is 20 μm; E, Morphological structure of renal corpuscle, the scale is 20 μm; F, PAS reaction was positive in proximal convoluted tubules while negative in renal corpuscles, the scale is 50 μm; G, The epithelial cells of the proximal convoluted tubule were rich in glycogen particles, which were showed brush-like outline on the apical surface of the epithelial cells, the scale is 20 μm. Ar, Artery; BE, Brush-like edge; TK, Trunk kidney; Ca, Capsule; Ci, Cilia; CS, Cervical segment; CSp, Capsular space; DC, Distal convoluted tubule; Ec, Erythrocyte; Gl, Glycogen; Mc, Melanocyte; Pc, Podocyte; PCT, Proximal convoluted tubule; PEC, Parietal epithelial cell; RC, Renal corpuscle; S, Spine; SB, Swim bladder; SC, Spinal cord; V, Vein.
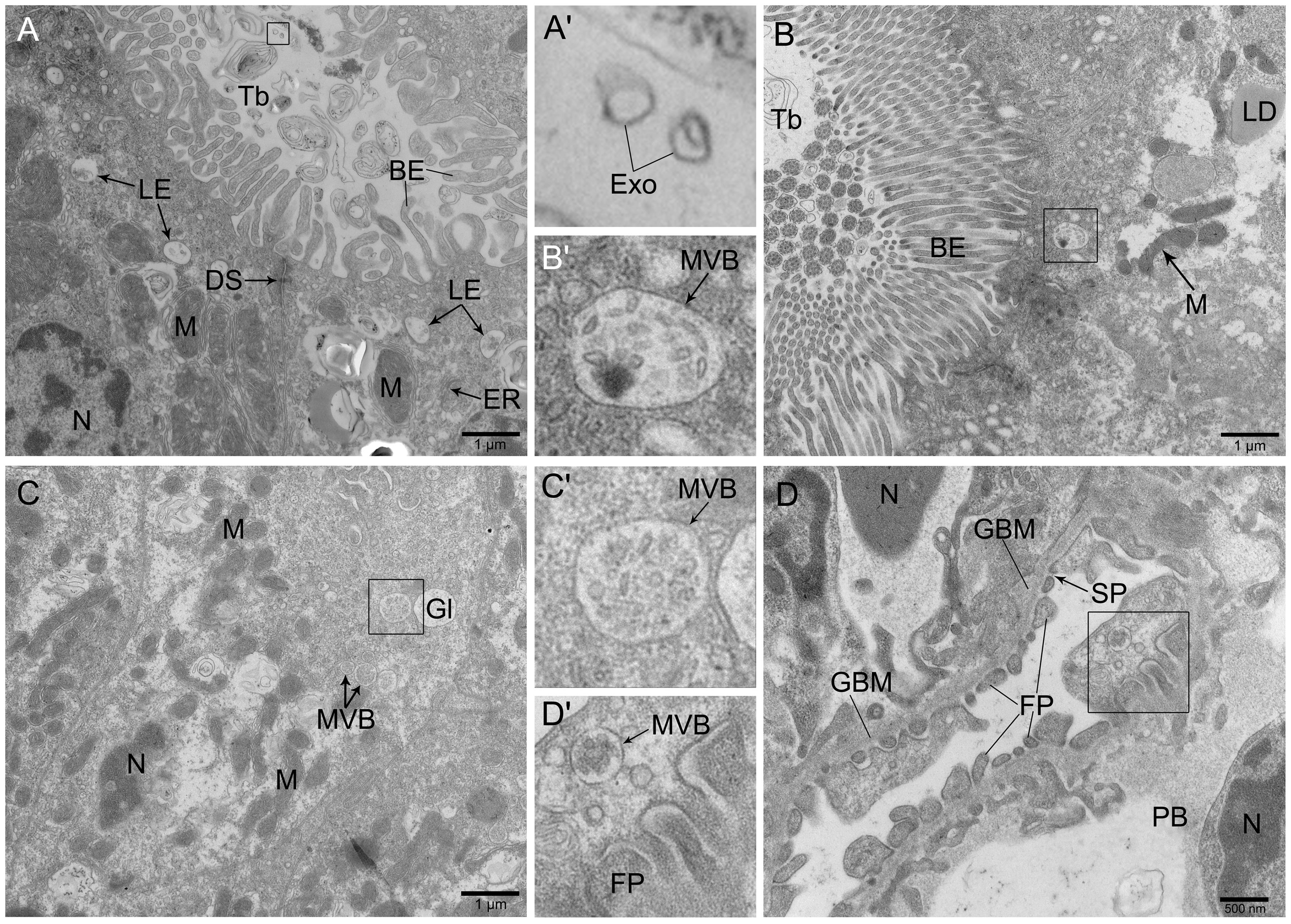
Figure 3 Ultrastructure of trunk kidney in zebrafish observed by transmission electron microscopeNote:A, The epithelial cells of convoluted tubule contained mitochondria and glycogen with abundant late endosomes in the apical surface, the scale is 1 μm; B, The brush-like structure at the free-end of the proximal convoluted tubule was clearly visible and arranged in a palisade shape, the scale is 1 μm; C, There were multiple vesicles distributed at the top-end of epithelial cells in the renal tubule, the scale is 1 μm; D, The structure of podocytes in the glomerulus was obvious, with multiple vesicles distributed, the scale is 500 nm. A', B', C' and D' were enlarged figures at the boxes in A, B, C and D, respectively. BE, Brush-like edge; GBM, Glomerular basement membrane; DS, Desmosome; Exo, Exosome; ER, Endoplasmic reticulum; FP, Foot process; Gl, Glycogen; LE, Late endosome; LD, Lipid droplets; M, Mitochondria; MVB, Multivesicular body; N, Nucleus; PB, Podocyte cell body; SP, Slit pore; Tb, Tube.
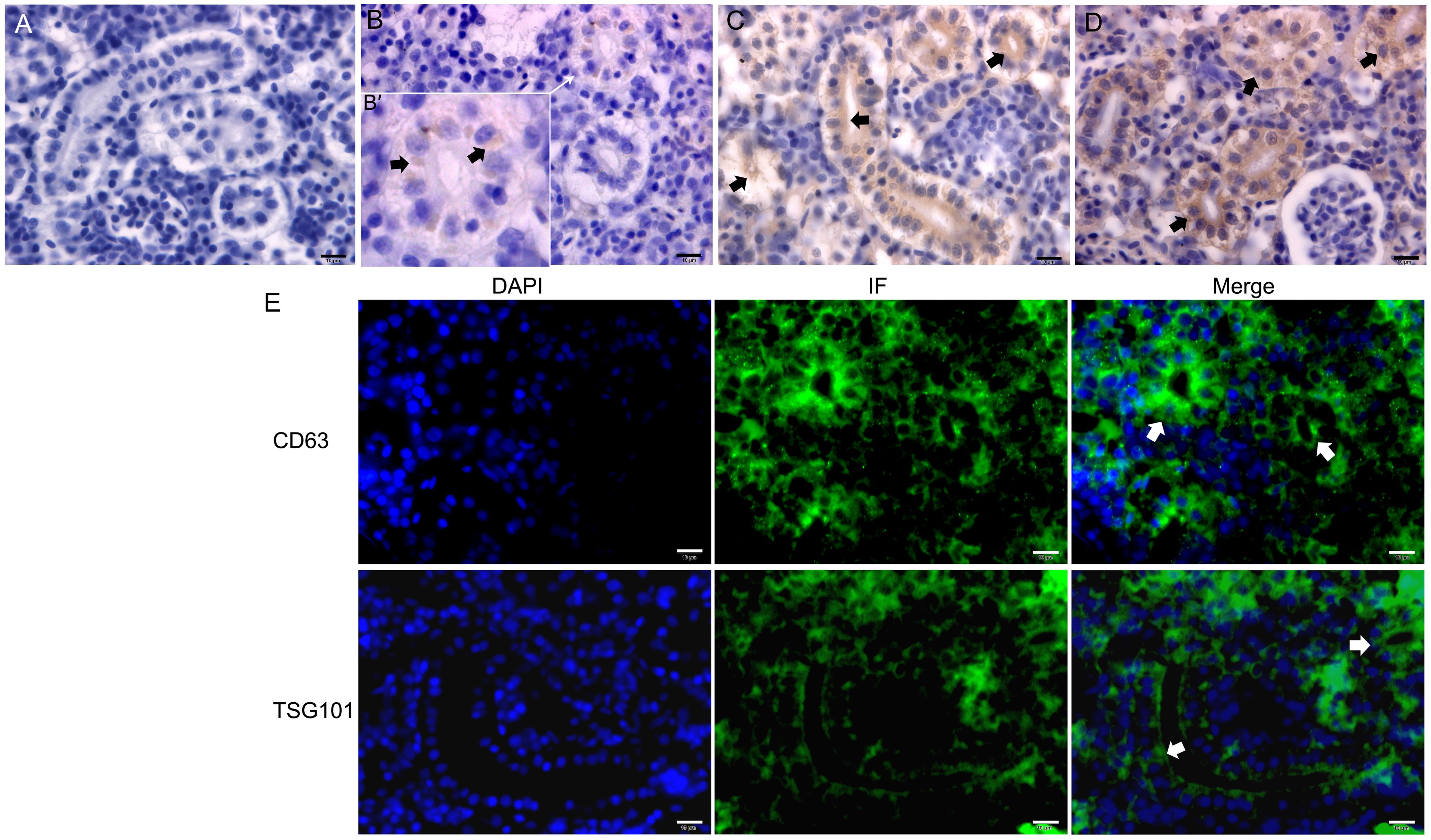
Figure 4 Immunohistochemistry (A-D) and immunofluorescence (E) of CD9、CD63 and TSG101 in the trunk kidney of zebrafishNote:A, Negative control of immunohistochemistry (DAB stain); B-D, Distribution of CD9, CD63 and TSG101 in the trunk kidney, respectively, and black short arrows showed positive reactions. The figure B' was the enlarged figure pointed by the white arrow of figure B: E, CD63 and TSG101 were widely distributed in the trunk kidney of zebrafish by immunofluorescence (IF) method (DAPI stain), particularly abundant in the apical surface of renal tubules (white short arrows). All of cale bars are 10 μm.
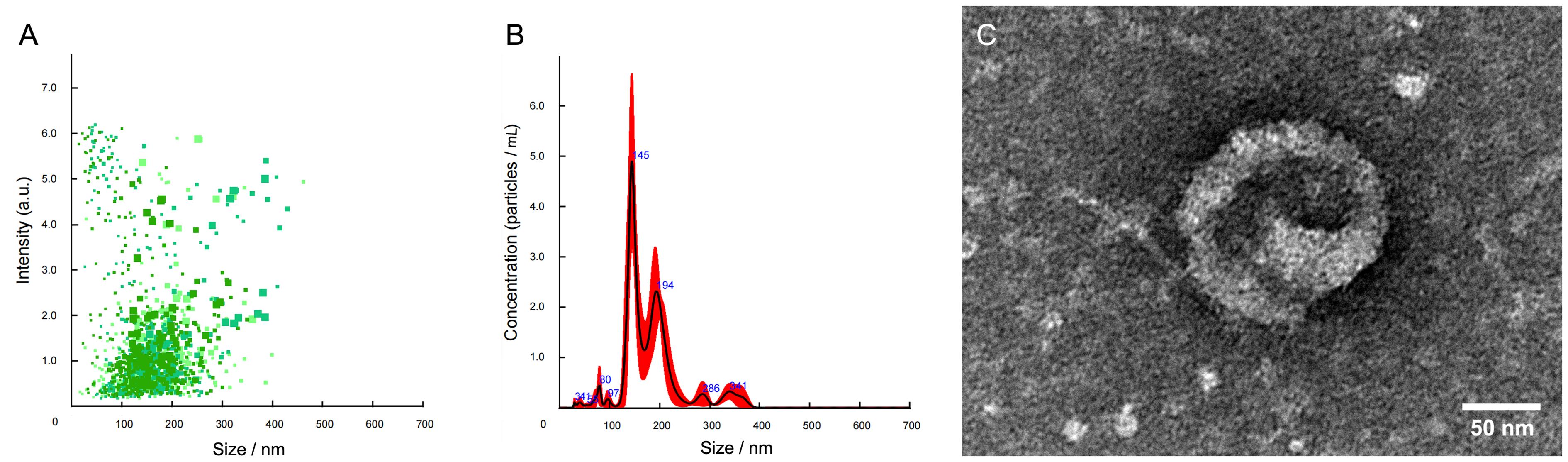
Figure 5 The trunk kidney exosomes in the adult zebrafish identified by nanoparticle tracking analysis (A-B) and transmission electron microscopy (C)Note:A, Scatter diagram of exosome strength/particle size in the trunk kidney of zebrafish; B, Distribution diagram of average exosome concentration/particle size in the trunk kidney of zebrafish; C, Ultrastructure of the trunk kidney exosome in zebrafish.
| 1 | RASOOLY R S, HENKEN D, FREEMAN N, et al. Genetic and genomic tools for zebrafish research: the NIH zebrafish initiative[J]. Dev Dyn, 2003, 228(3):490-496. DOI: 10.1002/dvdy.10366 . |
| 2 | LUBIN A, OTTERSTROM J, HOADE Y, et al. A versatile, automated and high-throughput drug screening platform for zebrafish embryos[J]. Biol Open, 2021, 10(9): bio058513. DOI: 10.1242/bio.058513 . |
| 3 | PATTON E E, ZON L I, LANGENAU D M. Zebrafish disease models in drug discovery: from preclinical modelling to clinical trials[J]. Nat Rev Drug Discov, 2021, 20(8):611-628. DOI: 10.1038/s41573-021-00210-8 . |
| 4 | 熊凤, 谢训卫, 潘鲁媛, 等. 国家斑马鱼资源中心的资源、技术和服务建设[J]. 遗传, 2018, 40(8):683-692. DOI: 10.16288/j.yczz.18-151 . |
| XIONG F, XIE X W, PAN L Y, et al. Development of resources, technologies and services at the China Zebrafish Resource Center[J]. Hereditas, 2018, 40(8):683-692. DOI: 10.16288/j.yczz.18-151 . | |
| 5 | 国家市场监督管理总局, 国家标准化管理委员会. 实验动物 实验鱼质量控制: GB/T 39649—2020[S]. 北京: 中国标准出版社, 2020. |
| State Administration for Market Regulation, Standardization Administration of the People's Republic of China. Laboratory animal-Quality control of laboratory fish: GB/T 39649-2020[S]. Beijing: Standards Press of China, 2020. | |
| 6 | WALLACE M A. Anatomy and physiology of the kidney[J]. AORN J, 1998, 68(5):800, 803-16, 819-20; quiz 821-824. DOI: 10.1016/s0001-2092(06)62377-6 . |
| 7 | LIN J X, CHEN Q S, HU J H. Color atlas of zebrafish histology and cytology[M]. Singapore: Springer, 2022. DOI:10.1007/978-981-16-9852-1 . |
| 8 | THÉRY C, WITWER K W, AIKAWA E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines[J]. J Extracell Vesicles, 2018, 7(1):1535750. DOI: 10.1080/20013078.2018.1535750 . |
| 9 | YANG D B, ZHANG W H, ZHANG H Y, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics[J]. Theranostics, 2020, 10(8):3684-3707. DOI: 10.7150/thno.41580 . |
| 10 | BAI X B, GUO Y N, SHI Y H, et al. In vivo multivesicular bodies and their exosomes in the absorptive cells of the zebrafish (Danio Rerio) gut[J]. Fish Shellfish Immunol, 2019, 88:578-586. DOI: 10.1016/j.fsi.2019.03.030 . |
| 11 | BAI X B, SHI Y H, TARIQUE I, et al. Multivesicular bodies containing exosomes in immune-related cells of the intestine in zebrafish (Danio rerio): Ultrastructural evidence[J]. Fish Shellfish Immunol, 2019, 95:644-649. DOI: 10.1016/j.fsi.2019.10.044 . |
| 12 | LÄSSER C, ELDH M, LÖTVALL J. Isolation and characterization of RNA-containing exosomes[J]. J Vis Exp, 2012(59): e3037. DOI: 10.3791/3037 . |
| 13 | WEN J, ZENG M R, YANG Y Y, et al. Exosomes in diabetic kidney disease[J]. Kidney Dis, 2023, 9(3):131-142. DOI: 10.1159/000529709 . |
| 14 | AGBORBESONG E, BISSLER J, LI X G. Liquid biopsy at the frontier of kidney diseases: application of exosomes in diagnostics and therapeutics[J]. Genes, 2023, 14(7):1367. DOI: 10.3390/genes14071367 . |
| 15 | RESENDE A D, LOBO-DA-CUNHA A, ROCHA E. Comparative summary of the fish renal structure, using the brown trout (Salmo trutta) as the reference model[J]. Microsc Microanal, 2012, 18(S5):47-48. DOI: 10.1017/s1431927612012895 . |
| 16 | 林国辉, 邱玫, 方展强. 剑尾鱼肾脏组织的超微结构观察[J]. 华南师范大学学报(自然科学版), 2011, 43(1): 105-108. |
| LIN G H, QIU M, FANG Z Q. Observation on the histology and ultrastructure of the kidney in xiphophorus helleri [J]. J South China Norm Univ Nat Sci Ed, 2011, 43(1): 105-108. | |
| 17 | VAN NIEL G, D'ANGELO G, RAPOSO G. Shedding light on the cell biology of extracellular vesicles[J]. Nat Rev Mol Cell Biol, 2018, 19(4):213-228. DOI: 10.1038/nrm.2017.125 . |
| 18 | KALLURI R, LEBLEU V S. The biology, function, and biomedical applications of exosomes[J]. Science, 2020, 367(6478): eaau6977. DOI: 10.1126/science.aau6977 . |
| 19 | BANG C, THUM T. Exosomes: new players in cell-cell communication[J]. Int J Biochem Cell Biol, 2012, 44(11):2060-2064. DOI: 10.1016/j.biocel.2012.08.007 . |
| 20 | VAN NIEL G, CARTER D R F, CLAYTON A, et al. Challenges and directions in studying cell-cell communication by extracellular vesicles[J]. Nat Rev Mol Cell Biol, 2022, 23(5):369-382. DOI: 10.1038/s41580-022-00460-3 . |
| 21 | SHEHZAD A, ISLAM S U, SHAHZAD R, et al. Extracellular vesicles in cancer diagnostics and therapeutics[J]. Pharmacol Ther, 2021, 223:107806. DOI: 10.1016/j.pharmthera. 2021.107806 . |
| 22 | ZHENG R, ZHANG K, TAN S Y, et al. Exosomal circLPAR1 functions in colorectal cancer diagnosis and tumorigenesis through suppressing BRD4 via METTL3-eIF3h interaction[J]. Mol Cancer, 2022, 21(1):49. DOI: 10.1186/s12943-021-01471-y . |
| 23 | WITWER K W, WOLFRAM J. Extracellular vesicles versus synthetic nanoparticles for drug delivery[J]. Nat Rev Mater, 2021, 6(2):103-106. DOI: 10.1038/s41578-020-00277-6 . |
| 24 | DUTTA D, KHAN N, WU J F, et al. Extracellular vesicles as an emerging frontier in spinal cord injury pathobiology and therapy[J]. Trends Neurosci, 2021, 44(6):492-506. DOI: 10.1016/j.tins.2021.01.003 . |
| 25 | FAN M, LIU H F, YAN H Y, et al. A CAR T-inspiring platform based on antibody-engineered exosomes from antigen-feeding dendritic cells for precise solid tumor therapy[J]. Biomaterials, 2022, 282:121424. DOI: 10.1016/j.biomaterials. 2022.121424 . |
| 26 | 白雨, 刁宗礼, 刘文虎. 外泌体在糖尿病肾脏疾病发病机制中的作用研究进展[J]. 当代医学, 2022, 28(10): 190-194. DOI: 10.3969/j.issn.1009-4393.2022.10.072 . |
| BAI Y, DIAO Z L, LIU W H. Advance of the role of exosomes in the pathogenesis of diabetic kidney disease[J]. Contemp Med, 2022, 28(10): 190-194. DOI: 10.3969/j.issn.1009-4393.2022.10.072 . | |
| 27 | CHEN Q H, TAKADA R, NODA C, et al. Different populations of Wnt-containing vesicles are individually released from polarized epithelial cells[J]. Sci Rep, 2016, 6:35562. DOI: 10.1038/srep35562 . |
| 28 | FÉVRIER B, RAPOSO G. Exosomes: endosomal-derived vesicles shipping extracellular messages[J]. Curr Opin Cell Biol, 2004, 16(4):415-421. DOI: 10.1016/j.ceb.2004.06.003 . |
| 29 | KLUMPERMAN J, RAPOSO G. The complex ultrastructure of the endolysosomal system[J]. Cold Spring Harb Perspect Biol, 2014, 6(10): a016857. DOI: 10.1101/cshperspect.a016857 . |
| 30 | DENG F Y, MILLER J. A review on protein markers of exosome from different bio-resources and the antibodies used for characterization[J]. J Histotechnol, 2019, 42(4):226-239. DOI: 10.1080/01478885.2019.1646984 . |
| [1] | ZHAO Xin, WANG Chenxi, SHI Wenqing, LOU Yuefen. Advances in the Application of Zebrafish in the Research of Inflammatory Bowel Disease Mechanisms and Drug Development [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 422-431. |
| [2] | Jinxian YANG, Shujuan WANG, Jinyun ZHAI, Shunxing ZHU. Downregulation of Micall2a Gene Expression Inhibited Vascular Development in Zebrafish [J]. Laboratory Animal and Comparative Medicine, 2023, 43(3): 282-287. |
| [3] | Zhigang TAN, Jinxin LIU, Chuya ZHENG, Wenfeng LIAO, Luping FENG, Hongli PENG, Xiu YAN, Zhenjian ZHUO. Advances and Applications in Animal Models of Neuroblastoma [J]. Laboratory Animal and Comparative Medicine, 2023, 43(3): 288-296. |
| [4] | Shirong JIN, Ye HUA, Huaxing ZI, Xufei DU, Jiwen BU. An Optimized Experimental Zebrafish Breeding Scheme for Significantly Enhancing Reproductive Efficiency and Service Life [J]. Laboratory Animal and Comparative Medicine, 2023, 43(3): 297-306. |
| [5] | FENG Yan, WU Wenqing, ZHANG Jingyuan, LI Yu, LI Leichen, YUAN Zheng, CUI Shufang. Isolation and Identification of Exosomes from Skin Fibroblasts of Naked Mole Rats [J]. Laboratory Animal and Comparative Medicine, 2020, 40(6): 506-512. |
| [6] | JIANG Xia, QIAN Haojie, WEI Xun, ZHENG Yuxuan, ZHOU Zhengyu. Research Progress in Construction and Application of Diabetes Model in Zebrafish [J]. Laboratory Animal and Comparative Medicine, 2020, 40(6): 547-552. |
| [7] | DI Yanan, ZHU Liying, QIAN Wen, PAN Wei. Application of Zebrafish as A Model Animal in Research of Human Eye Diseases [J]. Laboratory Animal and Comparative Medicine, 2020, 40(5): 440-. |
| [8] | WANG Xue, LIU Kechun, YANG Xueliang, MA Yukui, ZHANG Yun. Impacts of Okadaic Acid on Neurological Function and Behavior of Zebrafish Larvae#br# [J]. Laboratory Animal and Comparative Medicine, 2020, 40(3): 190-. |
| [9] | Li Ying-niang, Dai Wei, Sheng Jian. Analysis of Goiter in Zebrafish [J]. Laboratory Animal and Comparative Medicine, 2019, 39(6): 462-466. |
| [10] | WANG Li-mei, ZHANG Jian, LIU Zhong-hua, YANG Wei. Research Progress on Zebrafish Model of Retinoblastoma and rb1 Gene [J]. Laboratory Animal and Comparative Medicine, 2019, 39(3): 244-248. |
| [11] | ZHOU Bin, SHENG Zhe-jin, FENG Chen-zhuo, LI Li-mei. A Melanoma Zebrafish Model for Real-time Imaging in vivo [J]. Laboratory Animal and Comparative Medicine, 2018, 38(1): 22-28. |
| [12] | TIAN Fang, WANG Yu-zhu, XIA Min-jie, SUN Bing, DING Xun-cheng, LI Wei-hua, XU Hui-hui, HU Jing-ying. Optimization on Methodology of Histopathological Examination in Adult Zebrafish Visceral Tissues [J]. Laboratory Animal and Comparative Medicine, 2017, 37(6): 465-469. |
| [13] | JIN Lu, LI Yan, LI Zhi-cao, WU Xi-jun, ZHOU Yan-hua, HE Zhi-xu, SHU Li-ping. Effects of Irx5a Gene Overexpression on Early Hematopoietic in Zebrafish Embryos [J]. Laboratory Animal and Comparative Medicine, 2017, 37(4): 309-314. |
| [14] | LIN Jin-xing, FENG Li-ping, HU Jian-hua, GAO Cheng. Microscopical Observation on Pigment Cells in Fins and Scales of Zebrafish [J]. Laboratory Animal and Comparative Medicine, 2017, 37(2): 94-101. |
| [15] | ZHANG Cheng-da, ZHANG Li-li, ZHANG Li-jiang, CHEN Yun-xiang. Estabilishment of Cardiotoxicity Model in Zebrafish Induced by Doxorubicin [J]. Laboratory Animal and Comparative Medicine, 2015, 35(5): 362-366. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||