
Laboratory Animal and Comparative Medicine ›› 2025, Vol. 45 ›› Issue (3): 279-289.DOI: 10.12300/j.issn.1674-5817.2024.128
• Animal Models of Human Diseases • Previous Articles Next Articles
PAN Yicong( ), JIANG Wenhong(
), JIANG Wenhong( )(
)( ), HU Ming, QIN Xiao
), HU Ming, QIN Xiao
Received:2024-08-28
Revised:2024-12-18
Online:2025-06-25
Published:2025-06-25
Contact:
JIANG Wenhong
CLC Number:
PAN Yicong,JIANG Wenhong,HU Ming,et al. Optimization of Surgical Procedure and Efficacy Evaluation of Aortic Calcification Model in Rats with Chronic Kidney Disease[J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 279-289. DOI: 10.12300/j.issn.1674-5817.2024.128.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2024.128
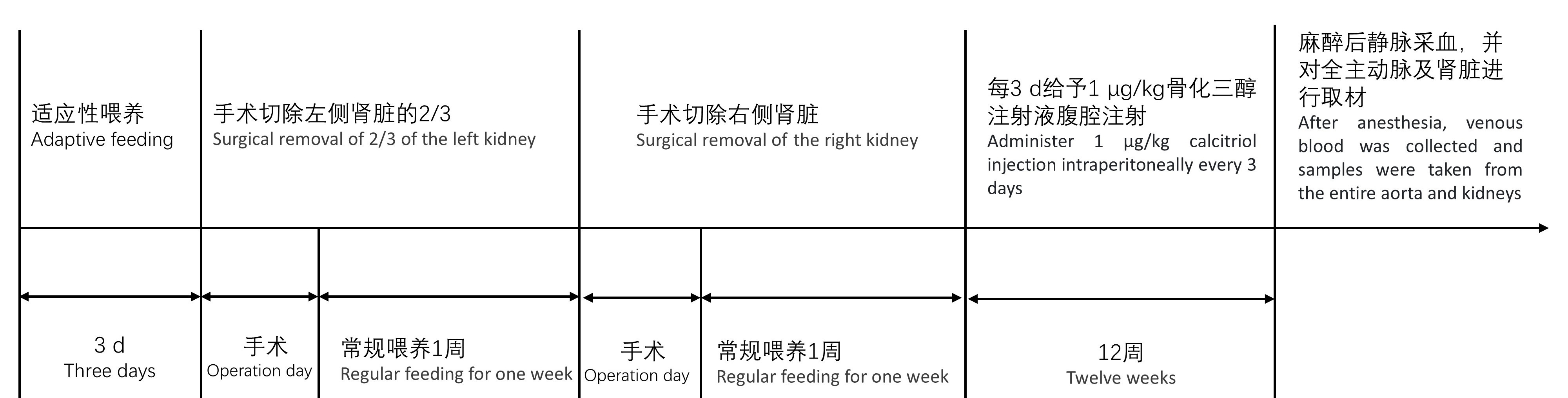
Figure1 Flowchart of the experimental process for modeling and evaluating aortic calcification in SD rats with chronic kidney diseaseNote:Eight-week-old SD rats were housed in SPF-grade animal barrier facilities and underwent left kidney two-thirds nephrectomy after three days of regular dietary adaptation. After surgery, they were routinely housed and underwent right kidney nephrectomy one week later. After one week, administer 1 μg/kg calcitriol injection every three days. After 12 weeks of injection, venous blood collection was performed, and samples were taken from the entire aorta and kidneys. Serum was used for blood biochemistry testing, and paraffin-embedded kidneys were sectioned in coronal side for HE staining. A portion of the aorta was sectioned in paraffin for Alizarin Red S staining and von Kossa staining, and a portion was frozen for real-time fluorescence quantitative PCR detection.
目标基因名称 Target gene name | NCBI基因序列号 NCBI reference sequence | 引物序列信息 Primer sequence information | 扩增片段大小/bp Amplification fragment size/bp |
|---|---|---|---|
| Sm22 (Tagln) | NC-086026.1 | F:5’-CAGATGGAACAGGTGGCTCAA-3’ | 161 |
| R:5’-GCCCAAAGCCATTACAGTCCTC-3’ | |||
| OPN (Spp1) | NC-086032.1 | F:5’-GCCGAGGTGATAGCTTGGCTTA-3’ | 145 |
| R:5’-TTGATAGCCTCATCGGACTCCTG-3’ | |||
| Runx2 | NC-086027.1 | F:5’-GGATGCCTTAGTGCCCAAATG-3’ | 120 |
| R:5’-CACCCTGTGAGGTGGCTGAA-3’ | |||
| β-actin | NC-086030.1 | F:5’-CACCCGCGAGTACAACCTTC-3’ | 207 |
| R:5’-CCCATACCCACCATCACACC-3’ |
Table 1 Primers for real-time fluorescent quantitative PCR
目标基因名称 Target gene name | NCBI基因序列号 NCBI reference sequence | 引物序列信息 Primer sequence information | 扩增片段大小/bp Amplification fragment size/bp |
|---|---|---|---|
| Sm22 (Tagln) | NC-086026.1 | F:5’-CAGATGGAACAGGTGGCTCAA-3’ | 161 |
| R:5’-GCCCAAAGCCATTACAGTCCTC-3’ | |||
| OPN (Spp1) | NC-086032.1 | F:5’-GCCGAGGTGATAGCTTGGCTTA-3’ | 145 |
| R:5’-TTGATAGCCTCATCGGACTCCTG-3’ | |||
| Runx2 | NC-086027.1 | F:5’-GGATGCCTTAGTGCCCAAATG-3’ | 120 |
| R:5’-CACCCTGTGAGGTGGCTGAA-3’ | |||
| β-actin | NC-086030.1 | F:5’-CACCCGCGAGTACAACCTTC-3’ | 207 |
| R:5’-CCCATACCCACCATCACACC-3’ |
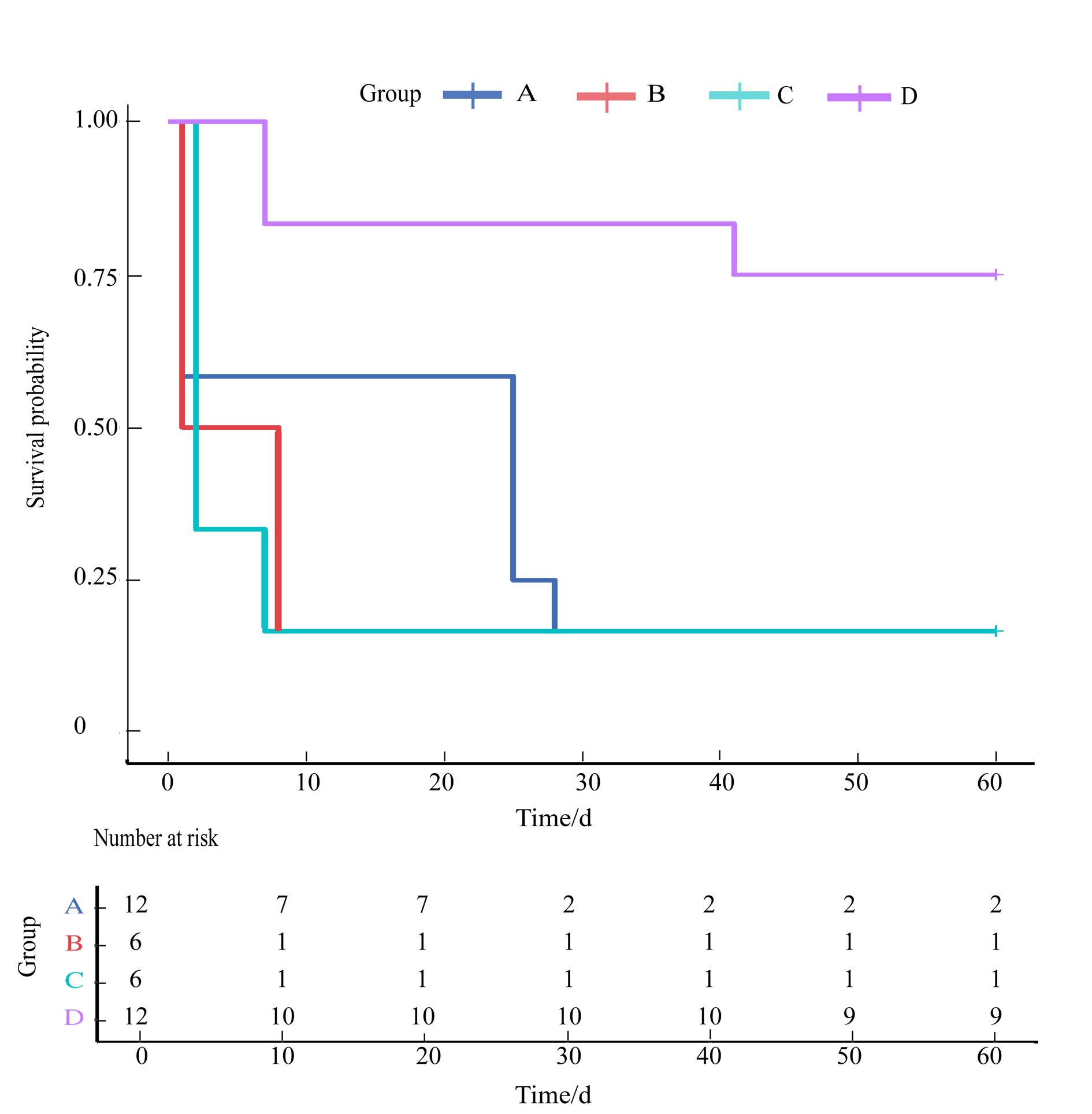
Figure 2 Kaplan-Meier survival curve analysis of rats in chronic renal insufficiency model groups constructed using different surgical methodsNote: The horizontal axis represents the time (in days) recorded from the second day after the removal of both kidneys. Upper of the figure shows that there is a significant difference in the cumulative survival rate of rats after 60 days of surgery using different surgical methods (log-rank test, P=0.007 7), The survival rate of rats in group D was the highest, with statistically significant differences compared to groups A, B, and C (P=0.003 0, P=0.007 4, P=0.003 0, respectively). Moreover, rats in groups A, B, and C experienced high mortality within 10 days after surgery.
血生化指标 Biochemistry index | 实验组(n=9) Experimental group | 对照组(n=12) Control group | t值 t value | P值 P value |
|---|---|---|---|---|
血清钙 c/(mmol·L-1) Serum calcium ions | 1.876±0.036 | 1.929±0.042 | 3.431 | 0.004 |
血清磷c/(mmol·L-1) Serum phosphate ions | 2.059±0.333 | 1.580±0.271 | 3.046 | 0.014 |
血清肌酐c/(μmol·L-1) Serum creatinine | 119.960±35.640 | 21.164±8.076 | 8.984 | <0.000 1 |
血清尿素氮c/(mmol·L-1) Serum urea nitrogen | 57.991±8.745 | 22.609±8.058 | 6.895 | <0.000 1 |
Table 2 Comparison of biochemical indicators between the experimental group and the control group
血生化指标 Biochemistry index | 实验组(n=9) Experimental group | 对照组(n=12) Control group | t值 t value | P值 P value |
|---|---|---|---|---|
血清钙 c/(mmol·L-1) Serum calcium ions | 1.876±0.036 | 1.929±0.042 | 3.431 | 0.004 |
血清磷c/(mmol·L-1) Serum phosphate ions | 2.059±0.333 | 1.580±0.271 | 3.046 | 0.014 |
血清肌酐c/(μmol·L-1) Serum creatinine | 119.960±35.640 | 21.164±8.076 | 8.984 | <0.000 1 |
血清尿素氮c/(mmol·L-1) Serum urea nitrogen | 57.991±8.745 | 22.609±8.058 | 6.895 | <0.000 1 |
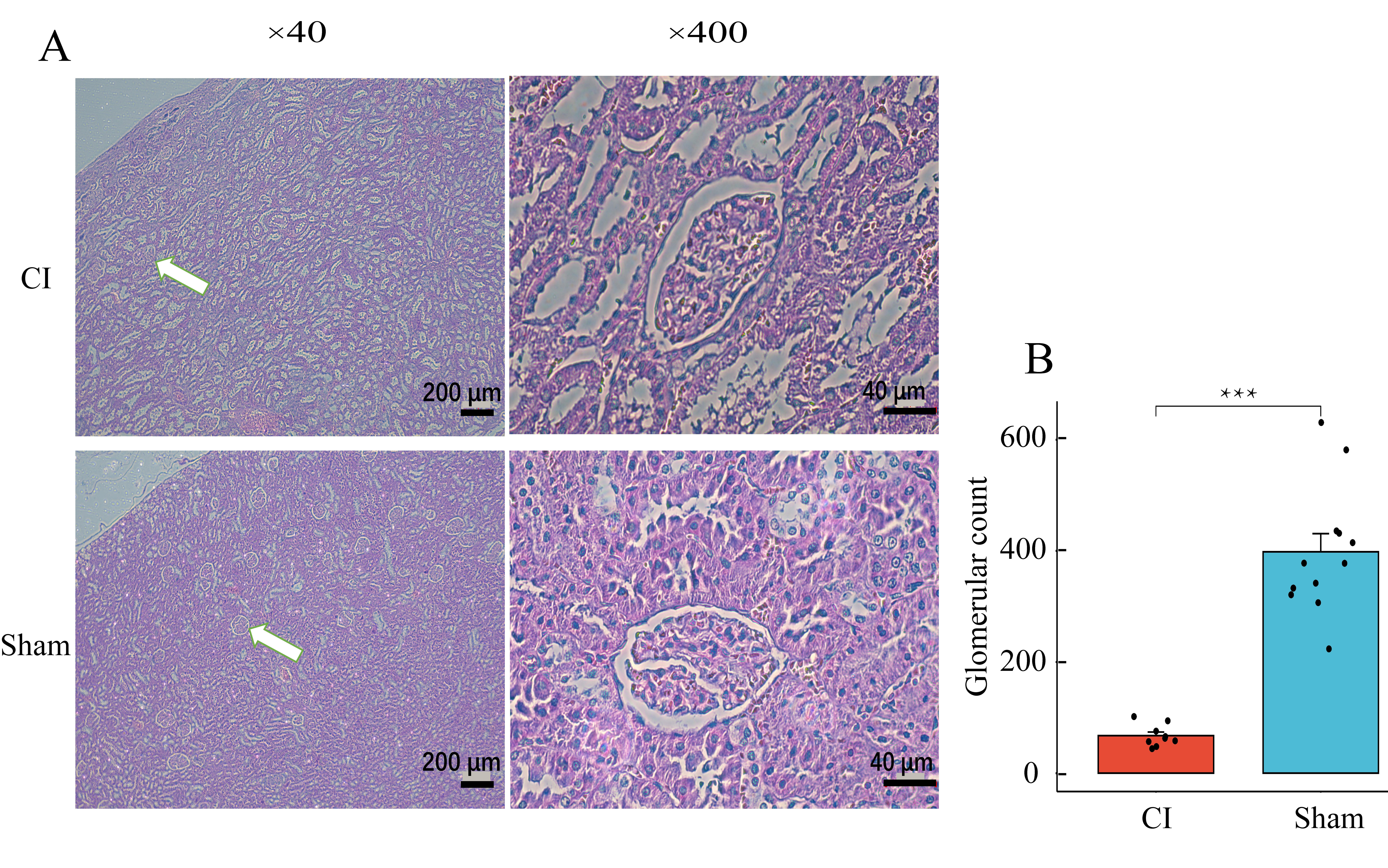
Figure 3 HE staining observation of kidneys from rats in the experimental and control groupsNote: The experimental group consisted of rats undergoing nephrectomy and calcitriol calcification induction for 3 months, while the control group consisted of rats undergoing sham surgery and DMSO injection for 3 months. The white arrow in the picture represents the glomerulus. The number of glomeruli in the experimental group (CI group, n=9) was significantly less than that in the control group (sham group, n=12),***P<0.001.
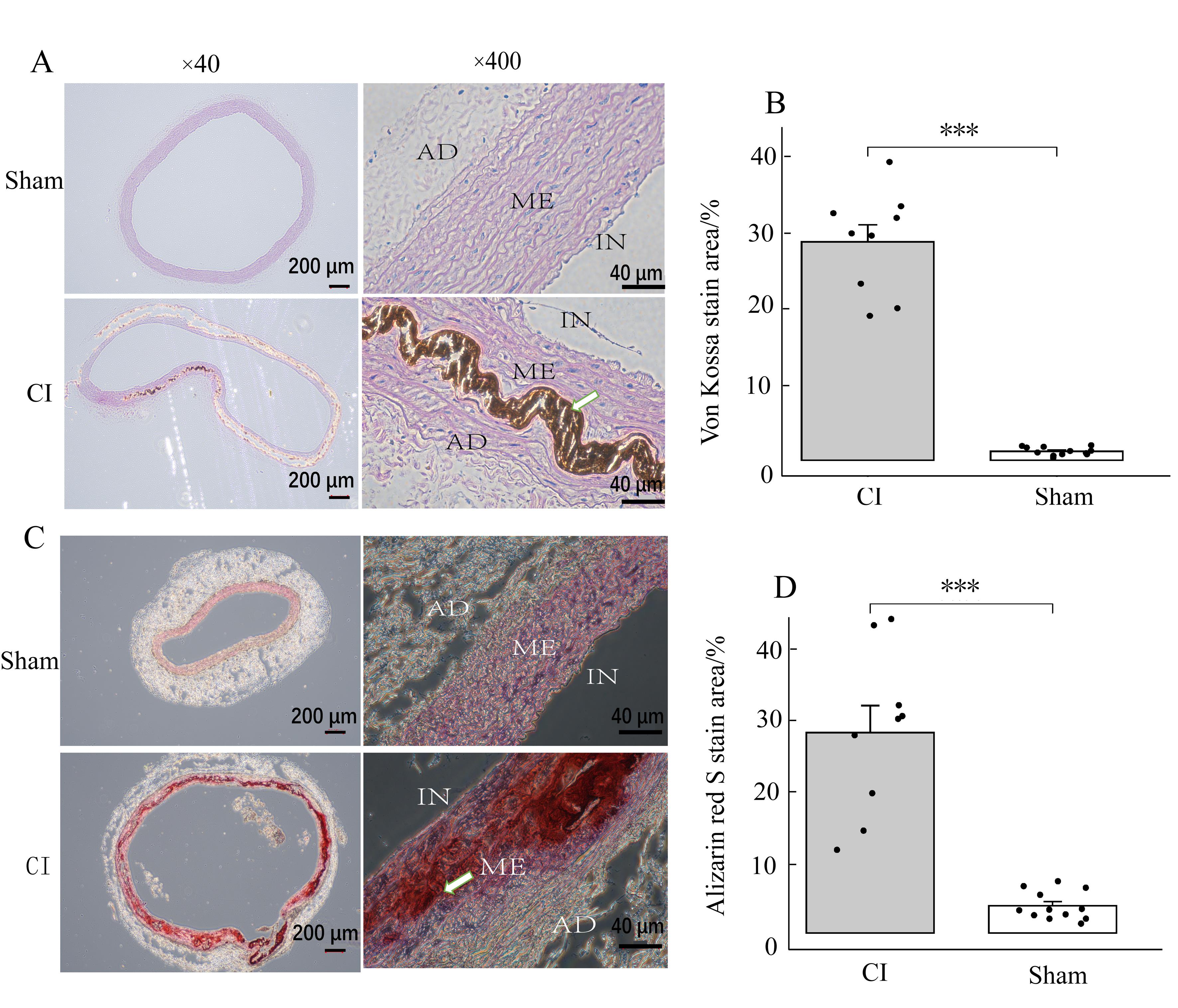
Figure 4 Observation of von Kossa and Alizarin red S staining in the aorta of rats from the experimental and control groupsNote: IN represents the intima of the aorta; ME stands for the media of the aortic; AD represents the adventitia of the aorta. Figures A and B show von Kossa staining; Figures C and D show Alizarin red S staining. The proportion of stained area in the experimental group (CI, n=9) was significantly larger than that in the control group (sham, n=12)(***P<0.001). The white arrow indicates arterial media calcification deposition under high magnification view.
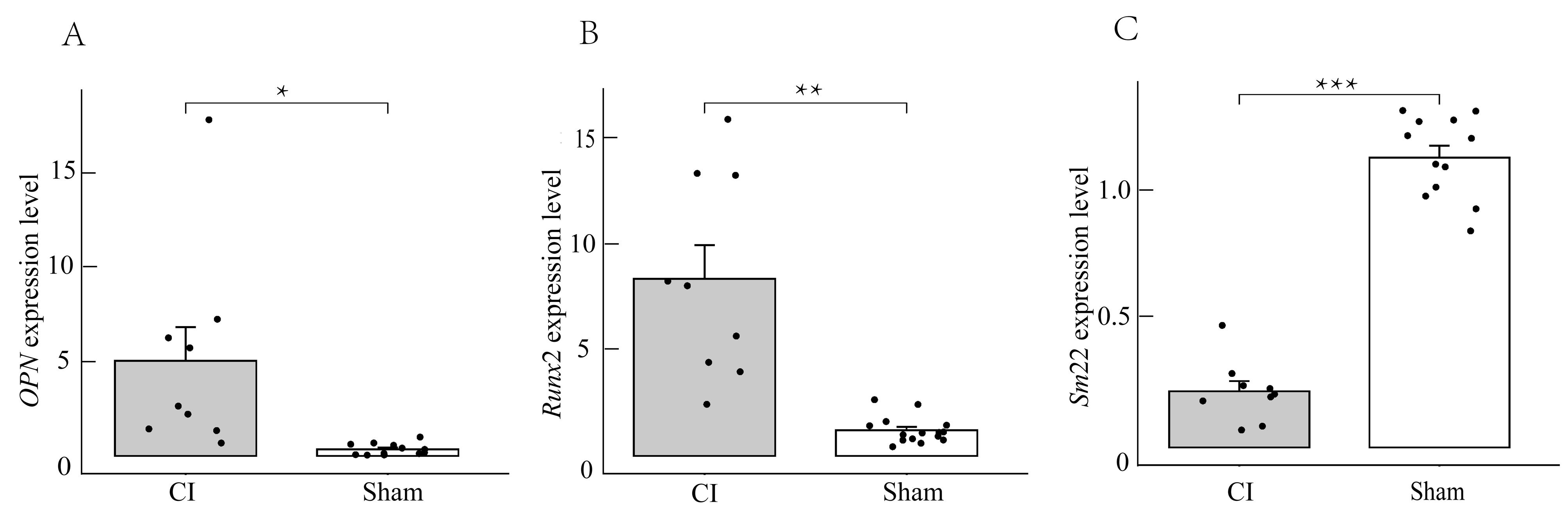
Figure 5 Real-time fluorescence quantitative PCR detection of OPN, Runx2, and Sm22 gene expression in aortic tissue of rats from the experimental and control groupsNote: Compared with the control group (sham), the expression level of Sm22, which reflects the vascular smooth muscle contraction phenotype, decreased in the experimental group (CI) (***P<0.001), while the expression levels of OPN and Runx2, which reflects the osteogenic differentiation phenotype of vascular smooth muscle, increased significantly ( * P<0.05, ** P<0.01).
| [1] | HUANG A R, GUO G Y, YU Y Q, et al. The roles of collagen in chronic kidney disease and vascular calcification[J]. J Mol Med, 2021, 99(1):75-92. DOI:10.1007/s00109-020-02014-6 . |
| [2] | 李贵森. 2019年«中国慢性肾脏病矿物质和骨异常诊治指南»解读[J]. 诊断学理论与实践, 2020, 19(3):229-231. DOI: 10.16150/j.1671-2870.2020.03.005 . |
| LI G S. Interpretation of Chinese Guideline for Diagnosis and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (2019 version)[J]. J Diagn Concepts Pract, 2020, 19(3):229-231. DOI: 10.16150/j.1671-2870.2020.03.005 . | |
| [3] | YE Y Z, CHEN A, LI L, et al. Repression of the antiporter SLC7A11/glutathione/glutathione peroxidase 4 axis drives ferroptosis of vascular smooth muscle cells to facilitate vascular calcification[J]. Kidney Int. 2022 102(6):1259-1275. DOI:10.1016/j.kint.2022.07.034 . |
| [4] | 柳潇雨. Shox2促进血管钙化的作用及机制研究[D]. 广州: 南方医科大学, 2023. DOI:10.27003/d.cnki.gojyu.2023.000180 . |
| LIU X Y. The role and mechanism of Shox2 in promoting vascular calcification[D]. Guangzhou: Southern Medical University, 2023. DOI:10.27003/d.cnki.gojyu.2023.000180 . | |
| [5] | 阙冬冬. 五羟色胺对慢性肾脏病大鼠血管钙化的作用及其机制研究[D]. 广州: 南方医科大学, 2023. DOI:10.27003/d.cnki.gojyu.2023.000170 . |
| QUE D D. Study on the effect and mechanism of serotonin on vascular calcification in rats with chronic kidney disease [D]. Guangzhou: Southern Medical University, 2023. DOI:10.27003/d.cnki.gojyu.2023.000170 . | |
| [6] | 冯丽芸. 二氢杨梅素抑制慢性肾脏病大鼠血管钙化及其机制研究[D]. 广州: 南方医科大学, 2022. DOI:10.27003/d.cnki.gojyu.2022.000227 . |
| FENG L Y. Study on the inhibition of vascular calcification and its mechanism by dihydromyricetin in rats with chronic kidney disease [D]. Guangzhou: Southern Medical University, 2022. DOI:10.27003/d.cnki.gojyu.2022.000227 . | |
| [7] | SHOBEIRI N, ADAMS M A, HOLDEN R M. Vascular calcification in animal models of CKD: a review[J]. Am J Nephrol, 2010, 31(6):471-481. DOI:10.1159/000299794 . |
| [8] | LIU Y J, GUO Y, BAO S M, et al. Bone marrow mesenchymal stem cell-derived exosomal microRNA-381-3p alleviates vascular calcification in chronic kidney disease by targeting NFAT5[J]. Cell Death Dis, 2022, 13(3):278. DOI:10.1038/s41419-022-04703-1 . |
| [9] | 苏培培. 不同剂量骨化三醇对慢性肾衰竭大鼠主动脉钙化的影响[D]. 唐山: 华北理工大学, 2021. DOI:10.27108/d.cnki.ghelu.2021.000272 . |
| SU P P. Effects of different doses of calcitriol on aortic calcification in rats with chronic renal failure [D]. Tangshan: North China University of Science and Technology, 2021. DOI:10.27108/d.cnki.ghelu.2021.000272 . | |
| [10] | WU X J, SHEN S J, WU J Y, et al. ENPP1 ameliorates vascular calcification via inhibiting the osteogenic transformation of VSMCs and generating PPi[J]. Open Med, 2023, 18(1):20230861. DOI:10.1515/med-2023-0861 . |
| [11] | CHEN C Z, LI Y D, LU H L, et al. Curcumin attenuates vascular calcification via the exosomal miR-92b-3p/KLF4 axis[J]. Exp Biol Med, 2022, 247(16):1420-1432. DOI:10.1177/1535370222 1095456 . |
| [12] | LIU X Y, CHEN A, LIANG Q C, et al. Spermidine inhibits vascular calcification in chronic kidney disease through modulation of SIRT1 signaling pathway[J]. Aging Cell, 2021, 20(6): e13377. DOI:10.1111/acel.13377 . |
| [13] | ZHANG X L, LI Y N, YANG P Z, et al. Trimethylamine-N-oxide promotes vascular calcification through activation of NLRP3 (nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3) inflammasome and NF-κB (nuclear factor κB) signals[J]. Arterioscler Thromb Vasc Biol, 2020, 40(3):751-765. DOI:10.1161/ATVBAHA.119.313414 . |
| [14] | ROWE P S, MCCARTHY E M, YU A L, et al. Correction of vascular calcification and hyperphosphatemia in CKD rats treated with ASARM peptide[J]. Kidney360, 2022, 3(10):1683-1698. DOI:10.34067/KID.0002782022 . |
| [15] | MA W Q, SUN X J, ZHU Y, et al. PDK4 promotes vascular calcification by interfering with autophagic activity and metabolic reprogramming[J]. Cell Death Dis, 2020, 11(11):991. DOI:10.1038/s41419-020-03162-w . |
| [16] | SATO H, GOTO M, NISHIMURA G, et al. Upacicalcet, a positive allosteric modulator of the calcium-sensing receptor, prevents vascular calcification and bone disorder in a rat adenine-induced secondary hyperparathyroidism model[J]. Bone, 2023, 167:116613. DOI:10.1016/j.bone.2022.116613 . |
| [17] | LI Z H, WU J, ZHANG X L, et al. CDC42 promotes vascular calcification in chronic kidney disease[J]. J Pathol, 2019, 249(4):461-471. DOI:10.1002/path.5334 . |
| [18] | CHANG J R, GUO J, WANG Y, et al. Intermedin1-53 attenuates vascular calcification in rats with chronic kidney disease by upregulation of α-Klotho[J]. Kidney Int, 2016, 89(3):586-600. DOI:10.1016/j.kint.2015.12.029 . |
| [19] | LEE S J, LEE I K, JEON J H. Vascular calcification-new insights into its mechanism[J]. Int J Mol Sci, 2020, 21(8):2685. DOI:10.3390/ijms21082685 . |
| [20] | 张佳莉, 张岩. 急性肾损伤动物模型构建方法与研究现状[J]. 中国实验动物学报, 2022, 30(7):955-965. DOI: 10.3969/j.issn.1005-4847.2022.07.011 . |
| ZHANG J L, ZHANG Y. Research approaches and status of animal models for acute kidney injury[J]. Acta Lab Anim Sci Sin, 2022, 30(7):955-965. DOI: 10.3969/j.issn.1005-4847.2022.07.011 . | |
| [21] | 陈娟, 易香伶, 罗佳, 等. 急性肾损伤动物模型及体外模型研究进展[J]. 华西医学, 2023, 38(5):777-783. DOI:10.7507/1002-0179.202210185 . |
| CHEN J, YI X L, LUO J, et al. Advances in animal models and in vitro models of acute kidney injury[J]. West China Med J, 2023, 38(5):777-783. DOI:10.7507/1002-0179.202210185 . | |
| [22] | HERRMANN J, BABIC M, TÖLLE M, et al. Research models for studying vascular calcification[J]. Int J Mol Sci, 2020, 21(6):2204. DOI:10.3390/ijms21062204 . |
| [23] | BAO Y W, YUAN Y, CHEN J H, et al. Kidney disease models: tools to identify mechanisms and potential therapeutic targets[J]. Zool Res, 2018, 39(2):72-86. DOI:10.24272/j.issn.2095-8137.2017.055 . |
| [24] | FU Y, TANG C Y, CAI J, et al. Rodent models of AKI-CKD transition[J]. Am J Physiol Renal Physiol, 2018, 315(4):F1098-F1106. DOI:10.1152/ajprenal.00199.2018 . |
| [25] | HERRMANN J, GUMMI M R, XIA M D, et al. Vascular calcification in rodent models-keeping track with an extented method assortment[J]. Biology, 2021, 10(6):459. DOI:10.3390/biology10060459 . |
| [1] | LIU Yayi, JIA Yunfeng, ZUO Yiming, ZHANG Junping, LÜ Shichao. Progress and Evaluation of Animal Model of Heart Qi-Yin Deficiency Syndrome [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 411-421. |
| [2] | QIN Chao, LI Shuangxing, ZHAO Tingting, JIANG Chenchen, ZHAO Jing, YANG Yanwei, LIN Zhi, WANG Sanlong, WEN Hairuo. Study on the 90-day Feeding Experimental Background Data of SD Rats for Drug Safety Evaluation [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 439-448. |
| [3] | LIAN Hui, JIANG Yanling, LIU Jia, ZHANG Yuli, XIE Wei, XUE Xiaoou, LI Jian. Construction and Evaluation of a Rat Model of Abnormal Uterine Bleeding [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 130-146. |
| [4] | YANG Jiahao, DING Chunlei, QIAN Fenghua, SUN Qi, JIANG Xusheng, CHEN Wen, SHEN Mengwen. Research Progress on Animal Models of Sepsis-Related Organ Injury [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 636-644. |
| [5] | HUANG Dongyan, WU Jianhui. Establishment Methods and Application Evaluation of Animal Models in Reproductive Toxicology Research [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 550-559. |
| [6] | ZHENG Yiqing, DENG Yasheng, FAN Yanping, LIANG Tianwei, HUANG Hui, LIU Yonghui, NI Zhaobing, LIN Jiang. Application Analysis of Animal Models for Pelvic Inflammatory Disease Based on Data Mining [J]. Laboratory Animal and Comparative Medicine, 2024, 44(4): 405-418. |
| [7] | WU Yue, LI Lu, ZHANG Yang, WANG Jue, FENG Tingting, LI Yitong, WANG Kai, KONG Qi. Integrative Analysis of Omics Data in Animal Models of Coronavirus Infection [J]. Laboratory Animal and Comparative Medicine, 2024, 44(4): 357-373. |
| [8] | Committee of Experts on Medical Animal Experiments, Chinese Research Hospital Association. Guidelines for the Selection of Animal Models and Preclinical Drug Trials for Spontaneous Intracerebral Hemorrhage (2024 Edition) [J]. Laboratory Animal and Comparative Medicine, 2024, 44(1): 3-30. |
| [9] | Shuwu XIE, Ruling SHEN, Jinxing LIN, Chun FAN. Progress in Establishment and Application of Laboratory Animal Models Related to Development of Male Infertility Drugs [J]. Laboratory Animal and Comparative Medicine, 2023, 43(5): 504-511. |
| [10] | Rui ZHANG, Meiyu LÜ, Jianjun ZHANG, Jinlian LIU, Yan CHEN, Zhiqiang HUANG, Yao LIU, Lanhua ZHOU. Research Progress on Establishing and Evaluation of Acne Animal Models [J]. Laboratory Animal and Comparative Medicine, 2023, 43(4): 398-405. |
| [11] | Jiahui YU, Qian GONG, Lenan ZHUANG. Animal Models of Pulmonary Arterial Hypertension and Their Application in Drug Research [J]. Laboratory Animal and Comparative Medicine, 2023, 43(4): 381-397. |
| [12] | Can LAI, Lele LI, Tala HU, Yan MENG. Recent Advances of Animal Models of Renal Interstitial Fibrosis [J]. Laboratory Animal and Comparative Medicine, 2023, 43(2): 163-172. |
| [13] | Ling HU, Zhibin HU, Yunqing HU, Yuqiang DING. Overview of Studies in Animal Models of Schizophrenia [J]. Laboratory Animal and Comparative Medicine, 2023, 43(2): 145-155. |
| [14] | Jian GE, Jingfen SUN, Yongjie WU. Taurine Has no Protective Effect on Rat Corneal Endothelial Cells Injured by Benzalkonium Chloride [J]. Laboratory Animal and Comparative Medicine, 2023, 43(1): 39-43. |
| [15] | Qin XU, Yan NI, Wenhui SHI, Jianying LI, Jiangwei LIU, Hongqiong ZHAO, Xinming XU. Analysis on Ileum and Colon Microflora of SPF Male SD Rats based on High-throughput Sequencing [J]. Laboratory Animal and Comparative Medicine, 2023, 43(1): 53-60. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||