
Laboratory Animal and Comparative Medicine ›› 2023, Vol. 43 ›› Issue (2): 103-111.DOI: 10.12300/j.issn.1674-5817.2022.154
• Animal Models of Human Diseases • Next Articles
Susu LIU, Yong WU, Yuan CAO, Haoyang ZHAO, Shijie ZHAI, Xiaowei SUN, Linli LI, Changfa FAN( )(
)( )
)
Received:2022-10-09
Revised:2022-11-27
Online:2023-04-25
Published:2023-04-25
Contact:
Changfa FAN
CLC Number:
Susu LIU,Yong WU,Yuan CAO,et al. Establishment of hKDR+/+ Humanized and Rag1-/- Gene Knockout Double Genetically Modified Mouse Model[J]. Laboratory Animal and Comparative Medicine, 2023, 43(2): 103-111. DOI: 10.12300/j.issn.1674-5817.2022.154.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2022.154
引物 Primer | 序列 (5’→3’) Sequence (5’→3’) |
|---|---|
| Rag1-T7 sgRNA-F | TAGGTGAAACGATTCCCACAGATG |
| Rag1-T7 sgRNA-R | AAACCATCTGTGGGAATCGTTTCA |
| Rag1-F | GGGGAAACCTTACCTAGAACAG |
| Rag1-R | AGAATTCCGTCGGGTGGAT |
| KDR-F | TTTATGTTTCAGGTTCAGGGGGAG |
| KDR-R | CCAGCACTGTCTAAATTCAACGGC |
| KDR-WF | GTATGTGTTTCTCTGCCCTCCTCG |
Table 1 Primers for model development
引物 Primer | 序列 (5’→3’) Sequence (5’→3’) |
|---|---|
| Rag1-T7 sgRNA-F | TAGGTGAAACGATTCCCACAGATG |
| Rag1-T7 sgRNA-R | AAACCATCTGTGGGAATCGTTTCA |
| Rag1-F | GGGGAAACCTTACCTAGAACAG |
| Rag1-R | AGAATTCCGTCGGGTGGAT |
| KDR-F | TTTATGTTTCAGGTTCAGGGGGAG |
| KDR-R | CCAGCACTGTCTAAATTCAACGGC |
| KDR-WF | GTATGTGTTTCTCTGCCCTCCTCG |
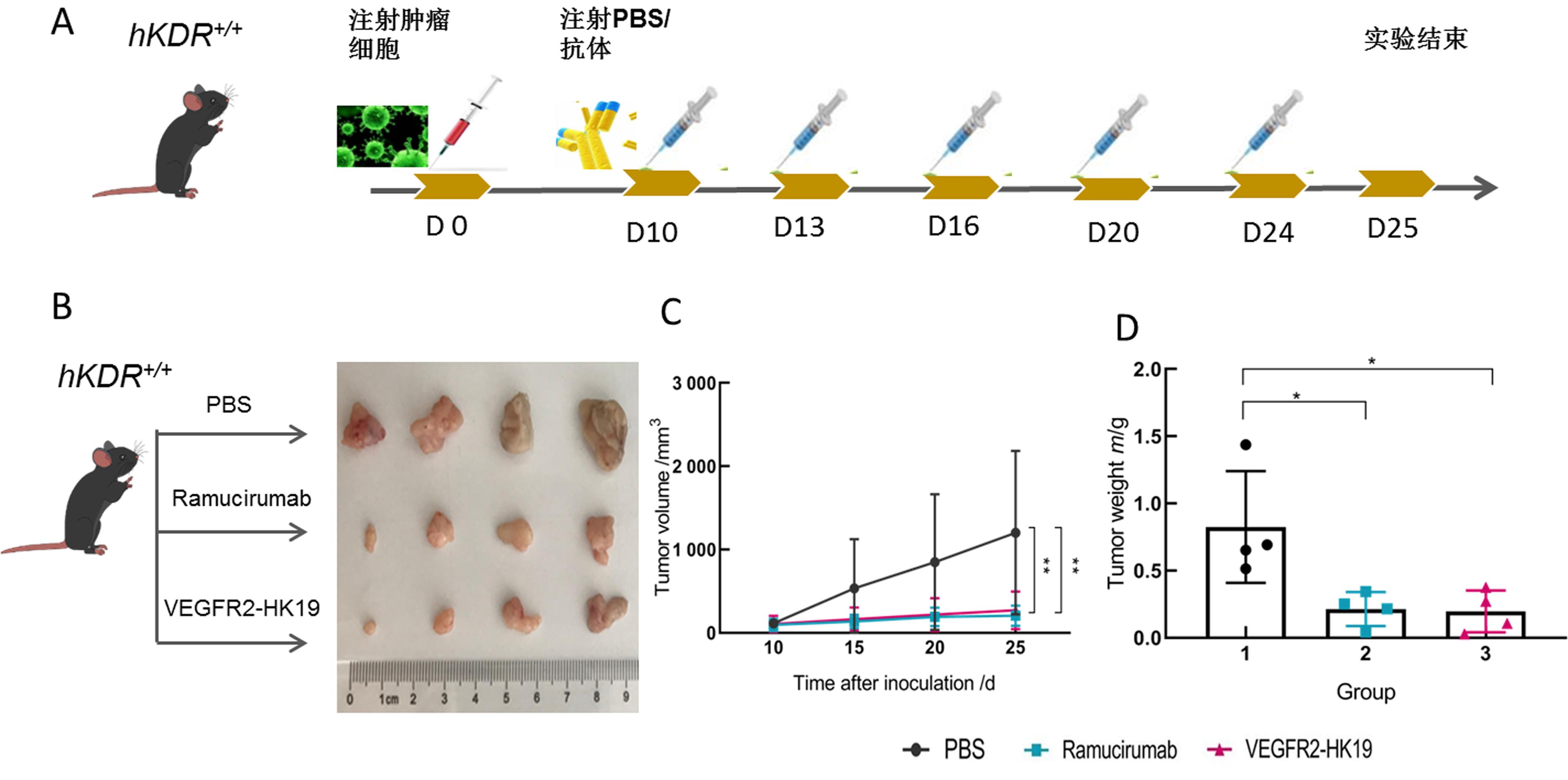
Figure 1 Antibody targeting VEGFR2 targets can inhibit tumor growth in hKDR+/+ mouseNote: A shows the experimental flow diagram of treating MC38 tumor bearing hKDR+/+ mice with humanized antibody or PBS (D0, D10, D13, D16, D20, D24和D25 show the day of injection and the 10th, 13th, 16th, 20th, 24th和25th day after the injection of tumor cells, respectively); B shows the tumors were dissected from each mouse in different groups at the end of the treatment period; C shows Ramucirumab and VEGFR-HK19 can significantly reduce tumor volume (n=4,**P<0.01); D shows the tumor weight in different groups at the end of the treatment (n=4, *P<0.05).
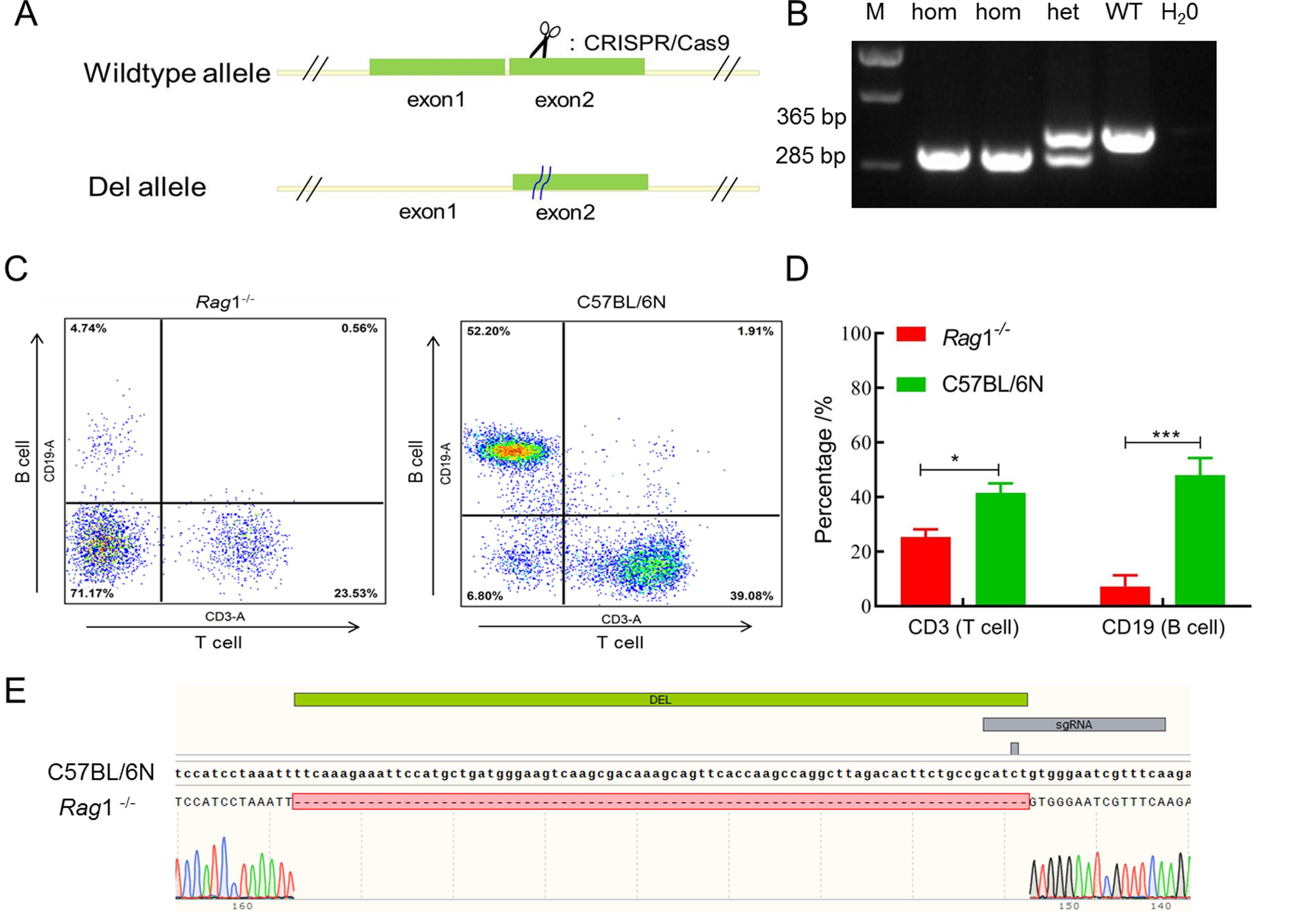
Figure 2 Design strategy and validation of Rag1-/- gene knockout miceNote:A shows model construction design; B shows genotype identification map of Rag1-/- mice (hom shows homozygous mice; het shows heterozygous mice; WT shows wild-type mice). C shows scatter diagrams of the percentage of T and B lymphocytes in peripheral blood of Rag1-/- mice and C57BL/6N wild-type mice. D shows the bar chart of percentage analysis of T and B lymphocytes in the two groups of mice (n=3, *P<0.05, ***P<0.001). E shows the sequence comparison of target fragments of the Rag1 gene. DEL shows the knockout segment.
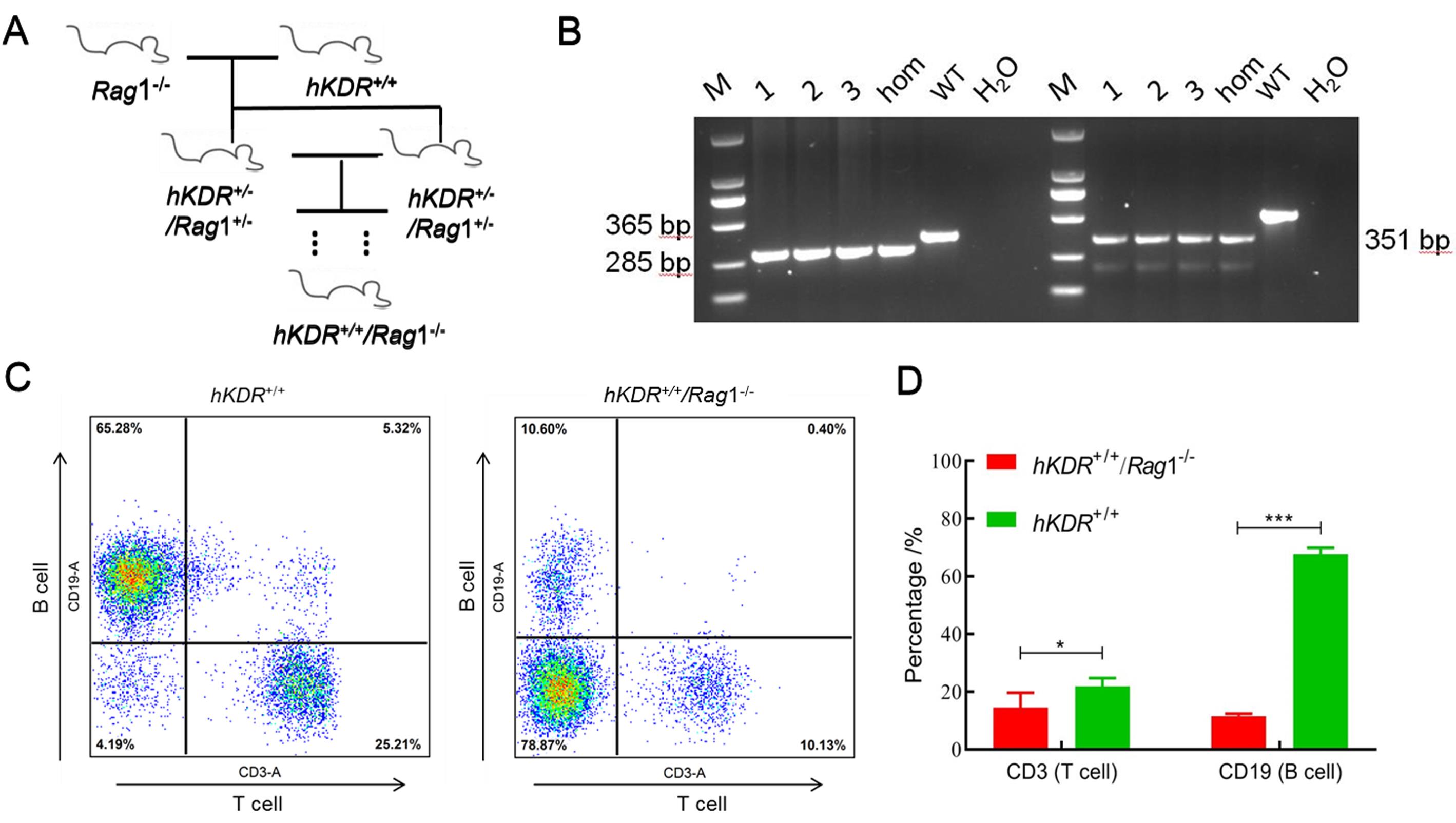
Figure 3 Design strategy and validation of hKDR+/+ humanized and Rag1-/- deficient double genetically modified mouse modelNote:A shows breeding process of hKDR+/+/Rag1-/- humanized double target gene-modified mice; B shows genotype identification of hKDR+/+/Rag1-/- humanized double target gene mice (1, 2, 3 show homozygous mice with two genes,hom shows homozygous mice, WT shows wild-type mice); C shows scatter diagrams of percentage of T and B lymphocytes in the peripheral blood of hKDR+/+/Rag1-/- humanized double target gene-modified mice; D shows the bar chart of percentage analysis of T and B lymphocytes in the two groups of mice(n = 3, *P<0.05,***P<0.001).
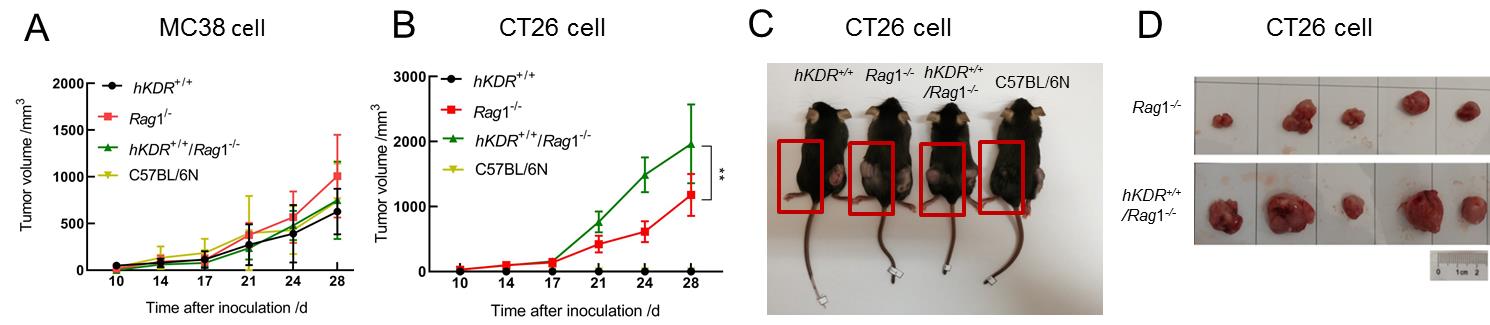
Figure 4 Tumor growth of MC38 and CT26 cells in mice with Rag1-/- gene deficiencyNote: A shows comparison of tumor volume changes in the four types of mice after transplantation of MC38 tumor cells; B shows comparison of tumor volume changes in the four types of mice after transplantation of CT26 tumor cells; C shows the appearances of tumor forming mice inoculated with CT26 cells (left, showed in the red boxes) and MC38 cells (right) at the end of the treatment period; D shows tumor samples of hKDR+/+/Rag1-/- and Rag1-/- mice inoculated with CT26 cells at the end of the experiment. n=5, **P<0.01.
| 1 | OLSSON A K, DIMBERG A, KREUGER J, et al. VEGF receptor signalling―in control of vascular function[J]. Nat Rev Mol Cell Biol, 2006, 7(5):359-371. DOI:10.1038/nrm1911 . |
| 2 | CHUNG A S, LEE J, FERRARA N. Targeting the tumour vasculature: insights from physiological angiogenesis[J]. Nat Rev Cancer, 2010, 10(7):505-514. DOI:10.1038/nrc2868 . |
| 3 | EBOS J M L, KERBEL R S. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis[J]. Nat Rev Clin Oncol, 2011, 8(4):210-221. DOI:10.1038/nrclinonc.2011.21 . |
| 4 | YAO L C, ARYEE K E, CHENG M S, et al. Creation of PDX-bearing humanized mice to study immuno-oncology[J]. Methods Mol Biol, 2019, 1953:241-252. DOI:10.1007/978-1-4939-9145-7_15 . |
| 5 | ITO R, KATANO I, KAWAI K J, et al. Highly sensitive model for xenogenic GVHD using severe immunodeficient NOG mice[J]. Transplantation, 2009, 87(11):1654-1658. DOI:10.1097/TP. 0b013e3181a5cb07 . |
| 6 | BREHM M A, KENNEY L L, WILES M V, et al. Lack of acute xenogeneic graft- versus-host disease, but retention of T-cell function following engraftment of human peripheral blood mononuclear cells in NSG mice deficient in MHC class Ⅰ and Ⅱ expression[J]. FASEB J, 2019, 33(3):3137-3151. DOI:10.1096/fj.201800636R . |
| 7 | FUGMANN S D, LEE A I, SHOCKETT P E, et al. The RAG proteins and V(D)J recombination: complexes, ends, and transposition[J]. Annu Rev Immunol, 2000, 18:495-527. DOI:10.1146/annurev.immunol.18.1.495 . |
| 8 | MOMBAERTS P, IACOMINI J, JOHNSON R S, et al. RAG-1-deficient mice have no mature B and T lymphocytes[J]. Cell, 1992, 68(5):869-877. DOI:10.1016/0092-8674(92)90030-g . |
| 9 | BENTZIEN F, STRUMAN I, MARTINI J F, et al. Expression of the antiangiogenic factor 16K hPRL in human HCT116 colon cancer cells inhibits tumor growth in Rag1 (-/-) mice[J]. Cancer Res, 2001, 61(19):7356-7362. |
| 10 | CAO Y, SUN C Y, HUO G T, et al. Novel hKDR mouse model depicts the antiangiogenesis and apoptosis-promoting effects of neutralizing antibodies targeting vascular endothelial growth factor receptor 2[J]. Cancer Sci, 2023, 114(1):115-128. DOI:10.1111/cas.15594 . |
| 11 | KARAMAN S, LEPPÄNEN V M, ALITALO K. Vascular endothelial growth factor signaling in development and disease[J]. Development, 2018, 145(14):dev151019. DOI:10.1242/dev.151019 . |
| 12 | CHU V T, WEBER T, GRAF R, et al. Efficient generation of Rosa26 knock-in mice using CRISPR/Cas9 in C57BL/6 zygotes[J]. BMC Biotechnol, 2016, 16:4. DOI:10.1186/s12896-016-0234-4 . |
| 13 | 白杰, 高志涛, 石龙, 等. 探讨PD-1抑制剂在不同小鼠肿瘤模型中的应答差异[J]. 解放军医学院学报, 2019, 40(4):357-363. DOI:10.3969/j.issn.2095-5227.2019.04.013 . |
| BAI J, GAO Z T, SHI L, et al. Differential PD-1 inhibitor efficacy among different mouse tumor models[J]. Acad J Chin PLA Med Sch, 2019, 40(4):357-363. DOI:10.3969/j.issn.2095-5227.2019.04.013 . | |
| 14 | TANIURA T, IIDA Y, KOTANI H, et al. Immunogenic chemotherapy in two mouse colon cancer models[J]. Cancer Sci, 2020, 111(10):3527-3539. DOI:10.1111/cas.14624 . |
| 15 | 林怡婷, 林雨虹, 阮梅, 等. MC38-luc稳转细胞株的构建及在小鼠结肠癌肝转移模型中的应用[J]. 福建医科大学学报, 2018, 52(5):305-310. |
| LIN Y T, LIN Y H, RUAN M, et al. Construction and validation of luciferase expressing MC38 cell in murine hepatic metastasis model of colon cancer[J]. J Fujian Med Univ, 2018, 52(5):305-310. | |
| 16 | HERBST R S, ARKENAU H T, SANTANA-DAVILA R, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial[J]. Lancet Oncol, 2019, 20(8):1109-1123. DOI:10.1016/S1470-2045(19)30458-9 . |
| 17 | CHATTERJEE S, HEUKAMP L C, SIOBAL M, et al. Tumor VEGF: VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer[J]. J Clin Invest, 2013, 123(4):1732-1740. DOI:10.1172/JCI65385 . |
| 18 | GIATROMANOLAKI A, KOUKOURAKIS M I, SIVRIDIS E, et al. Activated VEGFR2/KDR pathway in tumour cells and tumour associated vessels of colorectal cancer[J]. Eur J Clin Invest, 2007, 37(11):878-886. DOI:10.1111/j.1365-2362.2007.01866.x . |
| 19 | LU R M, CHIU C Y, LIU I J, et al. Novel human Ab against vascular endothelial growth factor receptor 2 shows therapeutic potential for leukemia and prostate cancer[J]. Cancer Sci, 2019, 110(12):3773-3787. DOI:10.1111/cas.14208 . |
| 20 | FRANKLIN M C, NAVARRO E C, WANG Y J, et al. The structural basis for the function of two anti-VEGF receptor 2 antibodies[J]. Structure, 2011, 19(8):1097-1107. DOI:10.1016/j.str.2011.01.019 . |
| [1] | Xi FANG, Qingqing AO, Chunhong LI, Yiqiang OUYANG, Songchao GUO, Bing HU. Metabolomics Analysis of Tupaia belangeri Breast Tumor Model [J]. Laboratory Animal and Comparative Medicine, 2024, 44(1): 52-61. |
| [2] | Yanjuan CHEN, Ruling SHEN. Progress in the Application of Animal Disease Models in the Medical Research on Colorectal Cancer [J]. Laboratory Animal and Comparative Medicine, 2023, 43(5): 512-523. |
| [3] | Shanshan ZHAI, Liang LIANG, Yingying CAO, Zhuxin LI, Qing WANG, Junyu TAO, Chenxia YUN, Jing LENG, Haibo TANG. Diagnosis of Trichoepithelioma in a Tree Shrew and Observation of Cell Biological Characteristics [J]. Laboratory Animal and Comparative Medicine, 2023, 43(4): 440-445. |
| [4] | Lujing MAO, Nana ZHANG, Hao LIU, Aihua SHI, Ziyu ZHU, Yi LÜ. Establishment and Comparison of Mouse Pancreatic Cancer Orthotopic Model for Minimally Invasive Ablation Therapy Study [J]. Laboratory Animal and Comparative Medicine, 2022, 42(2): 127-134. |
| [5] | SONG Weijie, ZHOU Yan, NIU Ruifang. Application of Laboratory Animal Models in Cancer Precision Medicine Research [J]. Laboratory Animal and Comparative Medicine, 2021, 41(6): 493-500. |
| [6] | ZHANG Boyang, CHEN Huanpeng, YU Wenlan, LIU Zhonghua, HUANG Chaofeng. Dynamic Changes of Tumor-infiltrating Immune Cells in Mice with MC38 Colon Cancer [J]. Laboratory Animal and Comparative Medicine, 2021, 41(5): 409-417. |
| [7] | XIAO Pan, NIU Tingxian, LUO Xiaohong, WANG Hongyi, GUO Xiaoyu, LU Lu, FENG Xiaoming. Changes of C-reactive Protein and Inflammatory Factors in Juema Pig Model with High Altitude Multiple Organ Dysfunction Syndrome [J]. Laboratory Animal and Comparative Medicine, 2021, 41(3): 238-243. |
| [8] | ZHANG Xiaojie, JIANG Lei. Molecular Mechanism of Resveratrol Against Myocardial Ischemia-Reperfusion Injury in Rats Based on Network Pharmacology [J]. Laboratory Animal and Comparative Medicine, 2020, 40(6): 496-505. |
| [9] | LUO Baohua, ZHANG Yongbin, LIU Xiaoqiu, SHI Changhong. Establishment and Applications of Tumor Organoid Models [J]. Laboratory Animal and Comparative Medicine, 2020, 40(6): 540-546. |
| [10] | ZHOU Xiaoli, ZHANG Qian, GAO Zheng, QIAN Zhiyong. Pathological Observation of Spontaneous Tumor in Aged SD Rats [J]. Laboratory Animal and Comparative Medicine, 2020, 40(5): 420-. |
| [11] | KANG Yu, YANG Xiaofang. Protective Effect and Mechanism of Nanoliposomal Quercetin on Focal Cerebral Ischemia-reperfusion Injury in Rats [J]. Laboratory Animal and Comparative Medicine, 2020, 40(2): 116-122. |
| [12] | WANG Aihong, WANG Mingquan, DU Juan. O-GlcNAc Glycosylation Level Positively Regulates Ang-2 Expression and Promotes Tumor Angiogenesis in Mice with Liver Cancer [J]. Laboratory Animal and Comparative Medicine, 2020, 40(1): 39-46. |
| [13] | CHEN Chao, CONG Wei, YANG Wen-jing, CUI Shu-fang. The Characteristics of Anti-chemical Cancer Inducing Factors in Naked Mole-rat [J]. Laboratory Animal and Comparative Medicine, 2019, 39(6): 484-488. |
| [14] | HU Bin-quan, CHU Hai-meng, SHI Chang-hong. Application of Patient-Derived Xenograft Models for Personalized Treatment of Pancreatic Cancer [J]. Laboratory Animal and Comparative Medicine, 2019, 39(6): 499-504. |
| [15] | MI Jin-xia, FANG Zhao-qin. Research Progress of Rabbit VX2 Tumor Model [J]. Laboratory Animal and Comparative Medicine, 2019, 39(2): 163-168. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||