
Laboratory Animal and Comparative Medicine ›› 2024, Vol. 44 ›› Issue (1): 52-61.DOI: 10.12300/j.issn.1674-5817.2023.094
• Animal Models of Human Diseases • Previous Articles Next Articles
Xi FANG1, Qingqing AO2, Chunhong LI1, Yiqiang OUYANG1, Songchao GUO1, Bing HU1( )
)
Received:2023-06-30
Revised:2023-12-12
Online:2024-02-25
Published:2024-02-25
Contact:
Bing HU
CLC Number:
Xi FANG,Qingqing AO,Chunhong LI,et al. Metabolomics Analysis of Tupaia belangeri Breast Tumor Model[J]. Laboratory Animal and Comparative Medicine, 2024, 44(1): 52-61. DOI: 10.12300/j.issn.1674-5817.2023.094.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2023.094
分组 Group | 动物数量 n Number of Tupaia belangeri | 体重/g Body weight/g | 体长/cm Body length/cm | 肿瘤质量/g Tumor weight/g | 肿瘤体积/mm3 Tumor size/mm3 |
|---|---|---|---|---|---|
DMBA造模未成瘤组 DMBA-induced group with breast cancer | 8 | 135.36±20.31 | 17.91±2.12 | 3.15±1.40 | 4 153.12±213.23 |
DMBA造模未成瘤组 DMBA-induced group without breast cancer | 12 | 131.11±11.54 | 17.68±1.32 | - | - |
正常对照组 Normal control | 12 | 123.65±10.54 | 17.73±0.65 | - | - |
Table 1 Basic information of Tupaia belangeri in three groups
分组 Group | 动物数量 n Number of Tupaia belangeri | 体重/g Body weight/g | 体长/cm Body length/cm | 肿瘤质量/g Tumor weight/g | 肿瘤体积/mm3 Tumor size/mm3 |
|---|---|---|---|---|---|
DMBA造模未成瘤组 DMBA-induced group with breast cancer | 8 | 135.36±20.31 | 17.91±2.12 | 3.15±1.40 | 4 153.12±213.23 |
DMBA造模未成瘤组 DMBA-induced group without breast cancer | 12 | 131.11±11.54 | 17.68±1.32 | - | - |
正常对照组 Normal control | 12 | 123.65±10.54 | 17.73±0.65 | - | - |
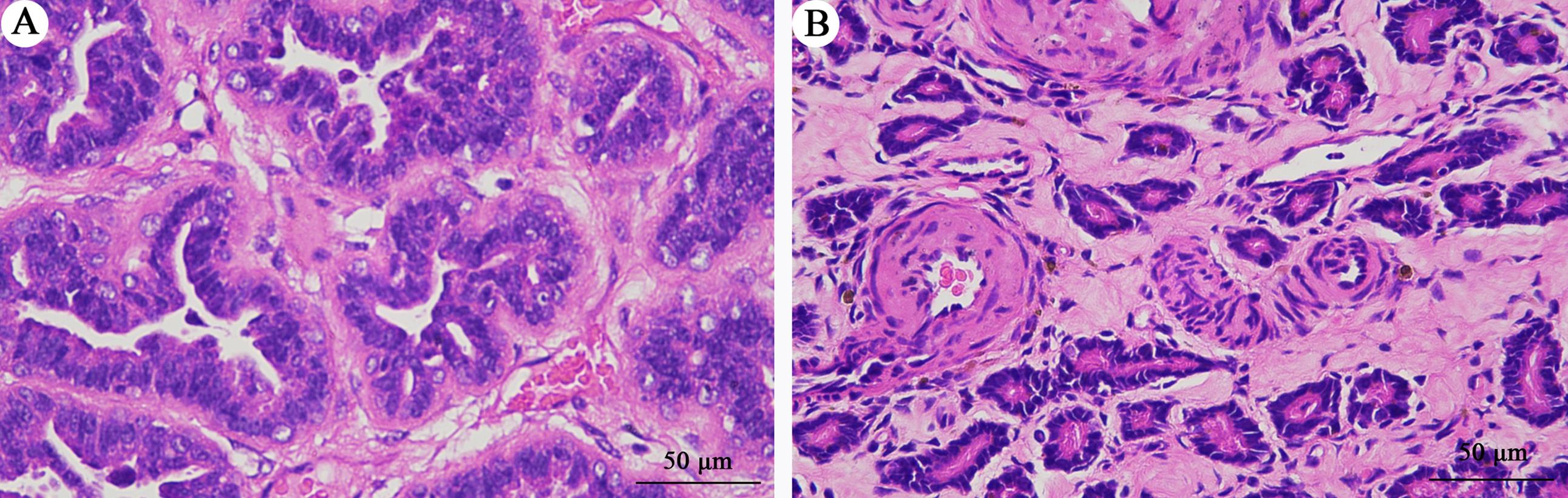
Figure 1 Pathological results of breast cancer in Tupaia belangeriNote:A, Breast tumour tissue of the DMBA-induced breast cancer group; B, Breast tissue of the normal group. Scale bars, 50 μm.

Figure 2 Total ion flow maps of serum from each group of Tupaia belangeriNote: A, DMBA-induced breast cancer group (n=8); B, DMBA-induced without breast cancer group (n=12); C, Normal control group (n=12).
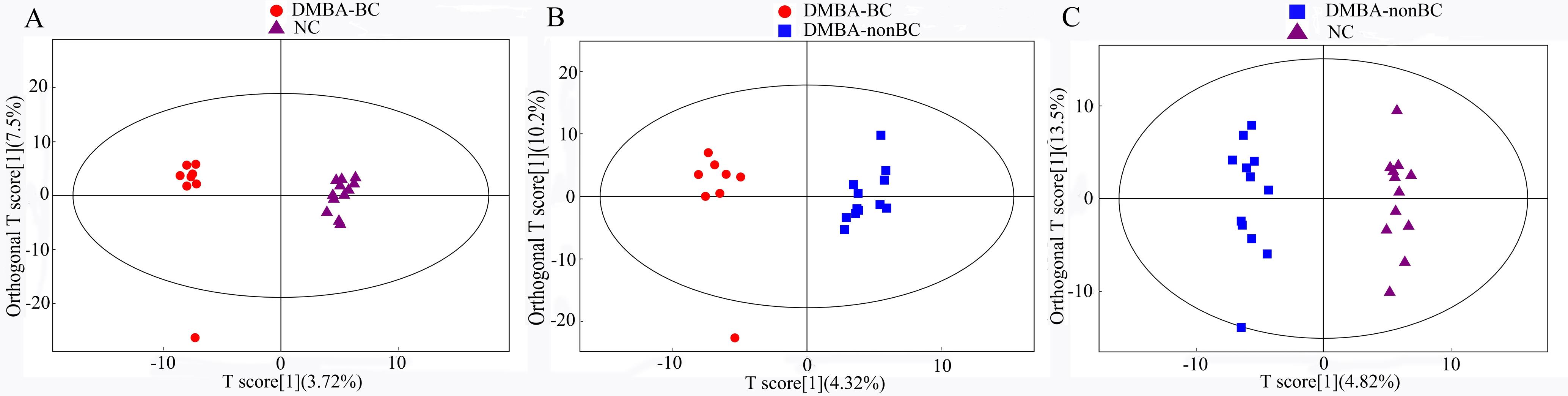
Figure 3 Score scatter plot of OPLS-DA model for each group of Tupaia belangeriNote:A, The OPLS-DA score plot of blood metabolites comparison between DMBA-induced breast cancer (DMBA-BC) group and normal control (NC) group; B, The OPLS-DA score plot of blood metabolites comparison between DMBA-induced with breast cancer (DMBA-BC) group and those without breast cancer (DMBA-nonBC); C, The OPLS-DA score plot of blood metabolites comparison between DMBA-induced without breast cancer (DMBA-nonBC) group and normal control (NC) group.
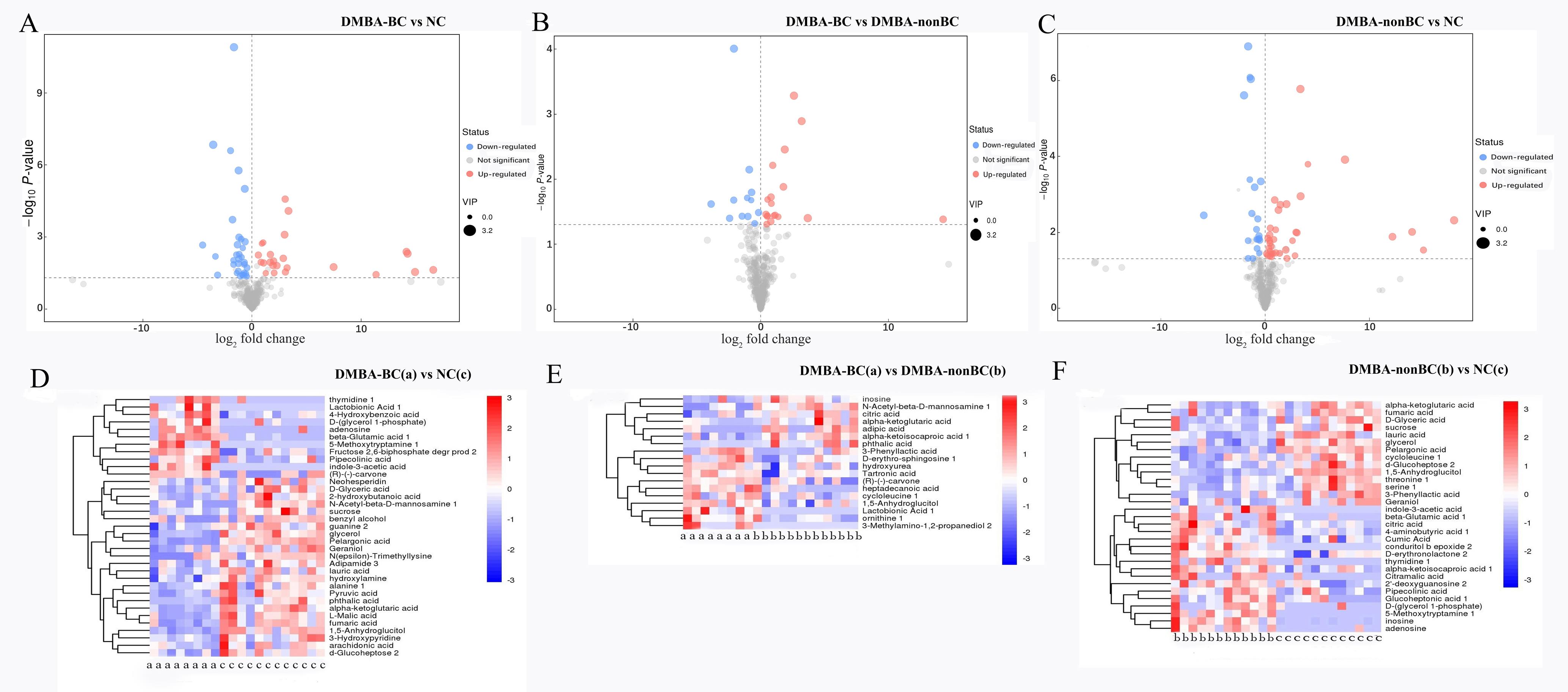
Figure 4 The volcano map (A-C) and heatmap of hierarchical clustering analysis (D-F) of differential metabolites between groups of Tupaia belangeriNote:A and D, DMBA-induced group with breast cancer (DMBA-BC) vs normal control (NC) group; B and E, DMBA-induced group with breast cancer (DMBA-BC) vs DMBA-induced group without breast cancer (DMBA-nonBC); C and F, DMBA-induced group without breast cancer (DMBA-nonBC) vs normal control (NC) group.
DMBA造模成瘤组vs正常对照组 DMBA-BC vs NC | DMBA造模成瘤组vsDMBA造模未成瘤组 DMBA-BC vs DMBA-nonBC | DMBA造模未成瘤组vs正常对照组 DMBA-nonBC vs NC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
代谢物 Metabolites | VIP | P | Log-FC | CT | 代谢物 Metabolites | VIP | P | Log-FC | CT | 代谢物 Metabolites | VIP | P | Log-FC | CT |
甘油酸-3-磷酸 Glycerate-3-phosphate | 2.762 | <0.001 | -0.650 | ↑ | 十七烷酸 Margaric acid | 2.236 | 0.013 | 1.783 | ↑ | 甲氧基色胺 Methoxytry ptamine | 3.020 | <0.001 | 7.659 | ↑ |
谷氨酸 Glutamate | 2.086 | <0.001 | 3.051 | ↑ | 香芹酮 Carvone | 2.096 | 0.019 | 0.812 | ↑ | 肌苷 Inosine | 1.280 | <0.001 | 4.104 | ↑ |
腺苷 Adenosine | 2.561 | 0.001 | 2.995 | ↑ | 羟基脲 Hydroxyurea | 1.160 | 0.021 | 0.539 | ↑ | 葡萄糖庚糖 Glucose heptose | 1.303 | <0.001 | -1.476 | ↓ |
2,6-二磷酸果糖 2,6-Fructose diphosphate | 1.273 | 0.002 | 1.035 | ↑ | 1-磷酸鞘氨醇 Sphingosine 1- phosphate | 1.431 | 0.024 | 0.839 | ↑ | 甘油 Glycerol | 2.420 | <0.001 | -0.419 | ↓ |
4-羟基苯甲酸 4-Hydroxyben- zoic acid | 1.101 | 0.002 | 0.916 | ↑ | 1,5-脱水葡萄糖醇 1,5-Dehydrated glucose alcohol | 1.878 | 0.035 | 0.418 | ↑ | 月桂酸 Lauric acid | 2.291 | 0.001 | -1.011 | ↓ |
壬酸 Pelargonic acid | 3.167 | <0.001 | -1.640 | ↓ | 鸟氨酸 Ornithine | 1.344 | 0.036 | 1.042 | ↑ | 腺苷 Adenosine | 2.737 | 0.001 | 3.399 | ↑ |
1,5-脱水葡萄糖醇 1, 5-Anhydro- gluosol | 2.779 | <0.001 | -1.216 | ↓ | 丙醇二酸 Tartronic acid | 1.375 | 0.037 | 0.508 | ↑ | 氨基丁酸 Aminobutyric acid | 2.140 | 0.001 | 0.914 | ↑ |
α-酮戊二酸 α-Ketoglutaric acid | 2.382 | <0.001 | -1.771 | ↓ | 3-苯基乳酸 3-Phenyllactic acid | 1.248 | 0.038 | 1.373 | ↑ | 苏氨酸 Threonine | 1.738 | 0.004 | -0.702 | ↓ |
富马酸 Fumaric acid | 2.301 | 0.001 | -1.174 | ↓ | N-乙酰基-D-氨基胺 N-acetyl-D- aminoamine | 2.648 | 0 | -2.090 | ↓ | 1,2-脱水肌醇 1,2-Dehydrated Inositol | 2.925 | 0.005 | 18.130 | ↑ |
羟胺 Hydroxylamine | 1.492 | 0.001 | -0.975 | ↓ | α-酮异己酸 α-Ketoisocaproic acid | 2.371 | 0.007 | -0.891 | ↓ | 丝氨酸 Serine | 1.568 | 0.008 | -0.830 | ↓ |
2-羟基丁酸 2-Hydroxybutyric acid | 2.176 | 0.002 | -0.625 | ↓ | 己二酸 Adipic acid | 1.757 | 0.021 | -2.106 | ↓ | 吲哚乙酸 Indoleacetic acid | 2.373 | 0.010 | 14.098 | ↑ |
L-苹果酸 L-malic acid | 2.313 | 0.002 | -1.319 | ↓ | 柠檬酸 Citric acid | 1.043 | 0.021 | -0.742 | ↓ | 胸苷 Thymidine | 1.882 | 0.010 | 2.960 | ↑ |
邻苯二甲酸 Phthalate | 1.860 | 0.002 | -4.509 | ↓ | 邻苯二甲酸 Phthalic acid | 2.109 | 0.024 | -3.879 | ↓ | 柠檬酸 Citric acid | 2.049 | 0.010 | 3.048 | ↑ |
丙酮酸 Pyruvate | 2.085 | 0.008 | -1.193 | ↓ | 肌苷 Inosine | 1.690 | 0.037 | -1.451 | ↓ | 甘油酸-3-磷酸 Glycerate-3- phosphate | 1.664 | 0.015 | -0.574 | ↓ |
花生四烯酸 Arachidonic acid | 1.356 | 0.033 | -0.543 | ↓ | α-酮戊二酸 α-Ketoglutaric acid | 1.978 | 0.038 | -0.996 | ↓ | 谷氨酸 Glutamate | 1.292 | 0.016 | 2.622 | ↑ |
Table 2 Key differential metabolites between DMBA-induced group with breast cancer and control group of Tupaia belangeri
DMBA造模成瘤组vs正常对照组 DMBA-BC vs NC | DMBA造模成瘤组vsDMBA造模未成瘤组 DMBA-BC vs DMBA-nonBC | DMBA造模未成瘤组vs正常对照组 DMBA-nonBC vs NC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
代谢物 Metabolites | VIP | P | Log-FC | CT | 代谢物 Metabolites | VIP | P | Log-FC | CT | 代谢物 Metabolites | VIP | P | Log-FC | CT |
甘油酸-3-磷酸 Glycerate-3-phosphate | 2.762 | <0.001 | -0.650 | ↑ | 十七烷酸 Margaric acid | 2.236 | 0.013 | 1.783 | ↑ | 甲氧基色胺 Methoxytry ptamine | 3.020 | <0.001 | 7.659 | ↑ |
谷氨酸 Glutamate | 2.086 | <0.001 | 3.051 | ↑ | 香芹酮 Carvone | 2.096 | 0.019 | 0.812 | ↑ | 肌苷 Inosine | 1.280 | <0.001 | 4.104 | ↑ |
腺苷 Adenosine | 2.561 | 0.001 | 2.995 | ↑ | 羟基脲 Hydroxyurea | 1.160 | 0.021 | 0.539 | ↑ | 葡萄糖庚糖 Glucose heptose | 1.303 | <0.001 | -1.476 | ↓ |
2,6-二磷酸果糖 2,6-Fructose diphosphate | 1.273 | 0.002 | 1.035 | ↑ | 1-磷酸鞘氨醇 Sphingosine 1- phosphate | 1.431 | 0.024 | 0.839 | ↑ | 甘油 Glycerol | 2.420 | <0.001 | -0.419 | ↓ |
4-羟基苯甲酸 4-Hydroxyben- zoic acid | 1.101 | 0.002 | 0.916 | ↑ | 1,5-脱水葡萄糖醇 1,5-Dehydrated glucose alcohol | 1.878 | 0.035 | 0.418 | ↑ | 月桂酸 Lauric acid | 2.291 | 0.001 | -1.011 | ↓ |
壬酸 Pelargonic acid | 3.167 | <0.001 | -1.640 | ↓ | 鸟氨酸 Ornithine | 1.344 | 0.036 | 1.042 | ↑ | 腺苷 Adenosine | 2.737 | 0.001 | 3.399 | ↑ |
1,5-脱水葡萄糖醇 1, 5-Anhydro- gluosol | 2.779 | <0.001 | -1.216 | ↓ | 丙醇二酸 Tartronic acid | 1.375 | 0.037 | 0.508 | ↑ | 氨基丁酸 Aminobutyric acid | 2.140 | 0.001 | 0.914 | ↑ |
α-酮戊二酸 α-Ketoglutaric acid | 2.382 | <0.001 | -1.771 | ↓ | 3-苯基乳酸 3-Phenyllactic acid | 1.248 | 0.038 | 1.373 | ↑ | 苏氨酸 Threonine | 1.738 | 0.004 | -0.702 | ↓ |
富马酸 Fumaric acid | 2.301 | 0.001 | -1.174 | ↓ | N-乙酰基-D-氨基胺 N-acetyl-D- aminoamine | 2.648 | 0 | -2.090 | ↓ | 1,2-脱水肌醇 1,2-Dehydrated Inositol | 2.925 | 0.005 | 18.130 | ↑ |
羟胺 Hydroxylamine | 1.492 | 0.001 | -0.975 | ↓ | α-酮异己酸 α-Ketoisocaproic acid | 2.371 | 0.007 | -0.891 | ↓ | 丝氨酸 Serine | 1.568 | 0.008 | -0.830 | ↓ |
2-羟基丁酸 2-Hydroxybutyric acid | 2.176 | 0.002 | -0.625 | ↓ | 己二酸 Adipic acid | 1.757 | 0.021 | -2.106 | ↓ | 吲哚乙酸 Indoleacetic acid | 2.373 | 0.010 | 14.098 | ↑ |
L-苹果酸 L-malic acid | 2.313 | 0.002 | -1.319 | ↓ | 柠檬酸 Citric acid | 1.043 | 0.021 | -0.742 | ↓ | 胸苷 Thymidine | 1.882 | 0.010 | 2.960 | ↑ |
邻苯二甲酸 Phthalate | 1.860 | 0.002 | -4.509 | ↓ | 邻苯二甲酸 Phthalic acid | 2.109 | 0.024 | -3.879 | ↓ | 柠檬酸 Citric acid | 2.049 | 0.010 | 3.048 | ↑ |
丙酮酸 Pyruvate | 2.085 | 0.008 | -1.193 | ↓ | 肌苷 Inosine | 1.690 | 0.037 | -1.451 | ↓ | 甘油酸-3-磷酸 Glycerate-3- phosphate | 1.664 | 0.015 | -0.574 | ↓ |
花生四烯酸 Arachidonic acid | 1.356 | 0.033 | -0.543 | ↓ | α-酮戊二酸 α-Ketoglutaric acid | 1.978 | 0.038 | -0.996 | ↓ | 谷氨酸 Glutamate | 1.292 | 0.016 | 2.622 | ↑ |

Figure 5 Analysis of different metabolic pathways among groups of Tupaia belangeriNote:A, Differential metabolic pathways between DMBA-induced group with breast cancer (DMBA-BC) and normal control (NC) group; B, Differential metabolic pathways between DMBA-induced (DMBA-BC) group with breast cancer and those without breast cancer (DMBA-nonBC); C, Differential metabolic pathways between DMBA-induced without breast cancer (DMBA-nonBC)group and normal control (NC) group.
| 1 | 郭心怡, 吕青. 2023年«NCCN乳腺癌风险降低指南»解读[J]. 中国胸心血管外科临床杂志, 2023, 30(6):787-804.DOI:10.7507/1007-4848.202303034 . |
| GUO X Y, LV Q. Interpretations of the NCCN guidelines for breast cancer risk reduction(version 2023)[J]. Chin J Clin Thorac Cardiovasc Surg, 2023, 30(6):787-804.DOI:10.7507/1007-4848.202303034 . | |
| 2 | 王英哲, 殷咏梅, 江泽飞. 2023年CSCO«乳腺癌诊疗指南»更新要点解读[J]. 中国肿瘤外科杂志, 2023, 15(3): 209-213, 218. DOI: 10.3969/j.issn.1674-4136.2023.03.001 . |
| WANG Y Z, YIN Y M, JIANG Z F. Interpretation of updated key points of Chinese society of clinical oncology(CSCO)breast cancer guidelines 2023[J]. Chin J Surg Oncol, 2023, 15(3): 209-213, 218. DOI: 10.3969/j.issn.1674-4136.2023.03.001 . | |
| 3 | 姜艺, 顾天豪, 夏添松. 特殊乳腺癌动物模型研究进展[J]. 南京医科大学学报(自然科学版), 2021, 41(2):292-295, 309.DOI:10.7655/NYDXBNS20210227 . |
| JIANG Y, GU T H, XIA T S. Research progress of special animal models of breast cancer[J]. J Nanjing Med Univ Nat Sci, 2021, 41(2):292-295, 309.DOI:10.7655/NYDXBNS20210227 . | |
| 4 | 夏巍, 赖永静, 杜龙, 等. 树鼩在人类肿瘤疾病动物模型中的应用进展[J]. 动物医学进展, 2019, 40(3):109-113. DOI: 10.16437/j.cnki.1007-5038.2019.03.020 . |
| XIA W, LAI Y J, DU L, et al. Application of tree shrew in animal models of human tumor diseases[J]. Prog Vet Med, 2019, 40(3):109-113. DOI: 10.16437/j.cnki.1007-5038.2019.03.020 . | |
| 5 | 贾杰, 代解杰. 树鼩在生物医学研究中的优势与挑战[J]. 实验动物与比较医学, 2019, 39(1): 3-8. DOI: 10.3969/j.issn.1674-5817.2019.01.002 . |
| JIA J, DAI J J. Advantages and challenges of tree shrews in biomedical research[J]. Lab Anim Comp Med, 2019, 39(1): 3-8. DOI: 10.3969/j.issn.1674-5817.2019.01.002 . | |
| 6 | OLIVARES O, DÄBRITZ J H M, KING A, et al. Research into cancer metabolomics: towards a clinical metamorphosis[J]. Semin Cell Dev Biol, 2015, 43:52-64. DOI: 10.1016/j.semcdb. 2015.09.008 . |
| 7 | 马小雨, 罗彩萍, 刘悦. 代谢组学在乳腺癌诊疗中应用的研究进展[J]. 药学实践与服务, 2023, 41(3):139-145.DOI:10.12206/j.issn.2097-2024.202109112 . |
| MA X Y, LUO C P, LIU Y. Application of metabonomics in breast cancer[J]. J Pharm Pract Serv, 2023, 41(3):139-145.DOI:10.12206/j.issn.2097-2024.202109112 . | |
| 8 | 石倩倩, 敖青青, 韦兰成, 等. 树鼩自发性乳腺癌的代谢组学分析[J]. 广西医科大学学报, 2021, 38(10):1860-1864. DOI: 10.16190/j.cnki.45-1211/r.2021.10.005 . |
| SHI Q Q, AO Q Q, WEI L C, et al. Metabonomics analysis of spontaneous breast cancer in tree shrew[J]. J Guangxi Med Univ, 2021, 38(10):1860-1864. DOI: 10.16190/j.cnki.45-1211/r.2021.10.005 . | |
| 9 | 韩春平, 许佳琪, 宋旭阳. 南阳地区育龄期女性乳腺癌流行特征及相关因素[J]. 中国卫生工程学, 2023, 22(2):217-219. DOI: 10.19937/j.issn.1671-4199.2023.02.021 . |
| HAN C P, XU J Q, SONG X Y. Epidemiological characteristics and related factors of breast cancer in women of childbearing age in Nanyang area[J]. Chin J Public Heath Eng, 2023, 22(2):217-219. DOI: 10.19937/j.issn.1671-4199.2023.02.021 . | |
| 10 | 徐小珊, 侯小明, 王伟, 等. DMBA诱导树鼩乳腺肿瘤(中缅树鼩)[J]. 现代生物医学进展, 2015, 15(2):228-232. DOI: 10.13241/j.cnki.pmb.2015.02.007 . |
| XU X S, HOU X M, WANG W, et al. DMBA induced breast tumors in tree shrews(Tupaia belangeri Chinese)[J]. Prog Mod Biomed, 2015, 15(2):228-232. DOI: 10.13241/j.cnki.pmb.2015.02.007 . | |
| 11 | 何保丽, 夏厚军, 角建林, 等. 人工诱导树鼩乳腺肿瘤的病理分析[J]. 中国比较医学杂志, 2016, 26(3): 6-10. DOI: 10.3969.j.issn.1671-7856.2016.03.002 . |
| HE B L, XIAO H J, JIAO J L, et al. Pathological analysis of the induced breast tumor models in tree shrew[J]. Chin J Comp Med, 2016, 26(3): 6-10. DOI: 10.3969.j.issn.1671-7856.2016.03.002 . | |
| 12 | 杜沛, 沈红艺, 李中平, 等. 大豆异黄酮对DMBA诱导高脂饮食幼龄大鼠乳腺肿瘤的作用[J]. 南京中医药大学学报, 2014, 30(5):498-500. DOI: 10.14148/j.issn.1672-0482.2014.05.060 . |
| DU P, SHEN H Y, LI Z P, et al. Effects of soy isoflavones and high-fat diet on genesis and progress of 7, 12-dimethylbenz(α)anthrancence-induced breast cancer in young female SD rats[J]. J Nanjing Univ Tradit Chin Med, 2014, 30(5):498-500. DOI: 10.14148/j.issn.1672-0482.2014.05.060 . | |
| 13 | HOSSEINPOUR Z, REZAEI TAVIRANI M, AKBARI M E. Stage analysis of breast cancer metabolomics: a system biology approach[J]. Asian Pac J Cancer Prev, 2023, 24(5):1571-1582. DOI: 10.31557/APJCP.2023.24.5.1571 . |
| 14 | JOBARD E, PONTOIZEAU C, BLAISE B J, et al. A serum nuclear magnetic resonance-based metabolomic signature of advanced metastatic human breast cancer[J]. Cancer Lett, 2014, 343(1):33-41. DOI: 10.1016/j.canlet.2013.09.011 . |
| 15 | ZHANG Y P, SHIPKOVA P A, WARRACK B M, et al. Metabolomic profiling and drug interaction characterization reveal riboflavin As a breast cancer resistance protein-specific endogenous biomarker that demonstrates prediction of transporter activity in vivo [J]. Drug Metab Dispos, 2023, 51(7):851-861. DOI: 10.1124/dmd.123.001284 . |
| 16 | KODAMA M, OSHIKAWA K, SHIMIZU H, et al. A shift in glutamine nitrogen metabolism contributes to the malignant progression of cancer[J]. Nat Commun, 2020, 11(1):1320. DOI: 10.1038/s41467-020-15136-9 . |
| 17 | XIAO D F, ZENG L M, YAO K, et al. The glutamine-alpha-ketoglutarate (AKG) metabolism and its nutritional implications[J]. Amino Acids, 2016, 48(9):2067-2080. DOI: 10.1007/s00726-016-2254-8 . |
| 18 | WISE D R, THOMPSON C B. Glutamine addiction: a new therapeutic target in cancer[J]. Trends Biochem Sci, 2010, 35(8):427-433. DOI: 10.1016/j.tibs.2010.05.003 . |
| 19 | 吴静, 杨睿, 刘树业. 代谢组学技术在乳腺癌标志物筛选中的应用进展[J]. 天津医药, 2018, 46(9):1019-1022. DOI: 10.11958/20180365 . |
| WU J, YANG R, LIU S Y. Application of metabonomics in screening markers for breast cancer[J]. Tianjin Med J, 2018, 46(9):1019-1022. DOI: 10.11958/20180365 . | |
| 20 | HOU J, REID N E, TROMBERG B J, et al. Kinetic analysis of lipid metabolism in breast cancer cells via nonlinear optical microscopy[J]. Biophys J, 2020, 119(2):258-264. DOI: 10.1016/j.bpj.2020.06.007 . |
| 21 | ZHAO Z W, XIAO Y J, ELSON P, et al. Plasma lysophosphatidylcholine levels: potential biomarkers for colorectal cancer[J]. J Clin Oncol, 2007, 25(19):2696-2701. DOI: 10.1200/JCO.2006.08.5571 . |
| 22 | 邱振东, 邓文宏, 洪育蒲, 等. ATP柠檬酸裂解酶对结肠癌细胞脂质代谢及肿瘤生物学行为的作用[J]. 中国普外基础与临床杂志, 2021, 28(1):48-52. DOI: 10.7507/1007-9424.202004133 . |
| QIU Z D, DENG W H, HONG Y P, et al. Effects of ATP citrate lyase on lipid metabolism and tumor biological behavior of colon cancer cells[J]. China Ind Econ, 2021, 28(1):48-52. DOI: 10.7507/1007-9424.202004133 . |
| [1] | Zhuxin LI, Liang LIANG, Yingying CAO, Shanshan ZHAI, Yinhan DAI, Xia HE, Junyu TAO, Jing LENG, Haibo TANG. Diagnosis of a Primary Pleomorphic Liposarcoma in a Tree Shrew(Tupaia belangeri subsp. yaoshanensis) [J]. Laboratory Animal and Comparative Medicine, 2023, 43(6): 647-653. |
| [2] | Taofeng LU, Hui ZHANG, Jie ZHOU, Qian LI, Shuguang WU, Yanjun WU. Effects of Pogostemon cablin on Serum Metabolomiceof Guizhou Miniature Pigs and It's mechanism [J]. Laboratory Animal and Comparative Medicine, 2023, 43(3): 253-261. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||