
Laboratory Animal and Comparative Medicine ›› 2023, Vol. 43 ›› Issue (6): 585-594.DOI: 10.12300/j.issn.1674-5817.2023.065
• Animal Experimental Techniques and Methods • Next Articles
Jiaoxiang WANG1,2,3, Yan WANG1,2,4, Ke HU1,2,3, Kaixiang XU1,2,4, Taiyun WEI1,2, Deling JIAO1,2,4, Heng ZHAO1,2,4, Hongye ZHAO1,2,4, HongJiang WEI1,2,3,4( )(
)( )
)
Received:2023-05-25
Revised:2023-08-14
Online:2023-12-25
Published:2023-12-25
Contact:
HongJiang WEI
CLC Number:
Jiaoxiang WANG,Yan WANG,Ke HU,et al. Establishment of PCR Identification Method for Pig Blood Type[J]. Laboratory Animal and Comparative Medicine, 2023, 43(6): 585-594. DOI: 10.12300/j.issn.1674-5817.2023.065.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2023.065
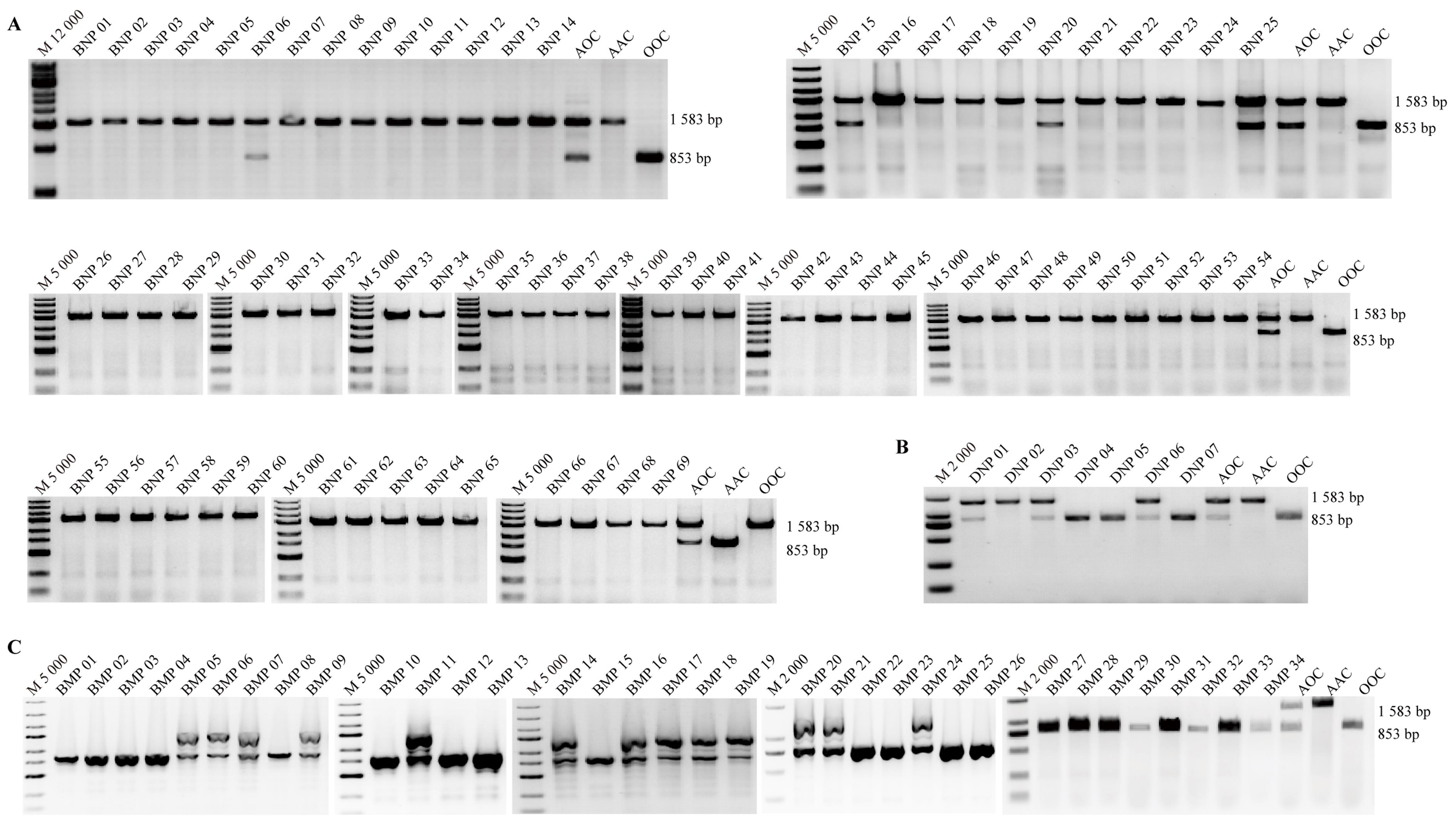
Figure 2 PCR identification results of blood types in different strains of pigsNote:A, The blood PCR identification results of 69 Banna miniature inbred pigs (BNP01-69); B, The blood PCR identification results of 7 Diannan small eared pigs (DNP01-7); C, The blood PCR identification results of 34 Bama Xiang pigs (BMP01-34). M12000, M5000 and M2000 are DNA Markers. AOC, AAC, and OOC respectively refer to the AO, AA, and OO blood group standard control samples that have been sequenced in our laboratory in the previous stage.
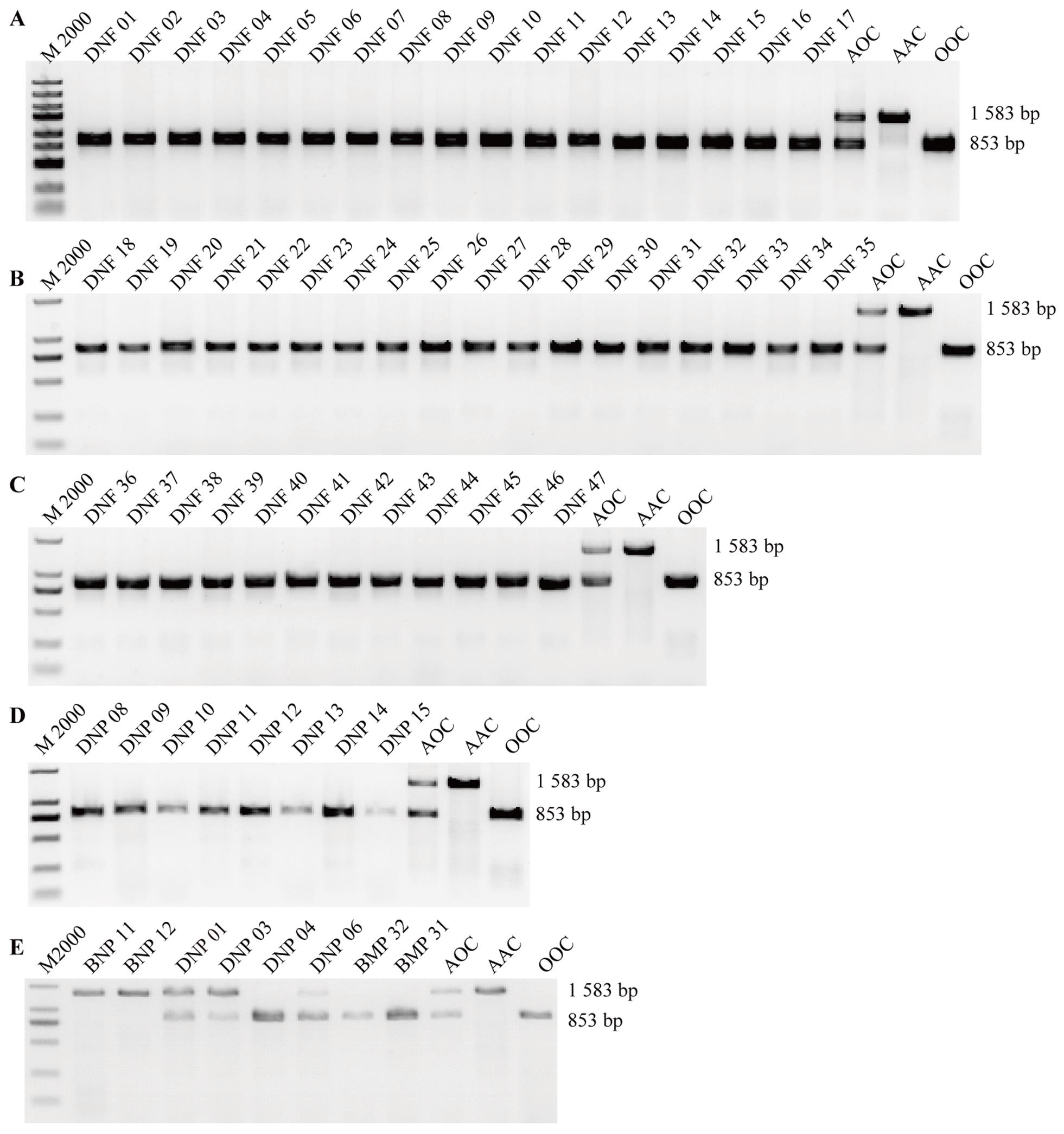
Figure 3 PCR identification of blood types in fetal fibroblasts and oral mucosal cells of Diannan cloned small eared pigsNote:A, PCR identification results of 17 wild-type Diannan small eared pig fetal fibroblasts (DNF1-17); B, PCR identification results of 18 porcine gene-editing fetuses fibroblasts of Diannan small eared pigs (DNF18-35); C, PCR identification results of 12 porcine gene-editing fetuses fibroblasts of Diannan small eared pigs (DNF36-47);D, PCR identification results of oral mucosal samples from 8 gene-editing cloned Diannan small eared pigs (DNP08-15); E, PCR identification results of oral mucosal samples from 8 wild- type pigs (BNP11-12, Two Banna miniature inbred pigs; DNP01,03,04 and 06, four Diannan small eared pigs; BMP31-32, two Bama Xiang pigs). M2000 is the DNA Marker. AOC, AAC, and OOC respectively refer to the AO, AA, and OO blood group standard control samples that have been sequenced in our laboratory in the previous stage.
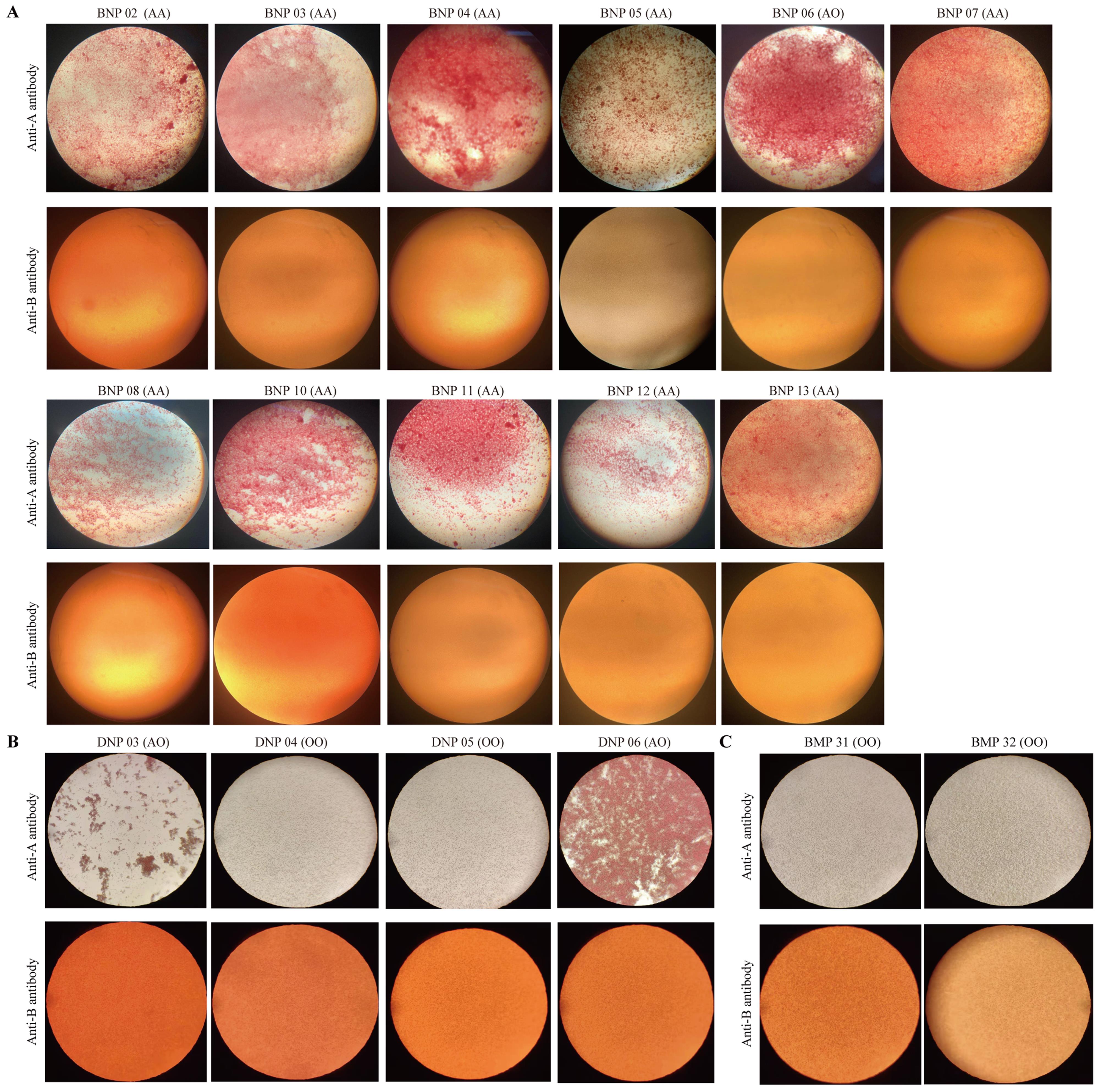
Figure 4 Results of the blood agglutination reaction for identifying pig blood typesNote:A, Blood agglutination reaction of 11 Banna miniature inbred pigs (BNP02-08, 10-13); B, Blood agglutination reaction of Diannan small eared pigs (DNP03-06); C, Blood agglutination reaction of 2 Bama Xiang pigs (BMP31-32). AA, AO, and OO refer to the results of PCR identification of blood types, which were consistent with the results of blood agglutination reaction.
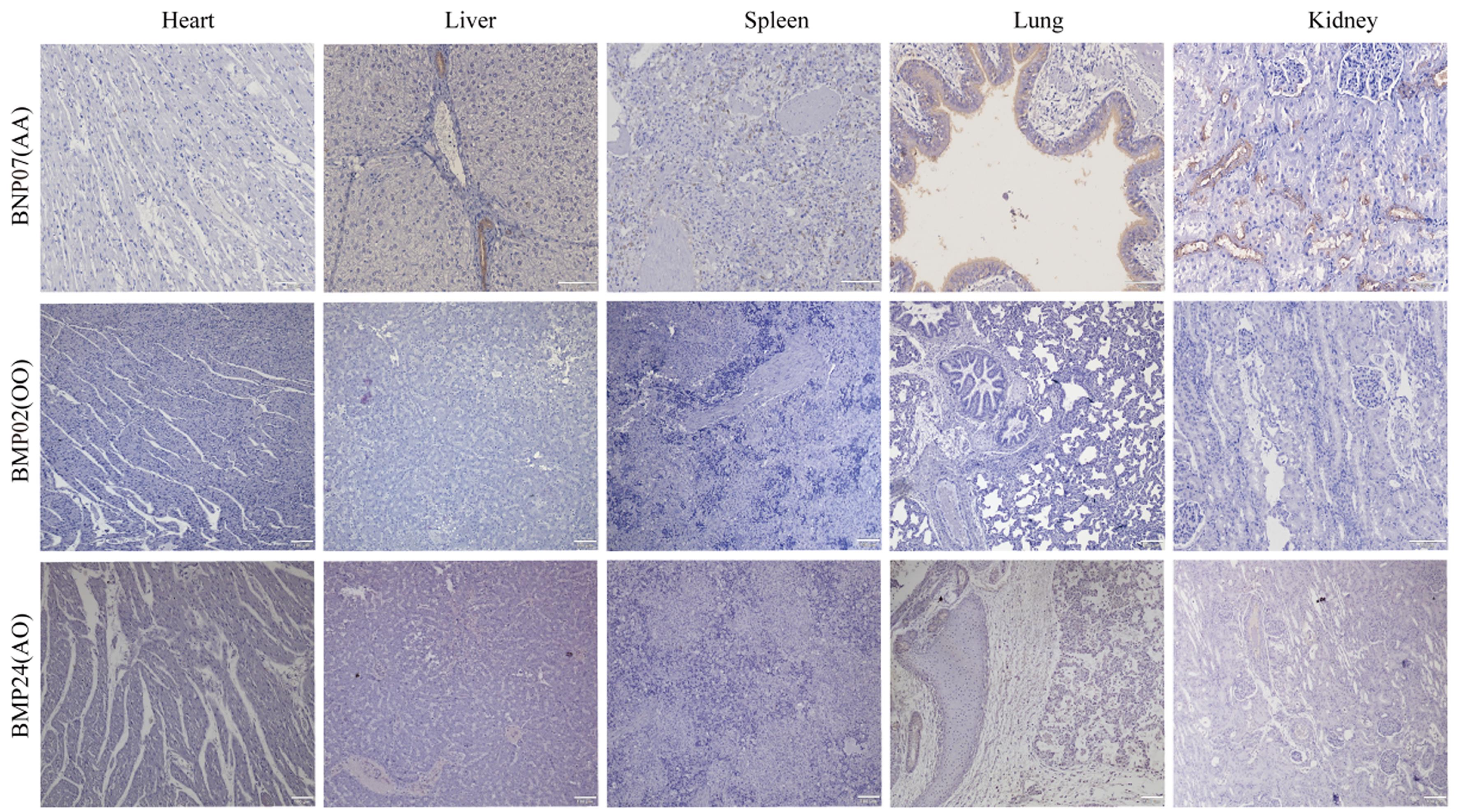
Figure 5 Results of immunohistochemical identification of pig blood types (DAB staining, all the bars are 100 μm)Note:BNP, Banna miniature inbred pigs; BMP, Bama Xiang pigs. AA, AO and OO refer to the results of blood type identification by PCR, which are consistent with the results of blood type identification by immunohistochemistry.
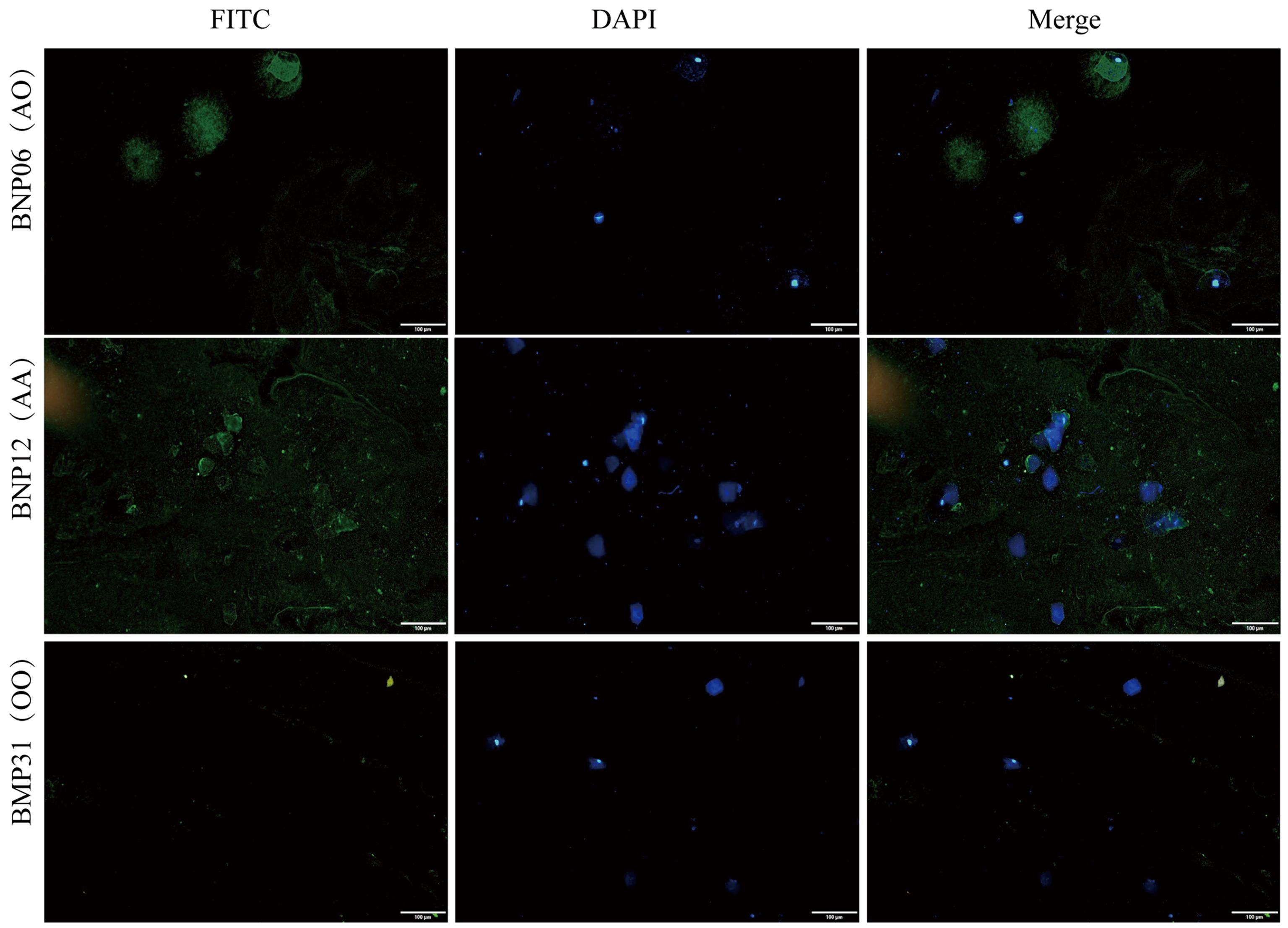
Figure 6 Results of immunofluorescence identification of pig blood types in oral mucosal samples (all the bars are 100 μm)Note: BNP06, 12 are two Banna miniature inbred pigs; BMP31 is one Bama Xiang pig. FITC, Fluorescein isothiocyanate; DAPI, 4',6-diamidine-2-phenylindole. AA, AO and OO refer to the results of blood PCR identification of blood types, which are consistent with the results of immunofluorescence identification of blood types.
| 序号Serial number | 编号Number | 血液 PCR Blood PCR | 血液凝集反应 Hemagglutination test | 免疫组织化学 Immunohistochemistry | 颊黏膜PCR PCR of buccal mucosal samples | 免疫荧光 Immunofluorescence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
抗A抗体Anti-A antibody | 抗B抗体Anti-B antibody | 心 Heart | 肝 Liver | 脾 Spleen | 肺 Lung | 肾 Kidney | ||||||
| 1 | BNP02 | AA | + | - | ||||||||
| 2 | BNP03 | AA | + | - | ||||||||
| 3 | BNP04 | AA | + | - | ||||||||
| 4 | BNP05 | AA | + | - | ||||||||
| 5 | BNP06 | AO | + | - | A | |||||||
| 6 | BNP07 | AA | + | - | - | A | A | A | A | |||
| 7 | BNP08 | AA | + | - | ||||||||
| 8 | BNP09 | AA | + | - | ||||||||
| 9 | BNP10 | AA | + | - | ||||||||
| 10 | BNP11 | AA | + | - | AA | |||||||
| 11 | BNP12 | AA | + | - | AA | A | ||||||
| 12 | DNP03 | AO | + | - | AO | |||||||
| 13 | DNP04 | OO | - | - | OO | |||||||
| 14 | DNP05 | OO | - | - | ||||||||
| 15 | DNP06 | AO | + | - | AO | |||||||
| 16 | BMP02 | OO | - | - | - | - | - | |||||
| 17 | BMP24 | AO | - | A | - | A | A | |||||
| 18 | BMP31 | OO | - | - | OO | - | ||||||
| 19 | BMP32 | OO | - | - | OO | |||||||
Table 1 Comparison of PCR, hemagglutination test, immunohistochemistry and immunofluorescence staining results
| 序号Serial number | 编号Number | 血液 PCR Blood PCR | 血液凝集反应 Hemagglutination test | 免疫组织化学 Immunohistochemistry | 颊黏膜PCR PCR of buccal mucosal samples | 免疫荧光 Immunofluorescence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
抗A抗体Anti-A antibody | 抗B抗体Anti-B antibody | 心 Heart | 肝 Liver | 脾 Spleen | 肺 Lung | 肾 Kidney | ||||||
| 1 | BNP02 | AA | + | - | ||||||||
| 2 | BNP03 | AA | + | - | ||||||||
| 3 | BNP04 | AA | + | - | ||||||||
| 4 | BNP05 | AA | + | - | ||||||||
| 5 | BNP06 | AO | + | - | A | |||||||
| 6 | BNP07 | AA | + | - | - | A | A | A | A | |||
| 7 | BNP08 | AA | + | - | ||||||||
| 8 | BNP09 | AA | + | - | ||||||||
| 9 | BNP10 | AA | + | - | ||||||||
| 10 | BNP11 | AA | + | - | AA | |||||||
| 11 | BNP12 | AA | + | - | AA | A | ||||||
| 12 | DNP03 | AO | + | - | AO | |||||||
| 13 | DNP04 | OO | - | - | OO | |||||||
| 14 | DNP05 | OO | - | - | ||||||||
| 15 | DNP06 | AO | + | - | AO | |||||||
| 16 | BMP02 | OO | - | - | - | - | - | |||||
| 17 | BMP24 | AO | - | A | - | A | A | |||||
| 18 | BMP31 | OO | - | - | OO | - | ||||||
| 19 | BMP32 | OO | - | - | OO | |||||||
| 1 | GRIFFITH B P, GOERLICH C E, SINGH A K, et al. Genetically modified Porcine-to-human cardiac xenotransplantation[J]. N Engl J Med, 2022, 387(1):35-44. DOI: 10.1056/NEJMoa2201422 . |
| 2 | NIU D, WEI H J, LIN L, et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9[J]. Science, 2017, 357(6357):1303-1307. DOI: 10.1126/science.aan4187 . |
| 3 | YUE Y N, XU W H, KAN Y N, et al. Extensive germline genome engineering in pigs[J]. Nat Biomed Eng, 2021, 5(2):134-143. DOI: 10.1038/s41551-020-00613-9 . |
| 4 | RYDBERG L. ABO-incompatibility in solid organ transplantation[J]. Transfus Med, 2001, 11(4):325-342. DOI: 10.1046/j.1365-3148.2001.00313.x . |
| 5 | BUSCH J, SPECHT S, EZZELARAB M, et al. Buccal mucosal cell immunohistochemistry: a simple method of determining the ABH phenotype of baboons, monkeys, and pigs[J]. Xenotransplantation, 2006, 13(1):63-68. DOI: 10.1111/j.1399-3089.2005.00255.x . |
| 6 | PETAZZI P, MIQUEL-SERRA L, HUERTAS S, et al. ABO gene editing for the conversion of blood type A to universal type O in Rhnull donor-derived human-induced pluripotent stem cells[J]. Clin Transl Med, 2022, 12(10): e1063. DOI: 10.1002/ctm2.1063 . |
| 7 | SMITH D M, NEWHOUSE M, NAZIRUDDIN B, et al. Blood groups and transfusions in pigs[J]. Xenotransplantation, 2006, 13(3):186-194. DOI: 10.1111/j.1399-3089.2006.00299.x . |
| 8 | YAMAMOTO F, YAMAMOTO M. Molecular genetic basis of porcine histo-blood group AO system[J]. Blood, 2001, 97(10):3308-3310. DOI: 10.1182/blood.v97.10.3308 . |
| 9 | TAWEECHAIPAISANKUL A, KIM G A, JIN J X, et al. Establishment and identification of cell lines from type O blood Korean native pigs and their efficiency in supporting embryonic development via somatic cell nuclear transfer[J]. J Vet Sci, 2018, 19(4):492-499. DOI: 10.4142/jvs.2018.19.4.492 . |
| 10 | CHOI M K, LE M T, CHO H, et al. Determination of complete sequence information of the human ABO blood group orthologous gene in pigs and breed difference in blood type frequencies[J]. Gene, 2018, 640:1-5. DOI: 10.1016/j.gene. 2017. 09.047 . |
| 11 | SONG W Q, ZHOU S H, SHAO L N, et al. Non-invasive fetal ABO genotyping in maternal plasma using real-time PCR[J]. Clin Chem Lab Med, 2015, 53(12):1943-1950. DOI: 10.1515/cclm-2015-0011 . |
| 12 | HSU F H, SERPEDIN E, CHEN Y D, et al. Stochastic modeling of the relationship between copy number and gene expression based on transcriptional logic[J]. IEEE Trans Biomed Eng, 2012, 59(1):272-280. DOI: 10.1109/TBME. 2011. 2173341 . |
| 13 | HERNÁNDEZ-GÓMEZ C, HERNÁNDEZ-LEMUS E, ESPINAL-ENRÍQUEZ J. The role of copy number variants in gene Co-expression patterns for luminal B breast tumors[J]. Front Genet, 2022, 13:806607. DOI: 10.3389/fgene.2022.806607 . |
| 14 | ORIOL R, YE Y, KOREN E, et al. Carbohydrate antigens of pig tissues reacting with human natural antibodies as potential targets for hyperacute vascular rejection in pig-to-man organ xenotransplantation[J]. Transplantation, 1993, 56(6):1433-1442. DOI: 10.1097/00007890-199312000-00031 . |
| 15 | ZHAO H, LI Y Y, WIRIYAHDAMRONG T, et al. Improved production of GTKO/hCD55/hCD59 triple-gene-modified Diannan miniature pigs for xenotransplantation by recloning[J]. Transgenic Res, 2020, 29(3):369-379. DOI: 10.1007/s11248-020-00201-2 . |
| 16 | 王娇祥, 范柠粼, 施德佳, 等. 通过微量细胞鉴定快速生产基因编辑猪[J]. 云南农业大学学报(自然科学), 2021, 36(5):811-819, 825. DOI: 10.12101/j.issn.1004-390X(n).202103095 . |
| WANG J X, FAN N L, SHI D J, et al. Rapid production of gene-editing pigs by identifying minimum numbers of positive fibroblasts for SCNT[J]. J Yunnan Agric Univ, 2021, 36(5):811-819, 825. DOI: 10.12101/j.issn.1004-390X(n).202103095 . | |
| 17 | ZENG R, ZENG Y Z. Molecular cloning and characterization of SLA-DR genes in the 133-family of the Banna mini-pig inbred line[J]. Animal Genet, 2005, 36(3):267-269. DOI: 10.1111/j.1365-2052.2005.01277.x . |
| 18 | WEI H J, QING Y B, PAN W R, et al. Comparison of the efficiency of Banna miniature inbred pig somatic cell nuclear transfer among different donor cells[J]. PLoS One, 2013, 8(2): e57728. DOI: 10.1371/journal.pone.0057728 . |
| [1] | LIN Zhenhua, CHU Xiangyu, WEI Zhenxi, DONG Chuanjun, ZHAO Zenglin, SUN Xiaoxia, LI Qingyu, ZHANG Qi. Evaluation of the Safety and Efficacy of Bone Cement in Experimental Pigs Using Vertebroplasty [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 466-472. |
| [2] | WANG Jiaoxiang, ZHANG Lu, CHEN Shuhan, JIAO Deling, ZHAO Heng, WEI Taiyun, GUO Jianxiong, XU Kaixiang, WEI Hongjiang. Construction and Functional Validation of GTKO/hCD55 Gene-Edited Xenotransplant Donor Pigs [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 379-392. |
| [3] | LIU Yuanyuan, XIN Wenshui, CHAO Zhe, CAO Zongxi, CAI Yifei, LI Qiang, LI Lingwei, LIU Guangliang. Identification and Analysis of MHCⅡ Genes in Wuzhishan Pigs [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 340-348. |
| [4] | WEI Yanye, SHEN Guo, ZHANG Pengfei, SHI Songping, HU Jiahao, ZHANG Xuzhe, HUA Huiyuan, HUA Guanyang, LU Hongzheng, ZENG Yong, JI Feng, WEI Zhumei. Dynamic Monitoring and Correlation Analysis of General Body Indicators, Blood Glucose, and Blood Lipid in Obese Cynomolgus Monkeys [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 30-36. |
| [5] | LIU Yishu, CAI Liping. Advances and Challenges of Using Experimental Pigs in Da Vinci Surgical Robot Training [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 667-674. |
| [6] | ZHU Chan, ZHANG Dongliang, ZHAO Deli, SHI Xueqin, QIAN Lei, ZHANG Xuan, JIN Yan, DUAN Wei, QI Ruocheng, LIU Chaohua, YANG Xuekang, HAN Juntao, PAN Dengke. Perioperative Animal Care for Xenotransplantation from Genetically Edited Pigs to Monkeys [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 495-501. |
| [7] | YANG Jin, YU Shiya, LIN Nan, FANG Yongchao, ZHAO Hu, QIU Jinwei, LIN Hongming, CHEN Huiyan, WANG Yu, WU Weihang. Effect of Modified Duodenal Exclusion Surgery on Glucose Metabolism in Rats with Type 2 Diabetes Mellitus [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 523-530. |
| [8] | QI Longju, CHEN Shiyuan, LIAO Zehua, SHI Yuanhu, SUN Yuyu, WANG Qinghua. Transcriptomic Analysis of Menstrual Blood-Derived Stem Cells Transplantation Combined with Exercise Training in Promoting Spinal Cord Injury Recovery in Rats [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 531-542. |
| [9] | XIAO Pan, WANG Hongyi, LU Lu, ZHANG Mei, CHEN Keming, SHEN Dongshuai, NIU Tingxian. Screening of Hypoxia-Sensitive and Hypoxia-Tolerant Wistar Rats and Preliminary Exploration of Hypoxia Sensitivity in Their G1 Generation [J]. Laboratory Animal and Comparative Medicine, 2024, 44(4): 374-383. |
| [10] | Jin LU, Jian WANG, Lian ZHU, Guofeng YAN, Zhengwen MA, Yao LI, Jianjun DAI, Yinqiu ZHU, Jing ZHOU. Establishment of Preeclampsia Model in Goat and Evaluation on Maternal Biological Characteristics [J]. Laboratory Animal and Comparative Medicine, 2023, 43(4): 371-380. |
| [11] | Jian WANG, Jin LU, Lingyun TAO, Hongkun FU, Cheng GAO, Baojin WU, Yao LI, Yufeng TAO. Analysis on the Standardized Expression of the Accredited Ability Scope for Hematology, Blood Biochemistry and Urinalysis in Laboratory Animal Clinical Testing [J]. Laboratory Animal and Comparative Medicine, 2022, 42(6): 511-517. |
| [12] | Jiaqi CHEN, Longbao LÜ, Feiyan ZHANG, Rui LI, Yijiang LI, Lihong LI, Xiaodi ZHANG. Application of Blood Microsampling and Its Implementation of the 3Rs in Non-clinical Studies of Drugs [J]. Laboratory Animal and Comparative Medicine, 2022, 42(4): 306-312. |
| [13] | Laien XUE. Anesthetic Effects of Zoletil and Xylazine on Bama Mini-pigs [J]. Laboratory Animal and Comparative Medicine, 2022, 42(1): 26-30. |
| [14] | DIWU Feihu, ZHAO Zhijing, DENG Yiheng. Mechanism of Hypertonic Saline to Reduce Cerebral Edema in Rats with Cerebral Hemorrhage by Protecting Blood-brain Barrier [J]. Laboratory Animal and Comparative Medicine, 2021, 41(4): 327-332. |
| [15] | XIAO Pan, NIU Tingxian, LUO Xiaohong, WANG Hongyi, GUO Xiaoyu, LU Lu, FENG Xiaoming. Changes of C-reactive Protein and Inflammatory Factors in Juema Pig Model with High Altitude Multiple Organ Dysfunction Syndrome [J]. Laboratory Animal and Comparative Medicine, 2021, 41(3): 238-243. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||