
Laboratory Animal and Comparative Medicine ›› 2023, Vol. 43 ›› Issue (2): 124-135.DOI: 10.12300/j.issn.1674-5817.2022.149
• Animal Models of Human Diseases • Previous Articles Next Articles
Ying TAN1,2( ), Wenping LIAO1,2, Qilong GAO3, Yong LI1,2, Xinhui SHI1,2, Jingkun WANG1,2(
), Wenping LIAO1,2, Qilong GAO3, Yong LI1,2, Xinhui SHI1,2, Jingkun WANG1,2( )(
)( )
)
Received:2022-09-22
Revised:2023-02-17
Online:2023-04-25
Published:2023-04-25
Contact:
Jingkun WANG
CLC Number:
Ying TAN,Wenping LIAO,Qilong GAO,et al. Physiological Indexes and Histopathology Analysis of Sodium Iodate-Induced Retinitis Pigmentosa in Rats[J]. Laboratory Animal and Comparative Medicine, 2023, 43(2): 124-135. DOI: 10.12300/j.issn.1674-5817.2022.149.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2022.149
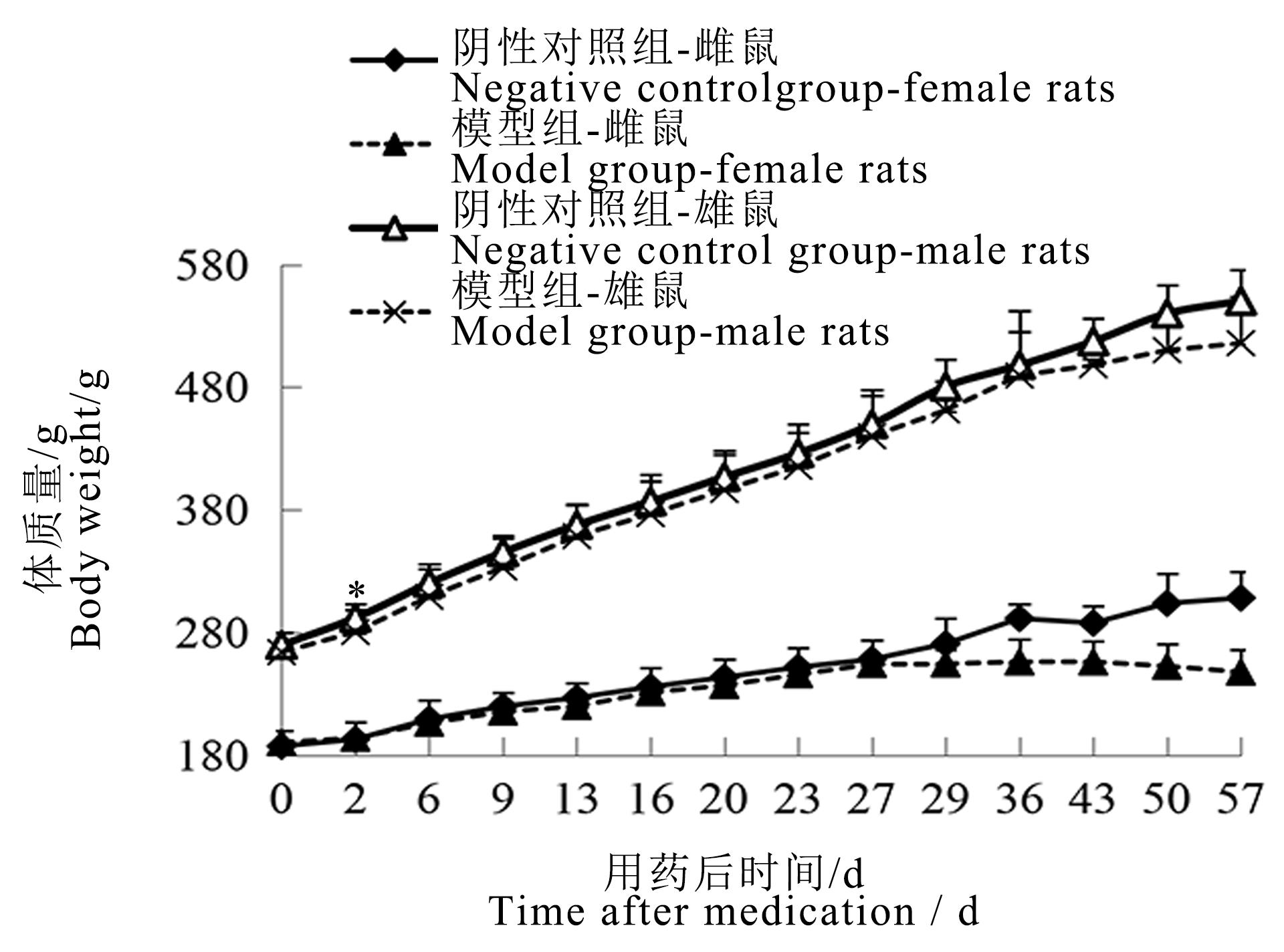
Figure 1 Body weight curve of male and female rats after sodium iodate injectionNote:The model group was intraperitoneally injected with 6 mg/mL sodium iodate solution once at the dose of 10 mL/kg;the negative control group was intraperitoneally injected with normal saline at the same volume and frequency. The number of rats in the two groups at each time point was 16, 16, 14, 12,12, 12, 12, 10, 10, 4, 4, 2, 2, 2 respectively for single sex. Compared with the negative control group, ?P<0.05.
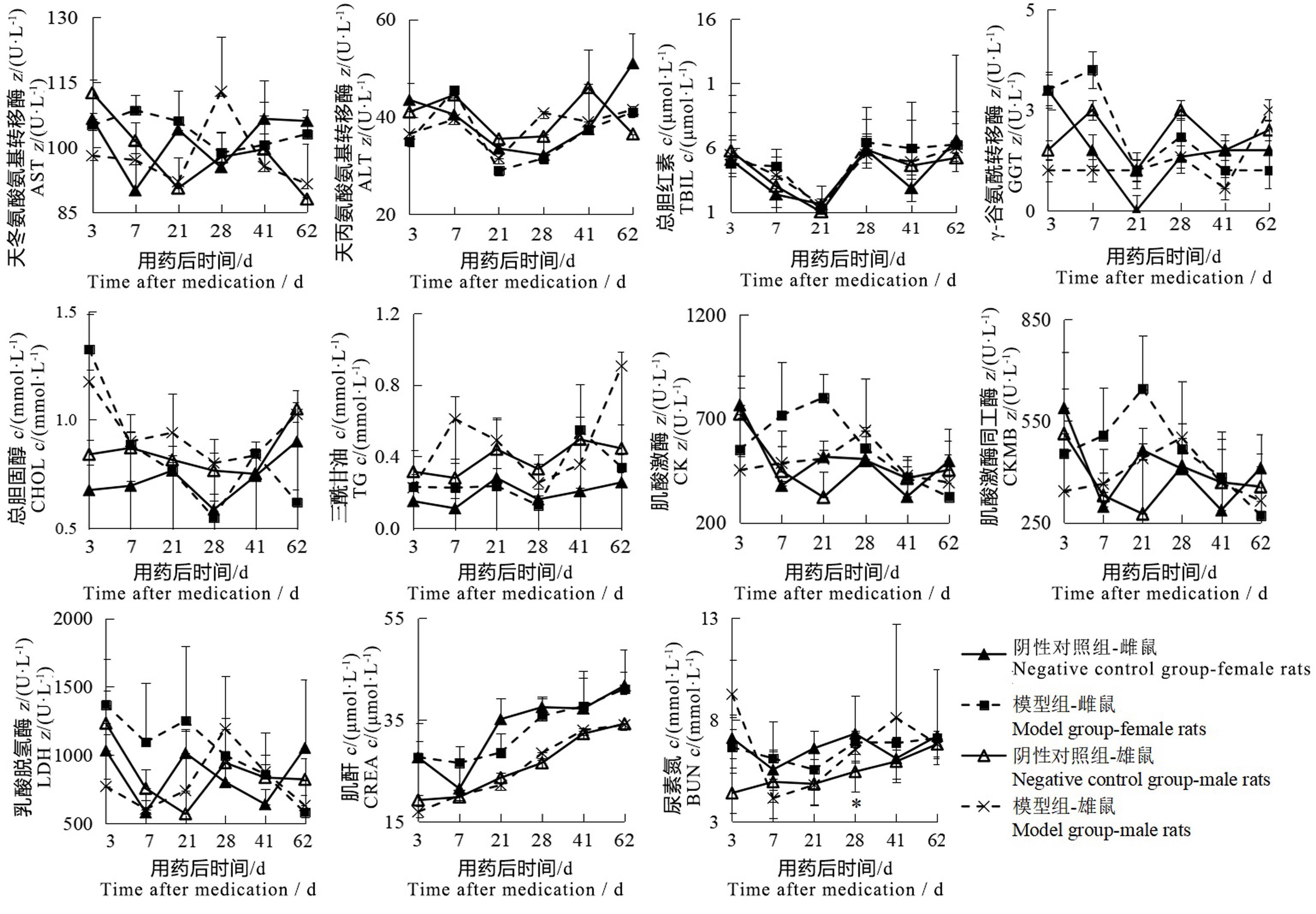
Figure 2 Changes of serum biochemical indexes of male and female rats after sodium iodate injectionNote: The model group was intraperitoneally injected with 6 mg/mL sodium iodate solution once at the dose of 10 mL/kg; the negative control group was intraperitoneally injected with normal saline at the same volume and frequency. The number of rats in the two groups at each time point was 2, 2, 2, 6, 2, 2 respectively for single sex. Compared with the negative control group, ?P<0.05.
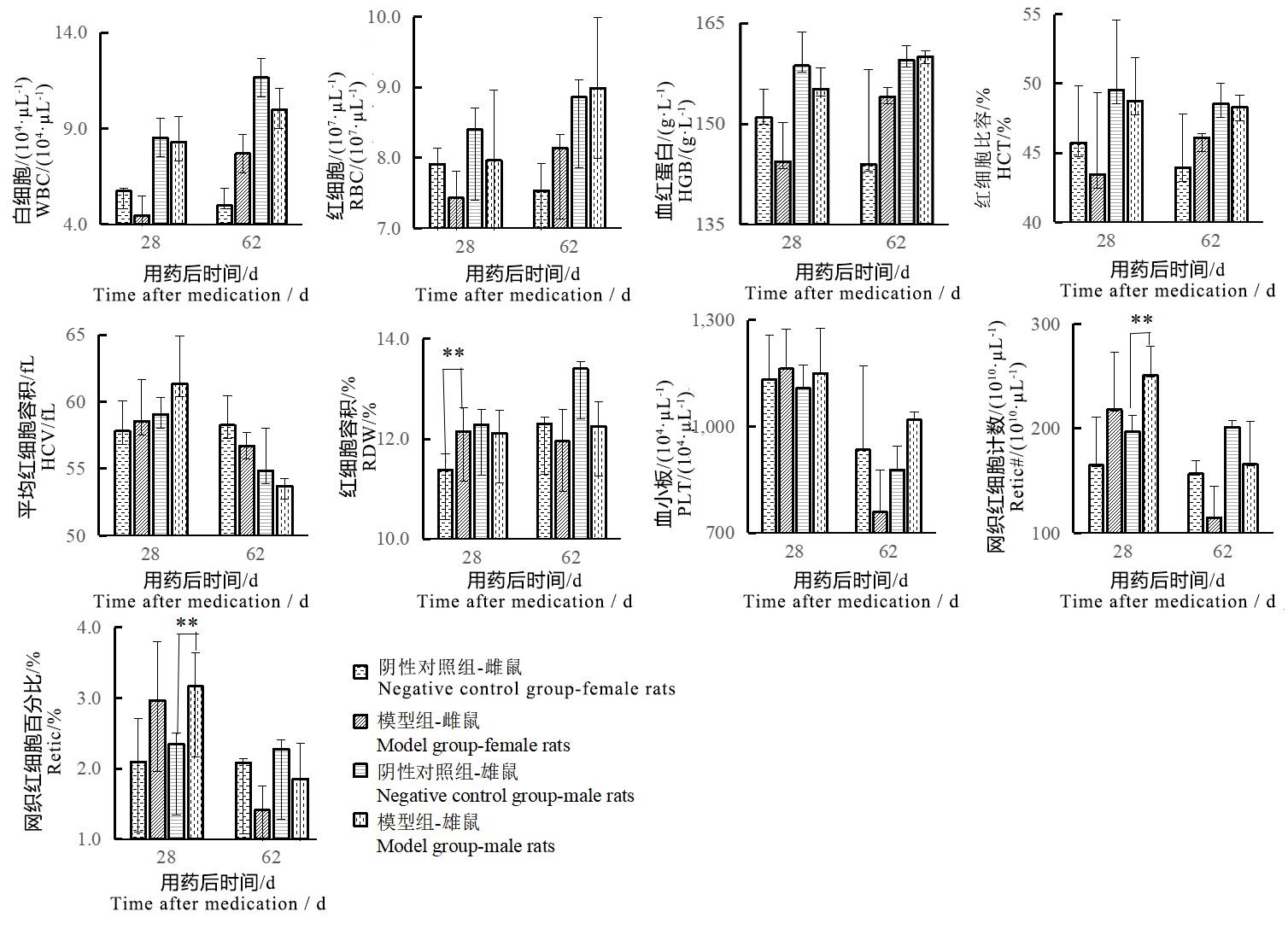
Figure 3 Changes of blood routine indexes of male and female rats after sodium iodate injectionNote:The model group was intraperitoneally injected with 6 mg/mL sodium iodate solution once at the dose of 10 mL/kg;the negative control group was intraperitoneally injected with normal saline at the same volume and frequency. The number of rats in the two groups at each time point was 2, 2, 2, 6, 2, 2 respectively for single sex. Compared with the negative control group, ??P<0.01.
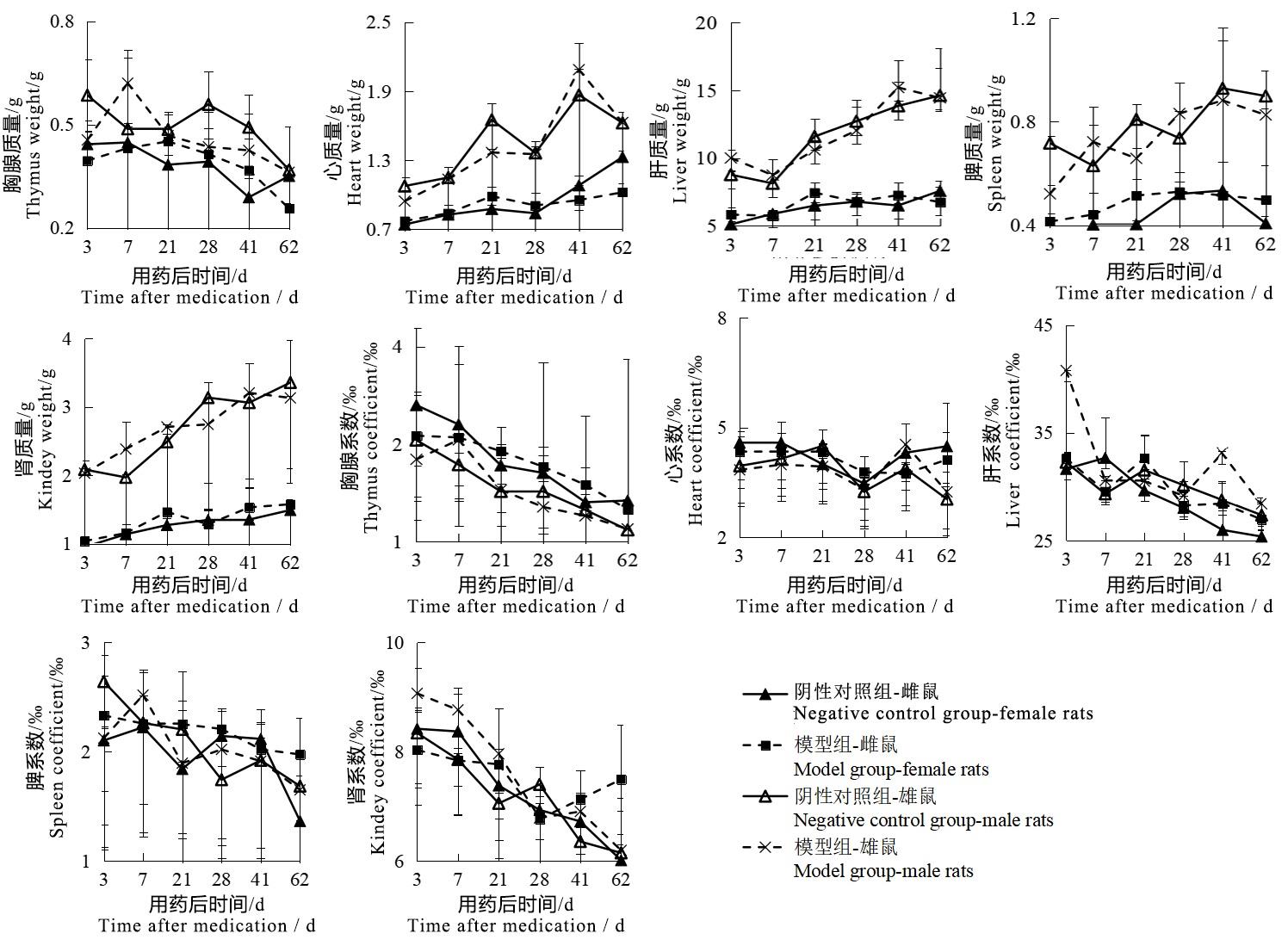
Figure 4 Changes of organ weight and coefficient of male and female rats after sodium iodate injectionNote:The model group was intraperitoneally injected with 6 mg/mL sodium iodate solution once at the dose of 10 mL/kg;the negative control group was intraperitoneally injected with normal saline at the same volume and frequency. The number of rats in the two groups at each time point was 2, 2, 2, 6, 2, 2 respectively for single sex.
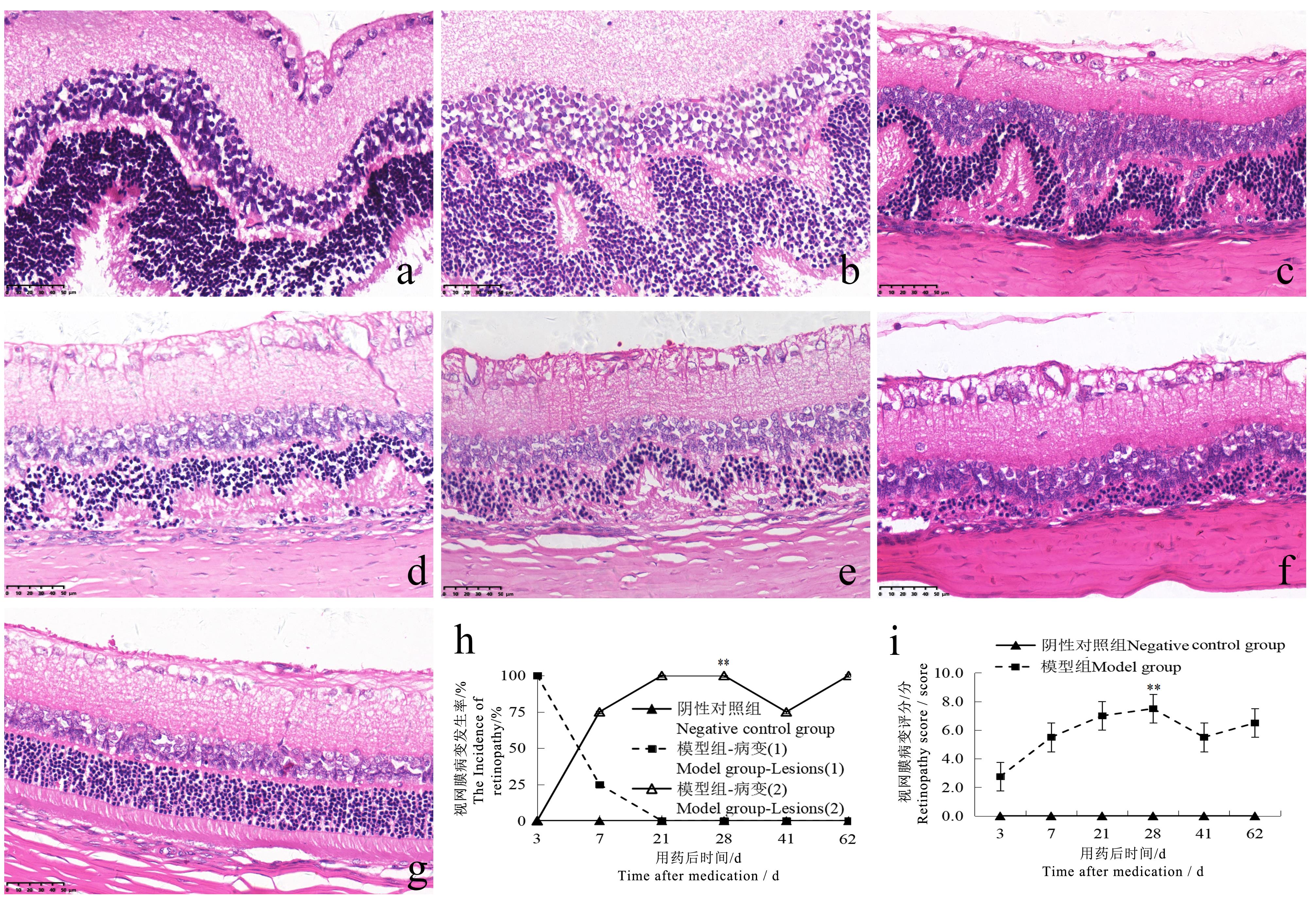
Figure 5 Retinopathy in rats at different time points after sodium iodate injection (HE staining, ×400)Note:The model group was intraperitoneally injected with 6 mg/mL sodium iodate solution once at the dose of 10 mL/kg; the negative control group was intraperitoneally injected with normal saline at the same volume and frequency. The number of rats in the two groups at each time point was 2, 2, 2, 6, 2, 2 respectively for single sex. a shows the retina of the model group appeared wave-like changes on the 3rd day after injection; b shows the retina of the model group appeared abnormal electrocardiogram (ECG)-like changes on the 7th day after injection, and the arrangement of each layer was disordered; c-f show the retina of the model group appeared abnormal ECG-like changes on the 21st, 28th, 41st and 62nd days after injection, with disordered arrangement of each layer and focal thinning of the outer nuclear layer. g shows normal retina in negative control group; h-i show the comparison of the incidence of retinopathy and HE score between the two groups (Lesion 1 shows wave-like changes in the retina, lesion 2 shows ECG-like changes in the retina). ??P<0.01, vs the negative control group. The scale size in the figure are 50 μm.
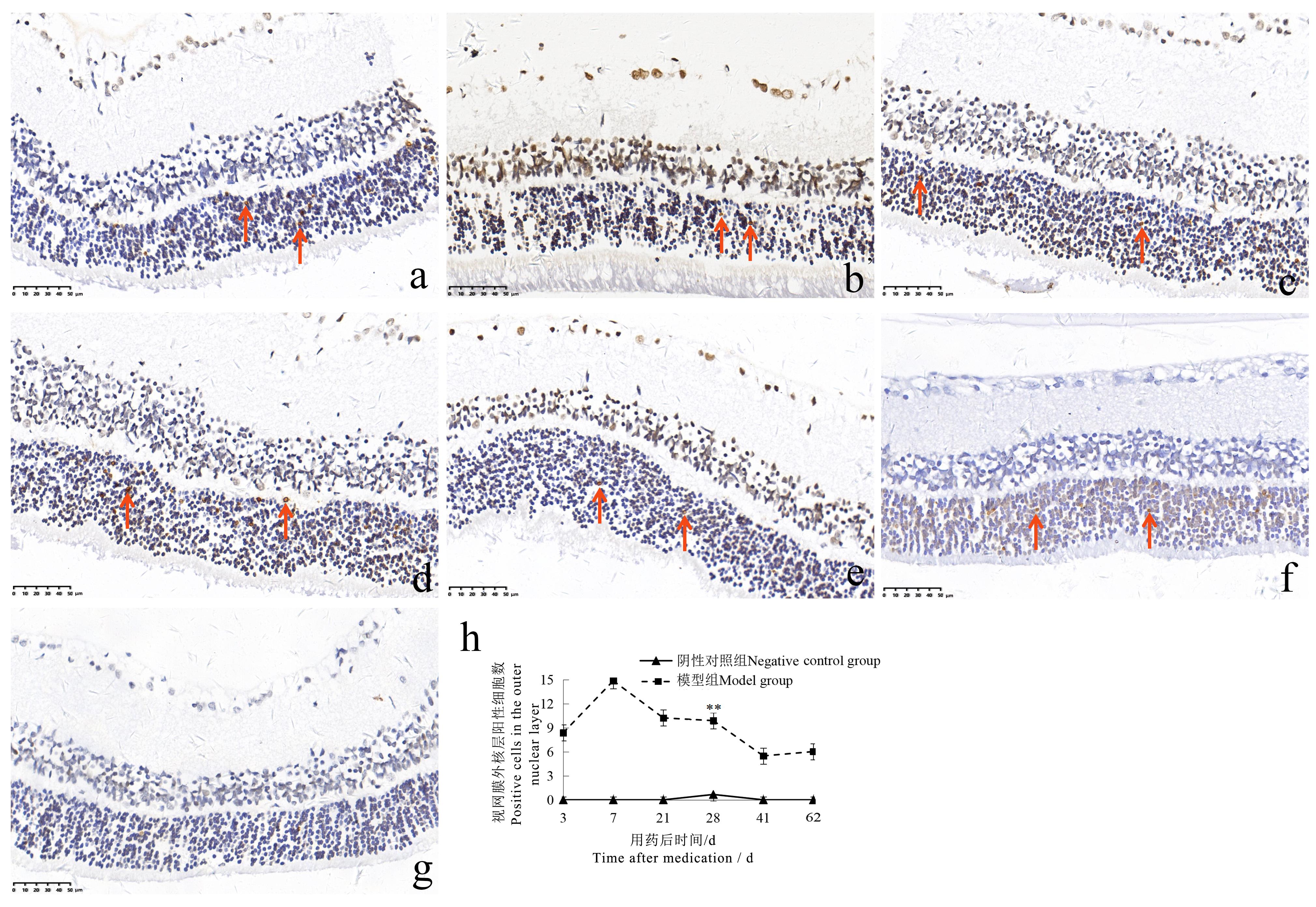
Figure 6 TUNEL staining of rat retina at different time points after sodium iodate injection (×400 )Note:The model group was intraperitoneally injected with 6 mg/mL sodium iodate solution once at the dose of 10 mL/kg;the negative control group was intraperitoneally injected with normal saline at the same volume and frequency. The number of rats in the two groups at each time point was 2, 2, 2, 6, 2, 2 respectively for single sex. a-f show that TUNEL staining was positive in the outer nuclear layer of the retina on the 3rd, 7th, 21st, 28th, 41st and 62nd days after injection in the model group (the arrows show TUNEL staining positive cells); g shows that the TUNEL staining of the retina in the negative control group was negative; h shows the comparison of the number of TUNEL staining positive cells in the outer nuclear layer of the two groups of rats. ??P<0.01, vs the negative control group. The scale size in the figure are 50 μm.
| 1 | 国家食品药品监督管理总局.«药物非临床研究质量管理规范»(国家食品药品监督管理总局令第34号)[EB/OL]. (2017-08-02)[2023-01-13]. . |
| 2 | ICH. M3(R2) Guidance on Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authori-zation for Pharmaceuticals[EB/OL]. (2009-06-11)[2023-01-13]. . |
| 3 | 范美花. 碘酸钠诱发的视网膜色素变性动物模型研究进展[J]. 中华实验眼科杂志, 2016, 34(9):860-864. DOI: 10.3760/cma.j.issn.2095-0160.2016.09.018 . |
| FAN M H. Progress in developing a sodium iodate-induced retinitis pigmentosa animal model[J]. Chin J Exp Ophthalmol, 2016, 34(9):860-864. DOI: 10.3760/cma.j.issn.2095-0160.2016.09.018 . | |
| 4 | 茅佩瑶, 茅希颖, 陈雪, 等. 碘酸钠诱导小鼠视网膜色素上皮变性模型的研究[J]. 现代生物医学进展, 2020, 20(9):1636-1641, 1662. DOI: 10.13241/j.cnki.pmb.2020.09.007 . |
| MAO P Y, MAO X Y, CHEN X, et al. Study on a mouse model of retinitis pigmentosa induced by sodium iodate[J]. Prog Mod Biomed, 2020, 20(9):1636-1641, 1662. DOI: 10.13241/j.cnki.pmb.2020.09.007 . | |
| 5 | LIU Y, LI Y, WANG C G, et al. Morphologic and histopathologic change of sodium iodate-induced retinal degeneration in adult rats[J]. Int J Clin Exp Pathol, 2019, 12(2):443-454. |
| 6 | 李鑫, 洪萌, 侯铁奇. 骨髓间充质干细胞对视网膜色素变性大鼠视网膜的修复作用[J]. 新乡医学院学报, 2021, 38(6):511-515. DOI: 10.7683/xxyxyxb.2021.06.003 . |
| LI X, HONG M, HOU T Q. Repair effect of bone marrow mesenchymal stem cells on the retina of rats with retinitis pigmentosa[J]. J Xinxiang Med Univ, 2021, 38(6):511-515. DOI: 10.7683/xxyxyxb.2021.06.003 . | |
| 7 | 蔡曾晓瑞, 胡相卡, 桂留铭, 等. 三七总皂苷对碘酸钠所致视网膜损伤的保护作用及机制研究[J]. 石河子大学学报(自然科学版), 2023, 41(1):113-119. DOI:10.13880/j.cnki.65-1174/n.2023.22.005 . |
| CAI Z X R, HU X K, GUI L M, et al. Study on protective effect and mechanism of panax notoginseng saponins on retina injury induced by sodium iodate[J]. Journal of Shihezi University(Natural Science),2023,41(1):113-119.DOI:10.13880/j.cnki.65-1174/n.2023.22.005 . | |
| 8 | 杜柏荣, 许衍峰, 韦志强. 维生素C通过调节巨噬细胞功能缓解大鼠黄斑变性的研究[J]. 实用临床医药杂志, 2022, 26(17):130-134. DOI: 10.7619/jcmp.20220296 . |
| DU B R, XU Y F, WEI Z Q. Vitamin C alleviates macular degeneration in rats by regulating function of macrophages[J]. J Clin Med Pract, 2022, 26(17):130-134. DOI: 10.7619/jcmp.20220296 . | |
| 9 | 许凯. 黄芪甲苷纳米乳眼用凝胶对实验性大鼠干性年龄相关性黄斑变性的作用研究[D]. 北京: 中国中医科学院, 2018. |
| XU K. Study on the effect of astragaloside IV nanoemulsion gel onexperimental dry age-related macular degeneration rat model[D]. Beijing: China Academy of Chinese Medical Sciences, 2018. | |
| 10 | 朱颖婷, 邓新国, 高杨, 等. 碘酸钠诱导大鼠视网膜损伤的病理改变和SOD、CAT的变化[J]. 中国病理生理杂志, 2010, 26(9):1851-1854. DOI: 10.3969/j.issn.1000-4718.2010.09.038 . |
| ZHU Y T, DENG X G, GAO Y, et al. Pathological changes and activity of SOD and CAT in the damaged retina induced by sodium iodate in rats[J]. Chin J Pathophysiol, 2010, 26(9):1851-1854. DOI: 10.3969/j.issn.1000-4718.2010.09.038 . | |
| 11 | 王立春, 刘建宗, 郭莹. 碘酸钠诱导兔视网膜色素上皮变性模型的研究[J]. 眼科新进展, 2015, 35(11):1032-1035. DOI: 10.13389/j.cnki.rao.2015.0282 . |
| WANG L C, LIU J Z, GUO Y. Experimental study on rabbit retinal pigment epithelial degeneration induced by sodium iodate[J]. Recent Adv Ophthalmol, 2015, 35(11):1032-1035. DOI: 10.13389/j.cnki.rao.2015.0282 . | |
| 12 | AHN S M, AHN J, CHA S, et al. Morphologic and electrophysiologic findings of retinal degeneration after intravitreal sodium iodate injection following vitrectomy in canines[J]. Sci Rep, 2020, 10(1):3588. DOI: 10.1038/s41598-020-60579-1 . |
| 13 | 陈若冰, 吴素琴, 姜玥, 等. 薯蓣皂苷元抑制碘酸钠诱发ARPE-19细胞氧化应激反应[J]. 中国老年学杂志, 2022, 42(11):2783-2786. DOI: 10.3969/j.issn.1005-9202.2022.11.053 . |
| CHEN R B, WU S Q, JIANG Y, et al. Diosgenin inhibits oxidative stress induced by sodium iodate in ARPE-19 cells[J]. Chin J Gerontol, 2022, 42(11): 2783-2786. DOI: 10.3969/j.issn.1005-9202.2022.11.053 . | |
| 14 | GRIGNOLO A, ORZALESI N, CALABRIA G A. Studies on the fine structure and the rhodopsin cycle of the rabbit retina in experimental degeneration induced by sodium iodate[J]. Exp Eye Res, 1966, 5(1):86-97. DOI: 10.1016/S0014-4835(66)80024-6 . |
| 15 | WANG J M, IACOVELLI J, SPENCER C, et al. Direct effect of sodium iodate on neurosensory retina[J]. Invest Ophthalmol Vis Sci, 2014, 55(3):1941-1953. DOI: 10.1167/iovs.13-13075 . |
| 16 | CHOWERS G, COHEN M, MARKS-OHANA D, et al. Course of sodium iodate-induced retinal degeneration in albino and pigmented mice[J]. Invest Ophthalmol Vis Sci, 2017, 58(4):2239-2249. DOI: 10.1167/iovs.16-21255 . |
| 17 | 黄文金, 陈志辉. 碘酸盐的毒理学研究进展[J]. 海峡预防医学杂志, 2002, 8(1):79-80. DOI: 10.3969/j.issn.1007-2705.2002.01.053 . |
| HUANG W J, CHEN Z H. Research progress in toxicology of iodate[J]. Strait J Prev Med, 2002, 8(1): 79-80. DOI: 10.3969/j.issn.1007-2705.2002.01.053 . | |
| 18 | 黄越, 陈乃耀, 赵雪聪, 等. 氧化应激参与放射性脑损伤的研究进展[J]. 神经解剖学杂志, 2019, 35(2):221-224. DOI: 10.16557/j.cnki.1000-7547.2019.02.019 . |
| HUANG Y, CHEN N Y, ZHAO X C, et al. Advances in research on oxidative stress involved in radiation-induced brain injury[J]. Chin J Neuroanat, 2019, 35(2):221-224. DOI: 10.16557/j.cnki.1000-7547.2019.02.019 . | |
| 19 | 廖月, 何毅怀, 罗亚文. 氧化应激在急性肝损伤中的作用[J]. 临床肝胆病杂志, 2022, 38(10):2402-2407. DOI: 10.3969/j.issn.1001-5256.2022.10.039 . |
| LIAO Y, HE Y H, LUO Y W. Role of oxidative stress in acute liver injury[J]. J Clin Hepatol, 2022, 38(10):2402-2407. DOI: 10.3969/j.issn.1001-5256.2022.10.039 . | |
| 20 | 胡英华, 贾炳超, 尚鑫, 等. 基于氧化应激通路的朱砂肾毒性机理研究及针灸对氧化应激的缓解作用[J]. 长春中医药大学学报, 2022, 38(5):513-517. DOI: 10.13463/j.cnki.cczyy.2022.05.012 . |
| HU Y H, JIA B C, SHANG X, et al. Study on the mechanism of cinnabar nephrotoxicity based on the oxidative stress pathway and the alleviating effect of acupuncture on oxidative stress[J]. J Chang Univ Chin Med, 2022, 38(5):513-517. DOI: 10.13463/j.cnki.cczyy.2022.05.012 . | |
| 21 | 刘和亮, 赵金垣. 氧化应激和肺损伤[J]. 中国职业医学, 2002, 29(5):49-51. DOI: 10.3969/j.issn.1000-6486.2002.05.024 . |
| LIU H L, ZHAO J Y. Oxidative stress and lung injury[J]. China Occup Med, 2002, 29(5):49-51. DOI: 10.3969/j.issn.1000-6486.2002.05.024 . | |
| 22 | 李敏启, 杜娟, 杨盼盼, 等. 氧化应激调控骨质疏松症的研究进展[J]. 山东大学学报(医学版), 2021, 59(6):16-24. DOI: 10.6040/j.issn.1671-7554.0.2021.0295 . |
| LI M Q, DU J, YANG P P, et al. Research progress of oxidative stress regulating osteoporosis[J]. J Shandong Univ Health Sci, 2021, 59(6):16-24. DOI: 10.6040/j.issn.1671-7554.0.2021.0295 . | |
| 23 | 宁佳, 胡欣, 程兴群. 变异链球菌氧化应激调控机制的研究进展[J]. 口腔疾病防治, 2023, 31(4):295-300. DOI:10.12016/j.issn.2096-1456.2023.04.012 . |
| NING J, HU X, CHENG X Q. Research progress on oxidative stress regulatory mechanisms in Streptococcus mutans[J]. J Prev Treat Stomatol Dis, 2023, 31(4):295-300. DOI: 10.12016/j. issn.2096-1456.2023.04.012 . | |
| 24 | WU N, ZHENG F, LI N, et al. RND3 attenuates oxidative stress and vascular remodeling in spontaneously hypertensive rat via inhibiting ROCK1 signaling[J]. Redox Biol, 2021, 48:102204. DOI: 10.1016/j.redox.2021.102204 . |
| 25 | 李楠, 王保和, 张晨, 等. 淫羊藿苷对心血管疾病作用机制研究进展[J]. 天津中医药, 2023, 40(1):126-130. DOI: 10.11656/j.issn.1672-1519.2023.01.22 . |
| LI N, WANG B H, ZHANG C, et al. Research progress on the mechanism of icariin on cardiovascular diseases[J]. Tianjin J Tradit Chin Med, 2023, 40(1):126-130. DOI: 10.11656/j.issn.1672-1519.2023.01.22 . | |
| 26 | 王香香, 孙建军, 刁明芳. 氧化应激在感音神经性耳聋中的作用机制[J]. 中国听力语言康复科学杂志, 2023, 21(1):56-60. DOI: 10.3969/j.issn.1672-4933.2023.01.014 . |
| WANG X X, SUN J J, DIAO M F. Mechanisms of oxidative stress in sensorineural deafness[J]. Chin Sci J Hear Speech Rehabil, 2023, 21(1):56-60. DOI: 10.3969/j.issn.1672-4933.2023.01.014 . | |
| 27 | 江楠, 马瑞红, 赵晓丽, 等. 氧化应激与生殖相关疾病研究进展[J]. 国际生殖健康/计划生育杂志, 2021, 40(5):402-406. DOI:10.12280/gjszjk.20210042 . |
| JIANG N, MA R H, ZHAO X L, et al. Research progress on oxidative stress and reproductive related diseases[J]. J Int Reproductive Health/family Plan, 2021, 40(5):402-406.DOI:10.12280/gjszjk.20210042 . | |
| 28 | 赵金枫, 颜士玉, 王瑞, 等. 氧化应激在三氯乙烯所致毒性效应中的作用[J]. 环境与职业医学, 2022, 39(12):1423-1429. DOI: 10.11836/JEOM22259 . |
| ZHAO J F, YAN S Y, WANG R, et al. Role of oxidative stress in trichloroethylene-induced toxicity[J]. J Environ Occup Med, 2022, 39(12):1423-1429. DOI: 10.11836/JEOM22259 . | |
| 29 | CARIDO M, ZHU Y, POSTEL K, et al. Characterization of a mouse model with complete RPE loss and its use for RPE cell transplantation[J]. Invest Ophthalmol Vis Sci, 2014, 55(8):5431-5444. DOI: 10.1167/iovs.14-14325 . |
| 30 | KIM H L, NAM S M, CHANG B J, et al. Ultrastructural changes and expression of PCNA and RPE65 in sodium iodate-induced acute retinal pigment epithelium degeneration model[J]. Neurochem Res, 2018, 43(5):1010-1019. DOI: 10.1007/s11064-018-2508-9 . |
| 31 | KIUCHI K, YOSHIZAWA K, SHIKATA N, et al. Morphologic characteristics of retinal degeneration induced by sodium iodate in mice[J]. Curr Eye Res, 2002, 25(6):373-379. DOI: 10.1076/ceyr.25.6.373.14227 . |
| [1] | QIN Chao, LI Shuangxing, ZHAO Tingting, JIANG Chenchen, ZHAO Jing, YANG Yanwei, LIN Zhi, WANG Sanlong, WEN Hairuo. Study on the 90-day Feeding Experimental Background Data of SD Rats for Drug Safety Evaluation [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 439-448. |
| [2] | LIU Liyu, JI Bo, LIU Xiaoxuan, FANG Yang, ZHANG Ling, GUO Tingting, QUAN Ye, LI Hewen, LIU Yitian. Exploration of Rat Fetal Lung Tissue Fixation Methods [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 432-438. |
| [3] | LIU Zhiwei, YANG Ran, LIAN Hao, ZHANG Yu, JIN Lilun. Cartilage Protection and Anti-Inflammatory Effects of Fraxetin on Monosodium Iodoacetate-Induced Rat Model of Osteoarthritis [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 259-268. |
| [4] | JIANG Meng, HAO Shulan, TONG Liguo, ZHONG Qiming, GAO Zhenfei, WANG Yonghui, WANG Xixing, JI Haijie. Dynamic Evaluation of Vinorelbine-Induced Phlebitis of Dorsalis Pedis Vein in a Rat Model [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 251-258. |
| [5] | PAN Yicong, JIANG Wenhong, HU Ming, QIN Xiao. Optimization of Surgical Procedure and Efficacy Evaluation of Aortic Calcification Model in Rats with Chronic Kidney Disease [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 279-289. |
| [6] | LIAN Hui, JIANG Yanling, LIU Jia, ZHANG Yuli, XIE Wei, XUE Xiaoou, LI Jian. Construction and Evaluation of a Rat Model of Abnormal Uterine Bleeding [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 130-146. |
| [7] | YIN Yulian, MA Lina, TU Siyuan, CHEN Ling, YE Meina, CHEN Hongfeng. Establishment and Evaluation of a Rat Model of Non-Puerperal Mastitis [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 587-596. |
| [8] | YANG Jin, YU Shiya, LIN Nan, FANG Yongchao, ZHAO Hu, QIU Jinwei, LIN Hongming, CHEN Huiyan, WANG Yu, WU Weihang. Effect of Modified Duodenal Exclusion Surgery on Glucose Metabolism in Rats with Type 2 Diabetes Mellitus [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 523-530. |
| [9] | QI Longju, CHEN Shiyuan, LIAO Zehua, SHI Yuanhu, SUN Yuyu, WANG Qinghua. Transcriptomic Analysis of Menstrual Blood-Derived Stem Cells Transplantation Combined with Exercise Training in Promoting Spinal Cord Injury Recovery in Rats [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 531-542. |
| [10] | ZHANG Naiqun, YUAN Piaopiao, CAO Linrong, YING Na, YANG Taotao. Application of PNR Detection in the Diagnosis and Drug-efficacy Evaluation of Diabetic Kidney Disease in Rats [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 543-549. |
| [11] | ZHENG Yiqing, DENG Yasheng, FAN Yanping, LIANG Tianwei, HUANG Hui, LIU Yonghui, NI Zhaobing, LIN Jiang. Application Analysis of Animal Models for Pelvic Inflammatory Disease Based on Data Mining [J]. Laboratory Animal and Comparative Medicine, 2024, 44(4): 405-418. |
| [12] | XIAO Pan, WANG Hongyi, LU Lu, ZHANG Mei, CHEN Keming, SHEN Dongshuai, NIU Tingxian. Screening of Hypoxia-Sensitive and Hypoxia-Tolerant Wistar Rats and Preliminary Exploration of Hypoxia Sensitivity in Their G1 Generation [J]. Laboratory Animal and Comparative Medicine, 2024, 44(4): 374-383. |
| [13] | Xiaoyu ZHU, Hantao YUAN, Sibo LI. MicroRNA-887-3p Inhibited MDM4 Expression and Proliferation but Promoted Apoptosis of Intervertebral Disc Annulus Fibrosus Cells in Rats [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 270-278. |
| [14] | Jinhua HU, Jingjie HAN, Min JIN, Bin HU, Yuefen LOU. Effects of Puerarin on Bone Density in Rats and Mice: A Meta-analysis [J]. Laboratory Animal and Comparative Medicine, 2024, 44(2): 149-161. |
| [15] | Liya ZHAO, Liju NI, Caiqin ZHANG, Jianping TANG, Yangzheng YAO, Yanyan NIE, Xiaoxue GU, Ying ZHAO. Establishing a Genetic Detection Protocol of Single Nucleotide Polymorphisms Panels in Inbred Rats Based on Multiplex PCR-LDR [J]. Laboratory Animal and Comparative Medicine, 2023, 43(5): 548-558. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||