
Laboratory Animal and Comparative Medicine ›› 2025, Vol. 45 ›› Issue (3): 251-258.DOI: 10.12300/j.issn.1674-5817.2024.163
• Animal Models of Human Diseases • Next Articles
JIANG Meng1( )(
)( ), HAO Shulan1, TONG Liguo1, ZHONG Qiming1, GAO Zhenfei2, WANG Yonghui2, WANG Xixing1(
), HAO Shulan1, TONG Liguo1, ZHONG Qiming1, GAO Zhenfei2, WANG Yonghui2, WANG Xixing1( )(
)( ), JI Haijie1(
), JI Haijie1( )(
)( )
)
Received:2024-11-06
Revised:2025-02-07
Online:2025-06-25
Published:2025-06-25
Contact:
WANG Xixing, JI Haijie
CLC Number:
JIANG Meng,HAO Shulan,TONG Liguo,et al. Dynamic Evaluation of Vinorelbine-Induced Phlebitis of Dorsalis Pedis Vein in a Rat Model[J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 251-258. DOI: 10.12300/j.issn.1674-5817.2024.163.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2024.163
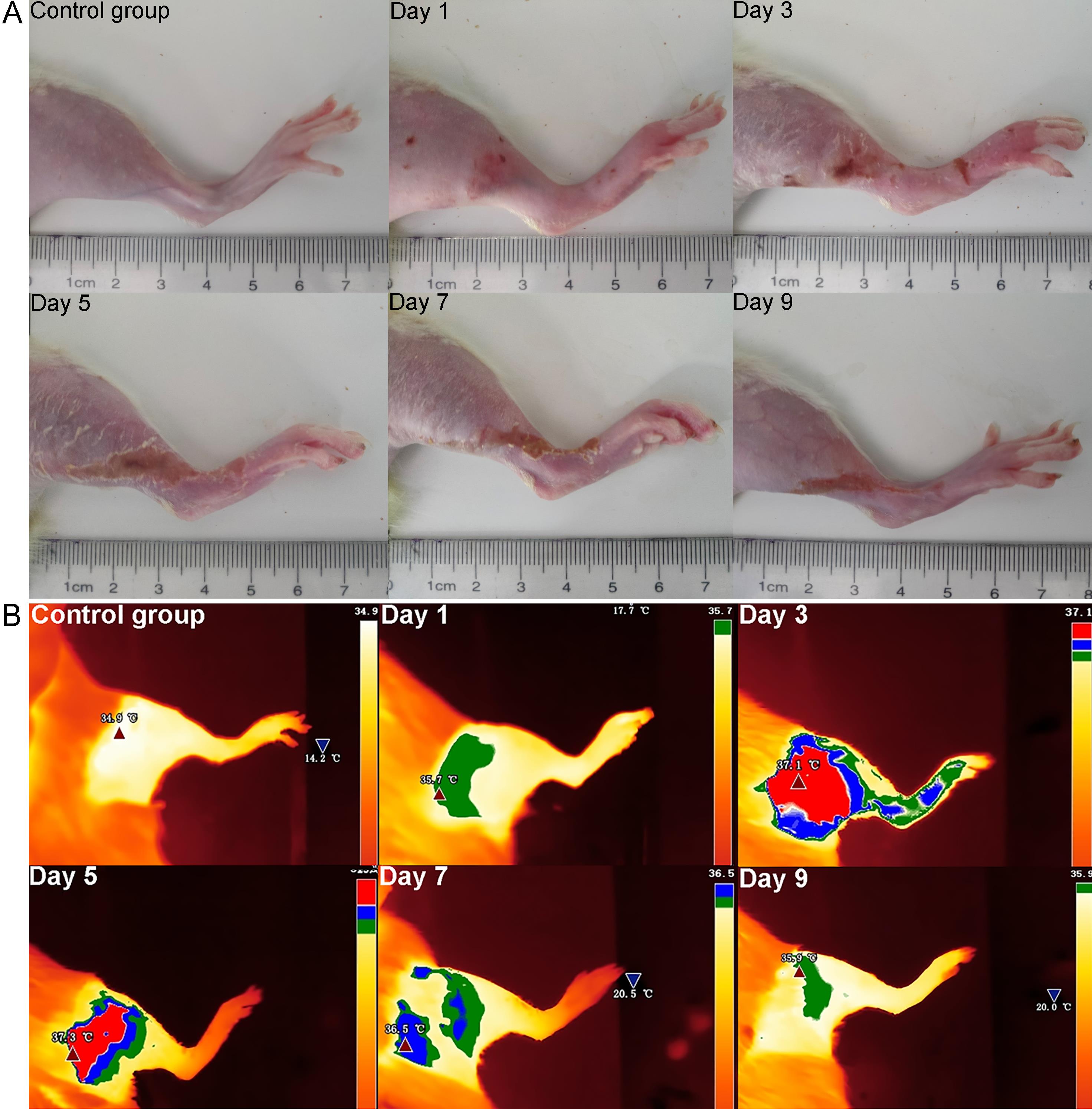
Figure 1 Appearances (A) and infrared thermal images (B) of the injured limbs of phlebitis model rats at different time pointsNote: A, appearance of the injured hindlimb; B, infrared thermal imaging (average temperature: >35.5 ℃ and ≤35.9 ℃, green; >35.9 ℃ and ≤36.5 ℃, blue; >36.5 ℃, red). The control group rats were injected with 0.1 mL of saline solution into the dorsal vein of the right hind limb, while the model group rats were injected with 0.1 mL of vinorelbine solution (4 mg/mL). Observation continued for 9 consecutive days. Day 1, Day 3, Day 5, Day 7, and Day 9 refer to the first, third, fifth, seventh, and ninth day after modeling, respectively.
时间点 Time points | 肿胀率/% Swelling rate/% | 承重占比/% Proportion of weight-bearing capacity/% | 平均温度/℃ Average temperature/℃ | |||||
|---|---|---|---|---|---|---|---|---|
对照组 Control group | 模型组 Model group | 对照组 Control group | 模型组 Model group | 对照组 Control group | 模型组 Model group | |||
| Day 1 | 0.91±0.89 | 25.49±7.93** | 51.44±0.04 | 44.58±0.86** | 34.35±0.58 | 34.75±0.68 | ||
| Day 3 | 0.99±0.53 | 81.89±15.75*** | 49.92±1.75 | 36.35±4.91*** | 33.85±0.42 | 35.17±0.81** | ||
| Day 5 | 1.51±0.30 | 63.03±1.41*** | 49.49±0.09 | 44.29±0.08** | 34.03±0.77 | 36.36±0.40*** | ||
| Day 7 | 1.58±0.44 | 29.75±5.91** | 49.90±1.85 | 47.71±0.25 | 35.10±0.43 | 35.28±0.80 | ||
| Day 9 | 0.30±0.17 | 15.41±0.33** | 50.86±1.49 | 49.67±1.19 | 34.40±0.44 | 34.88±0.63 | ||
Table 1 Swelling rate, weight-bearing ratio, and average temperature of the injured limb of phlebitis model rats at different time points
时间点 Time points | 肿胀率/% Swelling rate/% | 承重占比/% Proportion of weight-bearing capacity/% | 平均温度/℃ Average temperature/℃ | |||||
|---|---|---|---|---|---|---|---|---|
对照组 Control group | 模型组 Model group | 对照组 Control group | 模型组 Model group | 对照组 Control group | 模型组 Model group | |||
| Day 1 | 0.91±0.89 | 25.49±7.93** | 51.44±0.04 | 44.58±0.86** | 34.35±0.58 | 34.75±0.68 | ||
| Day 3 | 0.99±0.53 | 81.89±15.75*** | 49.92±1.75 | 36.35±4.91*** | 33.85±0.42 | 35.17±0.81** | ||
| Day 5 | 1.51±0.30 | 63.03±1.41*** | 49.49±0.09 | 44.29±0.08** | 34.03±0.77 | 36.36±0.40*** | ||
| Day 7 | 1.58±0.44 | 29.75±5.91** | 49.90±1.85 | 47.71±0.25 | 35.10±0.43 | 35.28±0.80 | ||
| Day 9 | 0.30±0.17 | 15.41±0.33** | 50.86±1.49 | 49.67±1.19 | 34.40±0.44 | 34.88±0.63 | ||
组别 Groups | 0级 0 grade | Ⅰ级 Ⅰ grade | Ⅱ级 Ⅱ grade | Ⅲ级 Ⅲ grade | Ⅳ级 Ⅳ grade | P值 P value |
|---|---|---|---|---|---|---|
| Control group | 8 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Day 1 | 0 (0.0) | 2 (25.0) | 6 (75.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Day 3 | 0 (0.0) | 0 (0.0) | 2 (25.0) | 3 (37.5) | 3 (37.5) | 0.001*** |
| Day 5 | 0 (0.0) | 0 (0.0) | 2 (25.0) | 4 (50.0) | 2 (25.0) | 0.001*** |
| Day 7 | 0 (0.0) | 0 (0.0) | 3 (37.5) | 5 (62.5) | 0 (0.0) | 0.014* |
| Day 9 | 0 (0.0) | 0 (0.0) | 3 (37.5) | 5 (62.5) | 0 (0.0) | 0.014* |
Table 2 Grading of phlebitis model rats at different time points
组别 Groups | 0级 0 grade | Ⅰ级 Ⅰ grade | Ⅱ级 Ⅱ grade | Ⅲ级 Ⅲ grade | Ⅳ级 Ⅳ grade | P值 P value |
|---|---|---|---|---|---|---|
| Control group | 8 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Day 1 | 0 (0.0) | 2 (25.0) | 6 (75.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Day 3 | 0 (0.0) | 0 (0.0) | 2 (25.0) | 3 (37.5) | 3 (37.5) | 0.001*** |
| Day 5 | 0 (0.0) | 0 (0.0) | 2 (25.0) | 4 (50.0) | 2 (25.0) | 0.001*** |
| Day 7 | 0 (0.0) | 0 (0.0) | 3 (37.5) | 5 (62.5) | 0 (0.0) | 0.014* |
| Day 9 | 0 (0.0) | 0 (0.0) | 3 (37.5) | 5 (62.5) | 0 (0.0) | 0.014* |
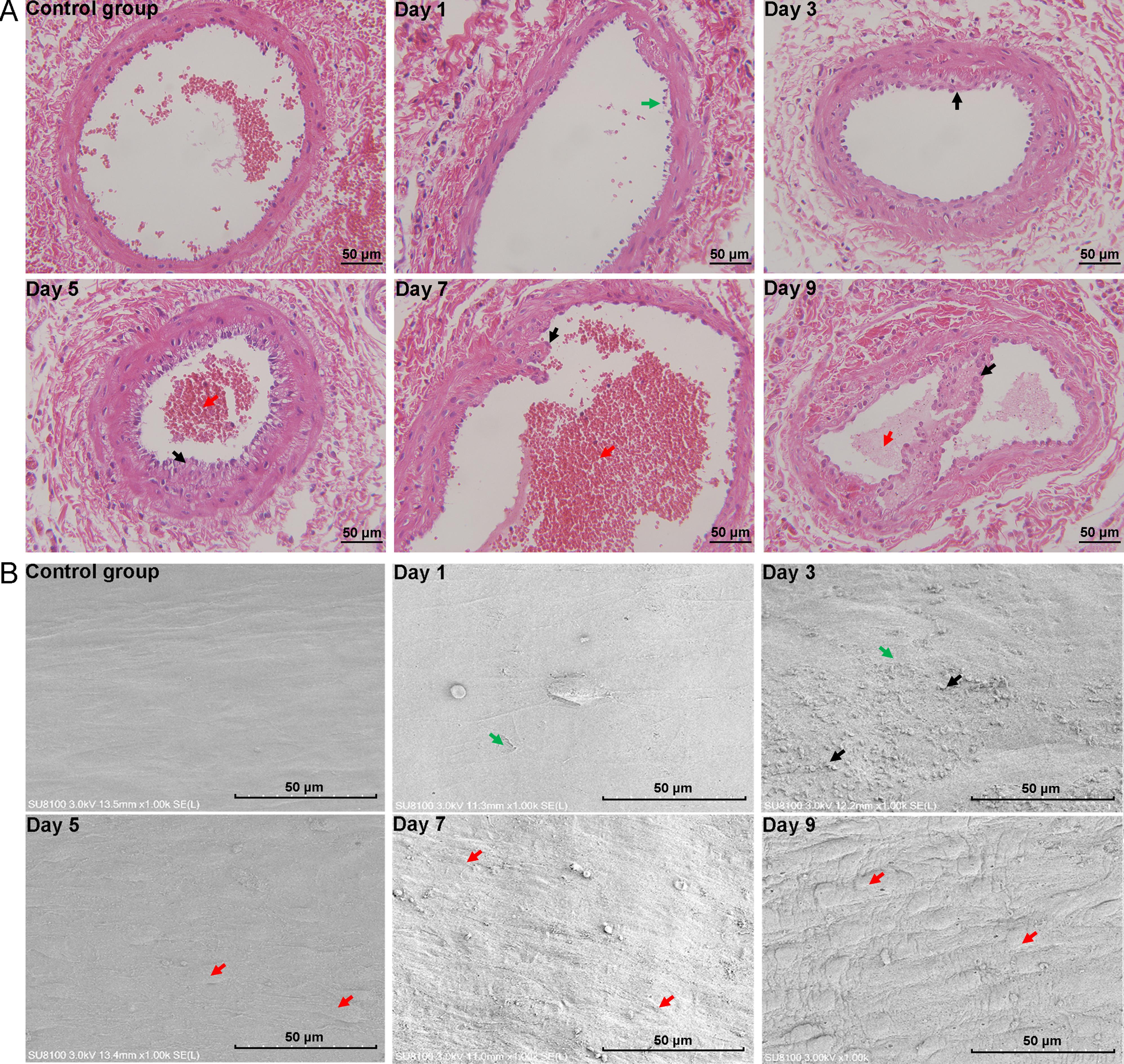
Figure 2 Histopathology of vein tissues (×400) and microstructure of vascular intima (×1 000) in phlebitis model rats at different time pointsNote: A, histopathological changes of vein tissue stained by hematoxylin-eosin (HE) (green arrows indicate irregular rupture of the inner membrane; black arrows indicate intimal fibrous hyperplasia; red arrows indicate thrombosis); B, microstructure of vascular intima observed by scanning electron microscopy (SEM) (green arrows indicate inner membrane rupture; black arrows indicate adhesion of blood cells; red arrows indicate elevated endothelial cells). The control group rats were injected with 0.1 mL of saline solution into the dorsal vein of the right hind limb, while the model group rats were injected with 0.1 mL of vinorelbine solution (4 mg/mL). Observation continued for 9 consecutive days. Day 1, Day 3, Day 5, Day 7, and Day 9 refer to the first, third, fifth, seventh, and ninth days after modeling, respectively.
| [1] | BIGDELI SHAMLOO M B, NASIRI M, MANEIY M, et al. Effects of topical sesame (Sesamum indicum) oil on the pain severity of chemotherapy-induced phlebitis in patients with colorectal cancer: a randomized controlled trial[J]. Complement Ther Clin Pract, 2019, 35:78-85. DOI:10.1016/j.ctcp.2019.01.016 . |
| [2] | GREGORY R K, SMITH I E. Vinorelbine: a clinical review[J]. Br J Cancer, 2000, 82(12):1907-1913. DOI:10.1054/bjoc.2000.1203 . |
| [3] | 刘花, 陈延绅, 尤慧柠, 等. 长春瑞滨药动学及毒副作用研究进展[J]. 中国药房, 2022, 33(11):1403-1408. DOI: 10.6039/j.issn.1001-0408.2022.11.21 . |
| LIU H, CHEN Y S, YOU H N, et al. Research progress on pharmacokinetics and toxicity of vinorelbine[J]. China Pharm, 2022, 33(11):1403-1408. DOI: 10.6039/j.issn.1001-0408.2022.11.21 . | |
| [4] | HUANG L, CHEN G, HU Q, et al. Construction of a rabbit model with vinorelbine administration via peripherally inserted central catheter and dynamic monitoring of changes in phlebitis and thrombosis[J]. Exp Ther Med, 2022, 23(3):212. DOI:10.3892/etm.2022.11135 . |
| [5] | GE G F, SHI W W, YU C H, et al. Baicalein attenuates vinorelbine-induced vascular endothelial cell injury and chemotherapeutic phlebitis in rabbits[J]. Toxicol Appl Pharmacol, 2017, 318:23-32. DOI:10.1016/j.taap.2017.01.013 . |
| [6] | 张咏梅, 宋凌霞, 王玲, 等. 虎杖膏预防高渗药液致兔耳缘静脉炎的实验研究[J]. 护理学杂志, 2013, 28(21):36-38. DOI: 10.3870/hlxzz.2013.21.036 . |
| ZHANG Y M, SONG L X, WANG L, et al. Experimental study on giant knotweed cream for prevention of inflammation of rabbit ear vein induced by intravenously administered hypertonic solution[J]. J Nurs Sci, 2013, 28(21):36-38. DOI: 10.3870/hlxzz.2013.21.036 . | |
| [7] | LIU P, YE L C, REN Y S, et al. Chemotherapy-induced phlebitis via the GBP5/NLRP3 inflammasome axis and the therapeutic effect of aescin[J]. Br J Pharmacol, 2023, 180(8):1132-1147. DOI:10.1111/bph.16002 . |
| [8] | WANG Z, MA L J, WANG X B, et al. Cimetidine attenuates vinorelbine-induced phlebitis in mice by militating E-selectin expression[J]. Cancer Chemother Pharmacol, 2014, 74(2):239-247. DOI:10.1007/s00280-014-2487-8 . |
| [9] | ZHANG H Y, GONG J, ZHANG S Y, et al. N-acetylcysteine attenuates the incidence of phlebitis induced by carbomer / vinorelbine gel[J]. Heliyon, 2023, 9(11): e21235. DOI:10.1016/j.heliyon.2023.e21235 . |
| [10] | 王淑敏, 郝淑兰, 冯玛莉, 等. 长春瑞滨诱导化疗性静脉炎大鼠模型的构建和评价[J]. 中国实验动物学报, 2023, 31(12):1539-1544. DOI: 10.3969/j.issn.1005-4847.2023.12.003 . |
| WANG S M, HAO S L, FENG M L, et al. Construction and evaluation of a chemotherapeutic phlebitis rat model induced by vinorelbine via the dorsalis pedis vein[J]. Acta Lab Anim Sci Sin, 2023, 31(12):1539-1544. DOI: 10.3969/j.issn.1005-4847.2023.12.003 . | |
| [11] | GORSKI L A, HADAWAY L, HAGLE M E, et al. Infusion therapy standards of practice, 8th edition[J]. J Infus Nurs, 2021, 44(1S ): S1-S224. DOI:10.1097/NAN.0000000000000396 . |
| [12] | 中华中医药学会. 化疗后静脉炎中医诊疗专家共识[EB/OL]. [2024-11-05]. https://www.cacm.org.cn/2023/01/05/21290/. |
| China Association of Chinese Medicine. Consensus of Traditional Chinese Medicine experts on diagnosis and treatment of phlebitis after chemotherapy[EB/OL]. [2024-11-05]. https://www.cacm.org.cn/2023/01/05/21290/. | |
| [13] | ALWORTH L C, KELLY L M, COOPER T L, et al. Unassisted blood collection from unanesthetized rats and gerbils[J]. Lab Anim (NY), 2012, 41(6):155-156. DOI:10.1038/laban0612-155 . |
| [14] | 郝淑兰, 王晞星, 吉海杰, 等. 一种化疗性静脉炎大鼠模型的构建方法: CN115568441A[P]. 2023-01-06. |
| HAO S L, WANG X X, JI H J, et al. Construction method of a rat model of chemotherapy-induced phlebitis: CN115568441A[P]. 2023-01-06. | |
| [15] | 姜萌, 郝淑兰, 仝立国, 等. 复方藤芷凝胶贴膏剂制备工艺及透皮主要成分研究[J]. 辽宁中医药大学学报, 2024, 26(9):57-63. DOI: 10.13194/j.issn.1673-842x.2024.09.011 . |
| JIANG M, HAO S L, TONG L G, et al. Research on the preparation process and transdermal main components of Fufang Tengzhi gel plaster [J]. J Liaoning Univ Tradit Chin Med, 2024, 26(9):57-63. DOI: 10.13194/j.issn.1673-842x.2024.09.011 . | |
| [16] | KIM H, KWAK S H, BYEON J Y, et al. An experimental and clinical study of flap monitoring with an analysis of the clinical course of the flap using an infrared thermal camera[J]. Bioengineering (Basel), 2024, 11(7):688. DOI:10.3390/bioengineering11070688 . |
| [17] | LEE H W, XU Y Y, HE L Q, et al. Role of venous endothelial cells in developmental and pathologic angiogenesis[J]. Circulation, 2021, 144(16):1308-1322. DOI:10.1161/CIRCULATIONAHA.121.054071 . |
| [18] | TOMBOR L S, JOHN D, GLASER S F, et al. Single cell sequencing reveals endothelial plasticity with transient mesenchymal activation after myocardial infarction[J]. Nat Commun, 2021, 12(1):681. DOI:10.1038/s41467-021-20905-1 . |
| [19] | JIANG M, ZHANG Y X, BU W J, et al. Piezo1 channel activation stimulates ATP production through enhancing mitochondrial respiration and glycolysis in vascular endothelial cells[J]. Br J Pharmacol, 2023, 180(14):1862-1877. DOI:10.1111/bph.16050 . |
| [1] | QIN Chao, LI Shuangxing, ZHAO Tingting, JIANG Chenchen, ZHAO Jing, YANG Yanwei, LIN Zhi, WANG Sanlong, WEN Hairuo. Study on the 90-day Feeding Experimental Background Data of SD Rats for Drug Safety Evaluation [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 439-448. |
| [2] | LIU Liyu, JI Bo, LIU Xiaoxuan, FANG Yang, ZHANG Ling, GUO Tingting, QUAN Ye, LI Hewen, LIU Yitian. Exploration of Rat Fetal Lung Tissue Fixation Methods [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 432-438. |
| [3] | LIU Zhiwei, YANG Ran, LIAN Hao, ZHANG Yu, JIN Lilun. Cartilage Protection and Anti-Inflammatory Effects of Fraxetin on Monosodium Iodoacetate-Induced Rat Model of Osteoarthritis [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 259-268. |
| [4] | PAN Yicong, JIANG Wenhong, HU Ming, QIN Xiao. Optimization of Surgical Procedure and Efficacy Evaluation of Aortic Calcification Model in Rats with Chronic Kidney Disease [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 279-289. |
| [5] | LIAN Hui, JIANG Yanling, LIU Jia, ZHANG Yuli, XIE Wei, XUE Xiaoou, LI Jian. Construction and Evaluation of a Rat Model of Abnormal Uterine Bleeding [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 130-146. |
| [6] | YIN Yulian, MA Lina, TU Siyuan, CHEN Ling, YE Meina, CHEN Hongfeng. Establishment and Evaluation of a Rat Model of Non-Puerperal Mastitis [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 587-596. |
| [7] | YANG Jin, YU Shiya, LIN Nan, FANG Yongchao, ZHAO Hu, QIU Jinwei, LIN Hongming, CHEN Huiyan, WANG Yu, WU Weihang. Effect of Modified Duodenal Exclusion Surgery on Glucose Metabolism in Rats with Type 2 Diabetes Mellitus [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 523-530. |
| [8] | QI Longju, CHEN Shiyuan, LIAO Zehua, SHI Yuanhu, SUN Yuyu, WANG Qinghua. Transcriptomic Analysis of Menstrual Blood-Derived Stem Cells Transplantation Combined with Exercise Training in Promoting Spinal Cord Injury Recovery in Rats [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 531-542. |
| [9] | ZHANG Naiqun, YUAN Piaopiao, CAO Linrong, YING Na, YANG Taotao. Application of PNR Detection in the Diagnosis and Drug-efficacy Evaluation of Diabetic Kidney Disease in Rats [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 543-549. |
| [10] | ZHENG Yiqing, DENG Yasheng, FAN Yanping, LIANG Tianwei, HUANG Hui, LIU Yonghui, NI Zhaobing, LIN Jiang. Application Analysis of Animal Models for Pelvic Inflammatory Disease Based on Data Mining [J]. Laboratory Animal and Comparative Medicine, 2024, 44(4): 405-418. |
| [11] | XIAO Pan, WANG Hongyi, LU Lu, ZHANG Mei, CHEN Keming, SHEN Dongshuai, NIU Tingxian. Screening of Hypoxia-Sensitive and Hypoxia-Tolerant Wistar Rats and Preliminary Exploration of Hypoxia Sensitivity in Their G1 Generation [J]. Laboratory Animal and Comparative Medicine, 2024, 44(4): 374-383. |
| [12] | Xiaoyu ZHU, Hantao YUAN, Sibo LI. MicroRNA-887-3p Inhibited MDM4 Expression and Proliferation but Promoted Apoptosis of Intervertebral Disc Annulus Fibrosus Cells in Rats [J]. Laboratory Animal and Comparative Medicine, 2024, 44(3): 270-278. |
| [13] | Jinhua HU, Jingjie HAN, Min JIN, Bin HU, Yuefen LOU. Effects of Puerarin on Bone Density in Rats and Mice: A Meta-analysis [J]. Laboratory Animal and Comparative Medicine, 2024, 44(2): 149-161. |
| [14] | Liya ZHAO, Liju NI, Caiqin ZHANG, Jianping TANG, Yangzheng YAO, Yanyan NIE, Xiaoxue GU, Ying ZHAO. Establishing a Genetic Detection Protocol of Single Nucleotide Polymorphisms Panels in Inbred Rats Based on Multiplex PCR-LDR [J]. Laboratory Animal and Comparative Medicine, 2023, 43(5): 548-558. |
| [15] | Lingzhi YU, Jianyun XIE, Liping FENG, Xiaofeng WEI. Establishment of Fluorescence qPCR Method for Detection of Staphylococcus Aureus and Its Application in Feces Detection of Rats and Mice [J]. Laboratory Animal and Comparative Medicine, 2023, 43(5): 566-573. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||