
Laboratory Animal and Comparative Medicine ›› 2024, Vol. 44 ›› Issue (5): 511-522.DOI: 10.12300/j.issn.1674-5817.2024.048
• Animal Models of Human Diseases • Previous Articles Next Articles
MENG Yu1,2( ), LIANG Dongli2(
), LIANG Dongli2( ), ZHENG Linlin2, ZHOU Yuanyuan2, WANG Zhaoxia2(
), ZHENG Linlin2, ZHOU Yuanyuan2, WANG Zhaoxia2( )
)
Received:2024-03-25
Revised:2024-06-03
Online:2024-10-25
Published:2024-10-25
Contact:
WANG Zhaoxia
CLC Number:
MENG Yu,LIANG Dongli,ZHENG Linlin,et al. Optimization and Evaluation of Conditions for Orthotopic Nude Mouse Models of Human Liver Tumor Cells[J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 511-522. DOI: 10.12300/j.issn.1674-5817.2024.048.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2024.048
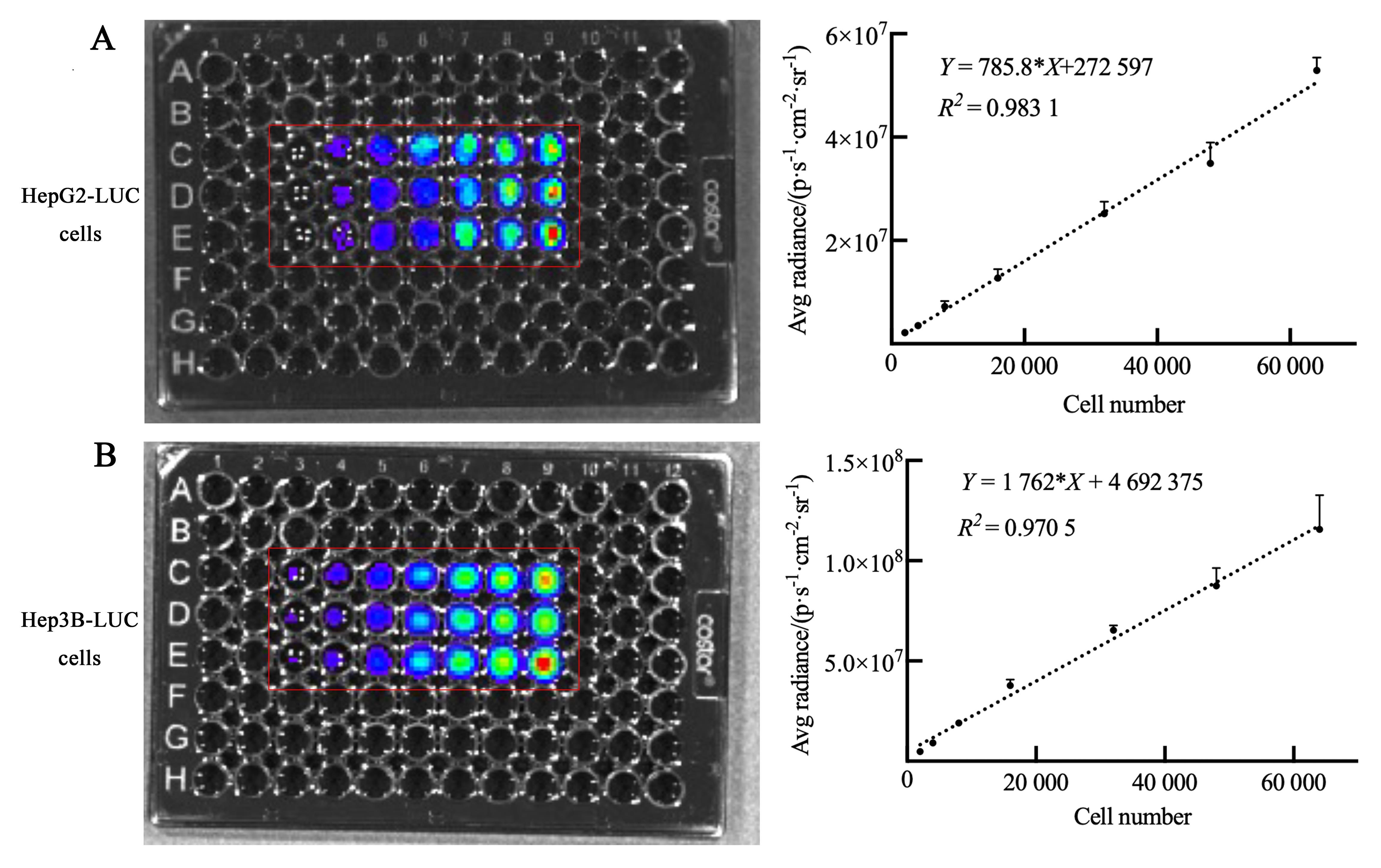
Figure 1 Cell bioluminescence efficiency of human liver tumor cell linesNote: A, Bioluminescence efficiency of human hepatoblastoma HepG2 cells labeled with luciferase reporter gene (LUC) after incubating with D-luciferin potassium; B, Bioluminescence efficiency of human hepatocellular carcinoma Hep3B cells labeled with LUC after incubating with D-luciferin potassium. Simple linear regression and one-way ANOVA test showed that the number of each cell was positively correlated with the average bioluminescence intensity. In the 96-well plate, the number of cells in each well from left to right was 2×103,4×103, 8×103, 1.6×104, 3.2×104, 4.8×104, and 6.4×104, n=3.
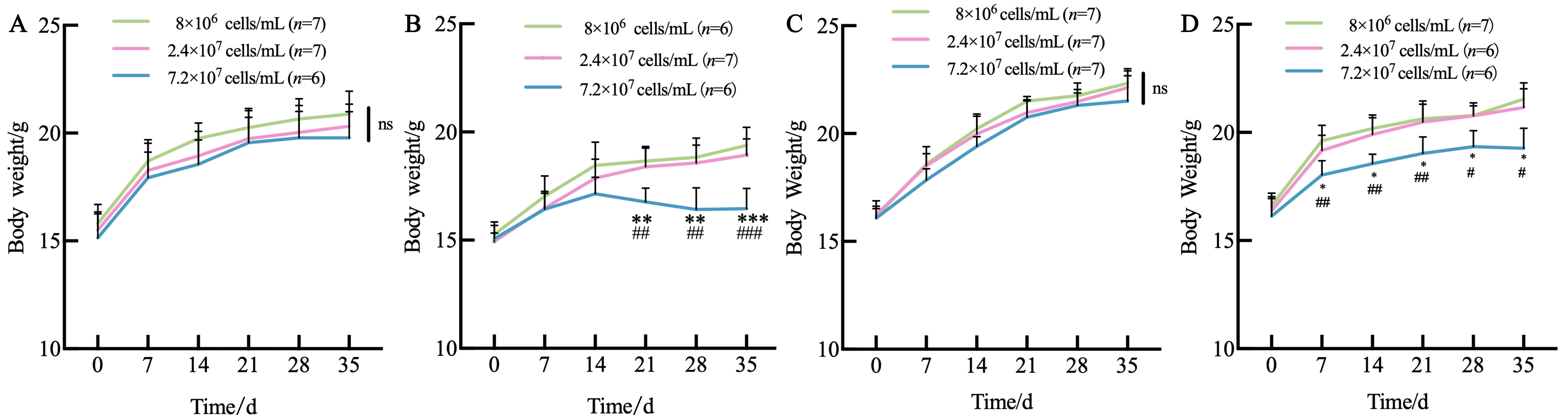
Figure 2 Body weight changes of nude mouse liver tumor models with different cell concentrations and suspension mediaNote:A-D represent body weight over time in nude mouse orthotopic tumor models of HepG2-LUC [human hepatoblastoma HepG2 cells labeled with luciferase reporter gene (LUC) and diluted with PBS into different cell concentrations], HepG2-LUC+Matrigel (human hepatoblastoma HepG2 cells labeled with LUC and diluted with 4 mg/mL Matrigel into different cell concentrations), Hep3B-LUC (human hepatocellular carcinoma Hep3B cells labeled with LUC and diluted with PBS into different cell concentrations), and Hep3B-LUC+Matrigel (human hepatocellular carcinoma Hep3B cells labeled with LUC and diluted with 4 mg/mL Matrigel into different cell concentrations) successively. Compared with the low-concentration (8×106 cells/mL) group of the same time, #P<0.05, ##P<0.01, ###P=0.000 1; Compared with the medium-concentration (2.4×107 cells/mL) group of the same time, #P<0.05, ##P<0.01, ###P=0.000 1; Compared among the three groups of the same time, nsP>0.05.
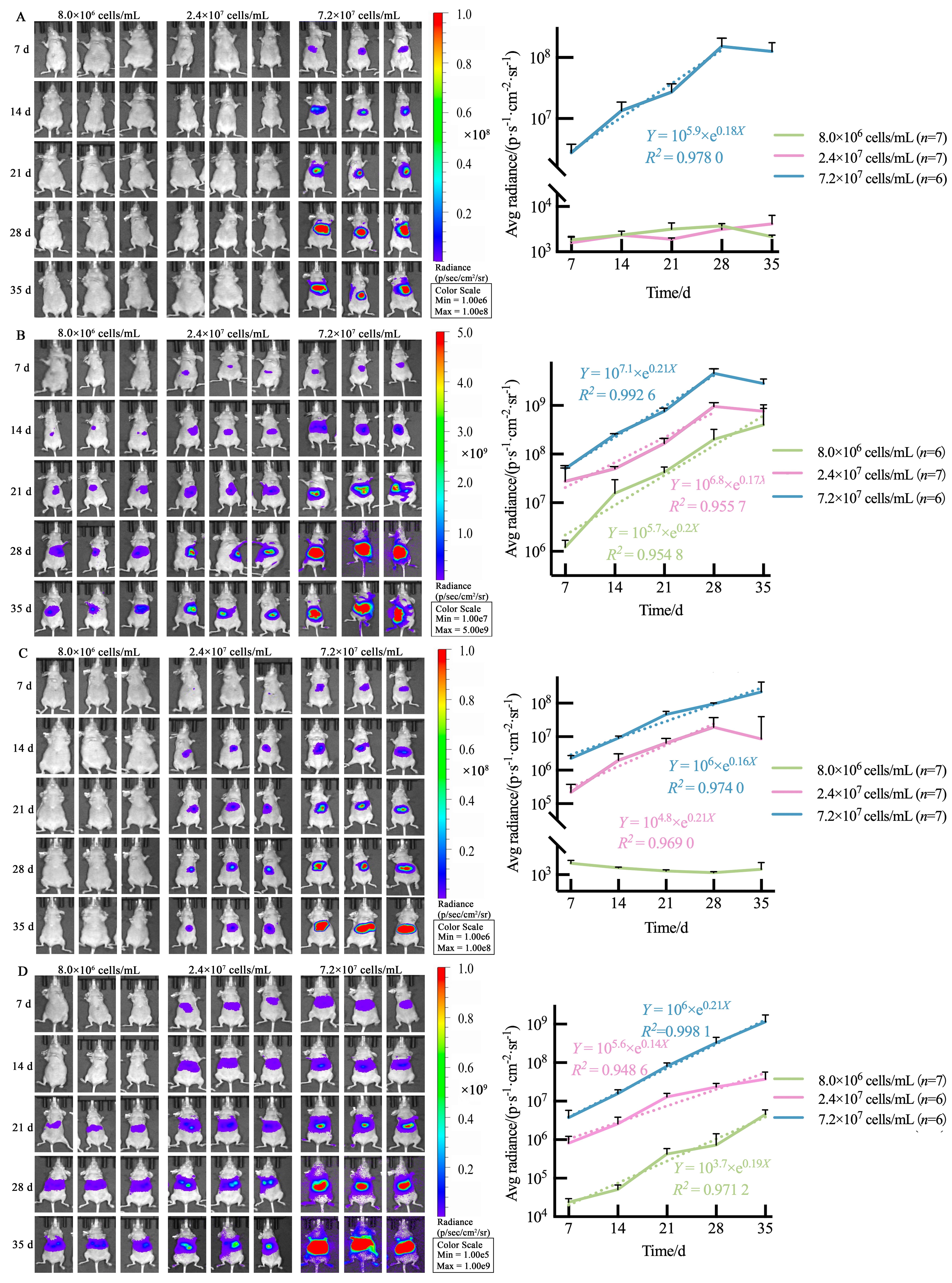
Figure 3 Bioluminescence monitoring of nude mouse liver tumor models with different cell concentrations and suspension mediaNote:A-D represent the weekly bioluminescence monitoring results of nude mouse orthotopic tumor models of HepG2-LUC [human hepatoblastoma HepG2 cells labeled with luciferase reporter gene (LUC) and diluted with PBS into different cell concentrations], HepG2-LUC+Matrigel (human hepatoblastoma HepG2 cells labeled with LUC and diluted with 4 mg/mL Matrigel into different cell concentrations), Hep3B-LUC (human hepatocellular carcinoma Hep3B cells labeled with LUC and diluted with PBS into different cell concentrations), and Hep3B-LUC+Matrigel (human hepatocellular carcinoma Hep3B cells labeled with LUC and diluted with 4 mg/mL Matrigel into different cell concentrations) successively, which show the change trends of the luminescence intensity over time after in-situ modeling (dotted lines indicate exponential growth).
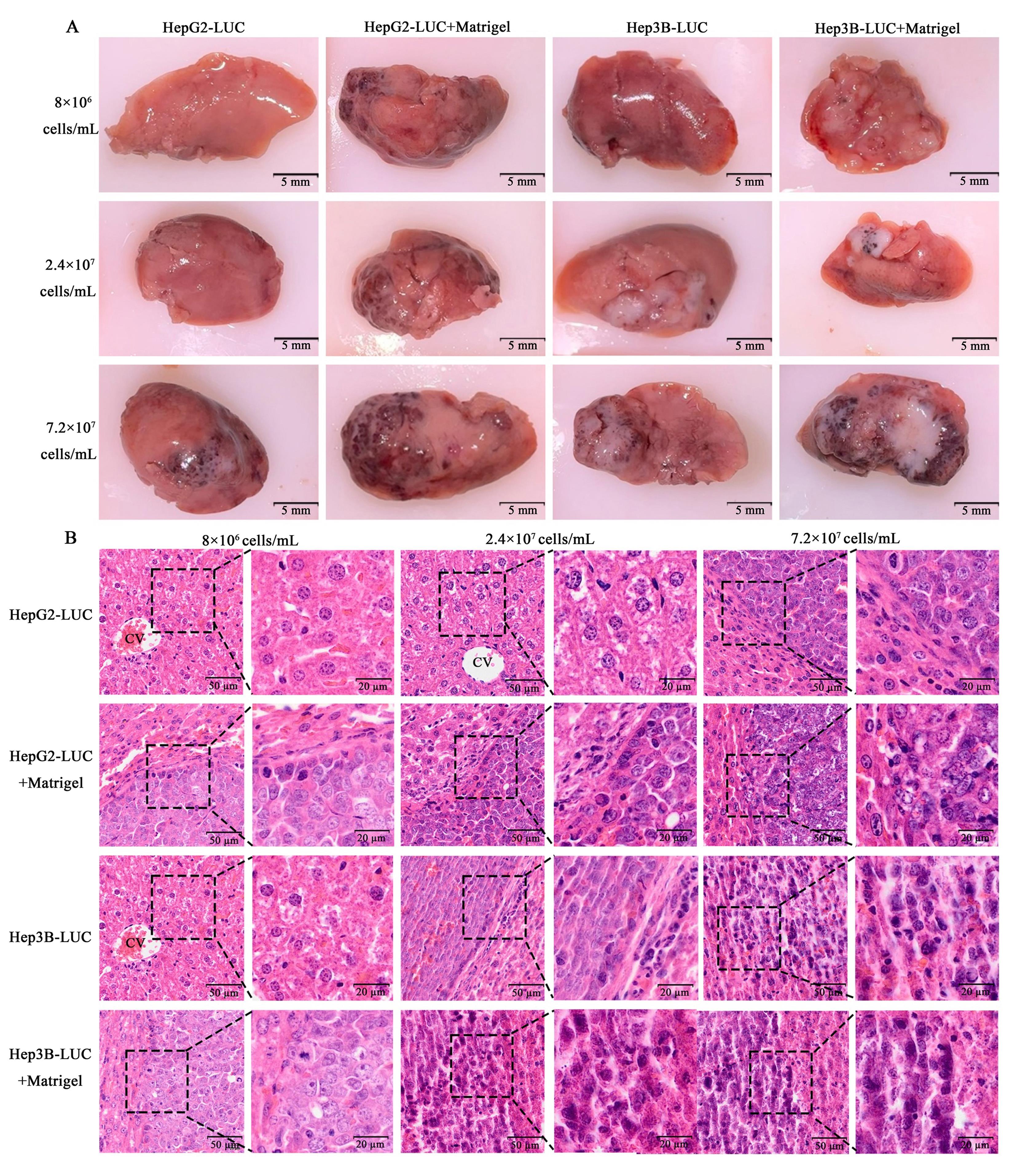
Figure 4 Gross images (A) and HE staining (B) of liver tissue in nude mouse liver tumor models with different cell concentrations and suspension mediaNote:HepG2-LUC, left liver lobe of nude mice 35 days after in-situ modeling with human hepatoblastoma HepG2 cells labeled with luciferase reporter gene (LUC) and diluted with PBS into different cell concentrations. HepG2-LUC+Matrigel, left liver lobe of nude mice 35 days after in-situ modeling with human hepatoblastoma HepG2 cells labeled with LUC and diluted with 4 mg/mL Matrigel into different cell concentrations. Hep3B-LUC, left liver lobe of nude mice 35 days after in-situ modeling with human hepatocellular carcinoma Hep3B cells labeled with LUC and diluted with PBS into different cell concentrations. Hep3B-LUC+Matrigel, left liver lobe of nude mice 35 days after in-situ modeling with human hepatocellular carcinoma Hep3B cells labeled with LUC and diluted with 4 mg/mL Matrigel into different cell concentrations. The scale size is 5 mm in figure A, and the scale sizes of the left and right graphs in each cell concentration group are 50 μm and 20 μm respectively in figure B.
| 1 | SELVAGGI F, CATALANO T, COTELLESE R, et al. Targeting Wnt/β-catenin pathways in primary liver tumours: from microenvironment signaling to therapeutic agents[J]. Cancers, 2022, 14(8):1912. DOI: 10.3390/cancers14081912 . |
| 2 | ZHENG H C, XUE H, YUN W J. An overview of mouse models of hepatocellular carcinoma[J]. Infect Agent Cancer, 2023, 18(1):49. DOI: 10.1186/s13027-023-00524-9 . |
| 3 | MARRERO J A, KULIK L M, SIRLIN C B, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases[J]. Hepatology, 2018, 68(2):723-750. DOI: 10.1002/hep.29913 . |
| 4 | EUROPEAN ASSOCIATION FOR THE STUDY OF THE LIVER. EASL clinical practice guidelines: management of hepatocellular carcinoma[J]. J Hepatol, 2018, 69(1):182-236. DOI: 10.1016/j.jhep.2018.03.019 . |
| 5 | MOLINA-SÁNCHEZ P, LUJAMBIO A. Experimental models for preclinical research in hepatocellular carcinoma[M]//Molecular and Translational Medicine. Cham: Springer International Publishing, 2019:333-358. DOI: 10.1007/978-3-030-21540-8_16 . |
| 6 | KALYAN A, NIMEIRI H, KULIK L. Systemic therapy of hepatocellular carcinoma: current and promising[J]. Clin Liver Dis, 2015, 19(2):421-432. DOI: 10.1016/j.cld.2015.01.009 . |
| 7 | CZAUDERNA P, ZBRZEZNIAK G, NAROZANSKI W, et al. Preliminary experience with arterial chemoembolization for hepatoblastoma and hepatocellular carcinoma in children[J]. Pediatr Blood Cancer, 2006, 46(7):825-828. DOI: 10.1002/pbc.20422 . |
| 8 | BROWN Z J, HEINRICH B, GRETEN T F. Mouse models of hepatocellular carcinoma: an overview and highlights for immunotherapy research[J]. Nat Rev Gastroenterol Hepatol, 2018, 15(9):536-554. DOI: 10.1038/s41575-018-0033-6 . |
| 9 | 雷会霞, 苗明三. 基于数据挖掘的肝癌动物模型应用分析[J]. 中药药理与临床, 2022, 38(3):186-190. DOI: 10.13412/j.cnki.zyyl.20210615.006 . |
| LEI H X, MIAO M S. Application analysis of liver cancer animal model based on data mining[J]. Pharmacol Clin Chin Mater Med, 2022, 38(3):186-190. DOI: 10.13412/j.cnki.zyyl.20210615.006 . | |
| 10 | 陈志刚, 纪志刚, 石冰冰, 等. 荧光素酶在膀胱肿瘤动物模型中的应用[J]. 北京医学, 2015, 37(11):1101-1103. DOI: 10.15932/j.0253-9713.2015.11.027 . |
| CHEN Z G, JI Z G, SHI B B, et al. Application of luciferase in animal model of bladder tumor[J]. Beijing Med J, 2015, 37(11):1101-1103. DOI: 10.15932/j.0253-9713.2015.11.027 . | |
| 11 | WU T, HEUILLARD E, LINDNER V, et al. Multimodal imaging of a humanized orthotopic model of hepatocellular carcinoma in immunodeficient mice[J]. Sci Rep, 2016, 6:35230. DOI: 10.1038/srep35230 . |
| 12 | GU C Y, LEE T K W. Preclinical mouse models of hepatocellular carcinoma: an overview and update[J]. Exp Cell Res, 2022, 412(2):113042. DOI: 10.1016/j.yexcr.2022.113042 . |
| 13 | ZHOU Z F, PENG F, LI J Y, et al. Intratumoral IL-12 gene therapy inhibits tumor growth in A HCC-hu-PBL-NOD/SCID murine model[J]. Onco Targets Ther, 2019, 12:7773-7784. DOI: 10.2147/OTT.S222097 . |
| 14 | HUANG Q X, HE S S, ZHAN D A. Osimertinib is a dual inhibitor of hepatocellular carcinoma and angiogenesis in an EGFR-independent manner, and synergizes with venetoclax[J]. J Cancer Res Clin Oncol, 2023, 149(12):10727-10735. DOI: 10.1007/s00432-023-04926-5 . |
| 15 | 黎凤明, 王静妮, 王春苗, 等. 人肝癌HepG2和Hep3B细胞的异质性与生物学行为关系的初探[J]. 广西医科大学学报, 2023, 40(3):398-405. DOI: 10.16190/j.cnki.45-1211/r.2023.03.009 . |
| LI F M, WANG J N, WANG C M, et al. Preliminary research on the relationship between heterogeneity and biological behavior of human hepatocellular carcinoma HepG2 and Hep3B cells[J]. J Guangxi Med Univ, 2023, 40(3):398-405. DOI: 10.16190/j.cnki.45-1211/r.2023.03.009 . | |
| 16 | 敬文宪, 张伶俐. 肿瘤研究中的实验动物福利问题探讨[J]. 中国实验动物学报, 2023, 31(9):1234-1240. DOI: 10.3969/j.issn.1005-4847.2023.09.015 . |
| JING W X, ZHANG L L. Discussing the welfare of laboratory animals in tumor research[J]. Acta Lab Animalis Sci Sin, 2023, 31(9):1234-1240. DOI: 10.3969/j.issn.1005-4847.2023.09.015 . | |
| 17 | 罗晓琴, 丁冠茗, 郑旭, 等. 小鼠肝癌原位移植性肿瘤动物模型的改良[J]. 中国比较医学杂志, 2021, 31(6):16-22. DOI: 10.3969/j.issn.1671-7856.2021.06.003 . |
| LUO X Q, DING G M, ZHENG X, et al. Improved mouse model of orthotopic transplantation for hepatocellular carcinoma[J]. Chin J Comp Med, 2021, 31(6):16-22. DOI: 10.3969/j.issn.1671-7856.2021.06.003 . | |
| 18 | ZHANG Y T, ZHONG A X, MIN J, et al. Biomimetic responsive nanoconverters with immune checkpoint blockade plus antiangiogenesis for advanced hepatocellular carcinoma treatment[J]. ACS Appl Mater Interfaces, 2024, 16(6):6894-6907. DOI: 10.1021/acsami.3c18140 . |
| 19 | 李泽山, 刘超, 申东方, 等. 虎杖提取物对小鼠肝癌原位移植瘤及miRNA-204-5p/Jarid2/PTEN信号通路的影响[J]. 智慧健康, 2022, 8(10):179-182, 192. DOI: 10.19335/j.cnki.2096-1219.2022.10.054 . |
| LI Z S, LIU C, SHEN D F, et al. Effects of Polygonum cuspidatum extract on orthotopic transplanted liver cancer and miRNA-204-5p/Jarid2/PTEN signaling pathway in mice[J]. Smart Healthc, 2022, 8(10):179-182, 192. DOI: 10.19335/j.cnki.2096-1219.2022.10.054 . | |
| 20 | 潘蕊, 喻锟, 张海亮, 等. 不同方法建立小鼠肝癌原位移植瘤模型差异性的探讨[J]. 中国实验动物学报, 2024, 32(3):329-336. DOI: 10.3969/j.issn.1005-4847.2024.03.006 . |
| PAN R, YU K, ZHANG H L, et al. Different transplantation models of hepatocellular carcinoma in mice[J]. Acta Lab Animalis Sci Sin, 2024, 32(3):329-336. DOI: 10.3969/j.issn.1005- 4847.2024.03.006 . | |
| 21 | 赵然, 刘羽, 高丽丽, 等. HepG2细胞系皮下接种与肝原位接种成瘤的比较研究[J]. 哈尔滨医科大学学报, 2010, 44(3):205-207, 211. DOI: 10.3969/j.issn.1000-1905.2010.03.002 . |
| ZHAO R, LIU Y, GAO L L, et al. Comparative study of subcutaneous injection and liver injection of HepG2 cells to develop tumor model[J]. J Harbin Med Univ, 2010, 44(3):205-207, 211. DOI: 10.3969/j.issn.1000-1905.2010.03.002 . | |
| 22 | 尹君, 李景丁莎, 左从林, 等. 人源性肝癌细胞小鼠原位移植瘤模型的建立及特点的比较研究[J]. 中国比较医学杂志, 2018, 28(12):68-74. DOI: 10.3969/j.issn.1671-7856.2018.12.012 . |
| YIN J, LIJING D S, ZUO C L, et al. Establishment of mouse orthotopic transplantation tumor models of human hepatoma and comparison of their characteristics[J]. Chin J Comp Med, 2018, 28(12):68-74. DOI: 10.3969/j.issn.1671-7856.2018.12.012 . | |
| 23 | 农宜熙, 黄俊玲, 黄赞松, 等. 人肝癌细胞株的特性及其实验应用[J]. 世界华人消化杂志, 2017, 25(2):159-165. DOI: 10.11569/wcjd.v25.i2.159 . |
| NONG Y X, HUANG J L, HUANG Z S, et al. Characteristics and experimental applications of human hepatocellular carcinoma cell lines[J]. World Chin J Dig, 2017, 25(2):159-165. DOI: 10.11569/wcjd.v25.i2.159 . | |
| 24 | ARZUMANIAN V A, KISELEVA O I, POVERENNAYA E V. The curious case of the HepG2 cell line: 40 years of expertise[J]. Int J Mol Sci, 2021, 22(23):13135. DOI: 10.3390/ijms222313135 . |
| 25 | ŠTAMPAR M, TOMC J, FILIPIČ M, et al. Development of in vitro 3D cell model from hepatocellular carcinoma (HepG2) cell line and its application for genotoxicity testing[J]. Arch Toxicol, 2019, 93(11):3321-3333. DOI: 10.1007/s00204-019-02576-6 . |
| 26 | KNOWLTON S, TASOGLU S. A bioprinted liver-on-a-chip for drug screening applications[J]. Trends Biotechnol, 2016, 34(9):681-682. DOI: 10.1016/j.tibtech.2016.05.014 . |
| 27 | BENTON G, ARNAOUTOVA I, GEORGE J, et al. Matrigel: from discovery and ECM mimicry to assays and models for cancer research[J]. Adv Drug Deliv Rev, 2014, 79-80:3-18. DOI: 10.1016/j.addr.2014.06.005 . |
| 28 | MULLEN P. The use of Matrigel to facilitate the establishment of human cancer cell lines as xenografts[J]. Methods Mol Med, 2004, 88:287-292. DOI: 10.1385/1-59259-406-9:287 . |
| 29 | MORENO J A, SANCHEZ A, HOFFMAN R M, et al. Fluorescent orthotopic mouse model of pancreatic cancer[J]. J Vis Exp, 2016(115):54337. DOI: 10.3791/54337 . |
| 30 | QUINTANA E, SHACKLETON M, SABEL M S, et al. Efficient tumour formation by single human melanoma cells[J]. Nature, 2008, 456(7222):593-598. DOI: 10.1038/nature07567 . |
| 31 | BEYREUTHER E, BRÜCHNER K, KRAUSE M, et al. An optimized small animal tumour model for experimentation with low energy protons[J]. PLoS One, 2017, 12(5): e0177428. DOI: 10.1371/journal.pone.0177428 . |
| 32 | BROUTIER L, MASTROGIOVANNI G, VERSTEGEN M M, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening[J]. Nat Med, 2017, 23(12):1424-1435. DOI: 10.1038/nm.4438 . |
| 33 | NUCIFORO S, FOFANA I, MATTER M S, et al. Organoid models of human liver cancers derived from tumor needle biopsies[J]. Cell Rep, 2018, 24(5):1363-1376. DOI: 10.1016/j.celrep.2018.07.001 . |
| 34 | LI L, KNUTSDOTTIR H, HUI K, et al. Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity[J]. JCI Insight, 2019, 4(2): e121490. DOI: 10.1172/jci.insight.121490 . |
| 35 | 郑南南, 黄钢. 小动物活体光学三维成像系统及其对乳腺癌的定量分析[J]. 激光生物学报, 2022, 31(3):215-223. DOI: 10.3969/j.issn.1007-7146.2022.03.004 . |
| ZHENG N N, HUANG G. Small animal living three-dimensional optical imaging system and its quantitative analysis of breast cancer[J]. Acta Laser Biol Sin, 2022, 31(3):215-223. DOI: 10.3969/j.issn.1007-7146.2022.03.004 . | |
| 36 | LI G, CHI C W, SHAO X F, et al. Application of molecular imaging technology in evaluating the inhibiting effect of apigenin in vivo on subcutaneous hepatocellular carcinoma[J]. Biochem Biophys Res Commun, 2017, 487(1): 122-7. DOI: 10.1016/j.bbrc.2017.04.029 . |
| 37 | 夏猛, 孙玉浩, 王萌, 等. 原发性肝癌常见动物模型的研究进展[J]. 临床肝胆病杂志, 2021, 37(8):1938-1942. DOI: 10.3969/j.issn.1001-5256.2021.08.042 . |
| XIA M, SUN Y H, WANG M, et al. Research advances in commonly used animal models of primary hepatocellular carcinoma[J]. J Clin Hepatol, 2021, 37(8):1938-1942. DOI: 10.3969/j.issn.1001-5256.2021.08.042 . | |
| 38 | BHATTACHARYA S D, MI Z Y, KIM V M, et al. Osteopontin regulates epithelial mesenchymal transition-associated growth of hepatocellular cancer in a mouse xenograft model[J]. Ann Surg, 2012, 255(2):319-325. DOI: 10.1097/SLA.0b013e31823e3a1c . |
| 39 | XU Q R, LIU X, LIU Z K, et al. MicroRNA-1296 inhibits metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by targeting SRPK1-mediated PI3K/AKT pathway[J]. Mol Cancer, 2017, 16(1):103. DOI: 10.1186/s12943-017-0675-y . |
| [1] | JIA Huan-huan, ZENG Ye-wen, LUO Ting, GONG Bao-yong, MAI Dong-mei, PAN Ying-chun, ZHAO Wei-bo. Effects of Different Concentrations of Chromium-containing Bedding Materials on Toxity of Blood and Organs in BALB/c Nude Mice and KM Mice [J]. Laboratory Animal and Comparative Medicine, 2019, 39(6): 454-461. |
| [2] | LIU Qian, LIU Song, Han Feng-feng, GUO Xue-jun. Establishment of Orthotopic Transplant Model of Human Lung Cancer and Observation of In vivo Biofluorescent Imaging in Nude Mice [J]. Laboratory Animal and Comparative Medicine, 2016, 36(4): 250-256. |
| [3] | ZHAO Yong-jiang, ZHU Miao-xin, YUAN Li-xin, SUN Lei, GENG Qin, LI Jing, YAO Ming, YAN Ming-xia. Dynamic Observation on In vivo Biofluorescent Imaging of Orthotopic Transplant Model of Human Colon Cancer in Nude Mice [J]. Laboratory Animal and Comparative Medicine, 2016, 36(3): 174-179. |
| [4] | ZHANG Yan, FAN Wen-xi, XU Yi-mei, SHI Sheng, GUI You-jun, YAN Shun-sheng. Effect of β-carboline derivative on Subcutaneous Transplant Hepatocellular Carcinoma Growth in BALB/c Nude Mice [J]. Laboratory Animal and Comparative Medicine, 2013, 33(4): 290-295. |
| [5] | WAN Bo-shun, YU Jing-xian, CHEN Yue-yu, WANG Xiao-min, ZHAO Fang-yu, YAO Ming, YAN Ming-xia. Establishment of Colon Cancer Mouse Model and its Biological Characteristics [J]. Laboratory Animal and Comparative Medicine, 2013, 33(2): 99-105. |
| [6] | PENG Xiu-Hua, SHEN Yan, XU Chun-Hua, YANG Yu-Qin, ZHOU Wen-Jiang. Dynamic Observation On Growth of Subcutaneous and Orthotopic Gastric Tumor by in vivo Fluorescence Imager [J]. Laboratory Animal and Comparative Medicine, 2011, 31(5): 371-375. |
| [7] | PENG Xiu-hua, SHEN Yan, XU Chun-hua, ZHOU Wen-jiang. Establishment of Subcutaneous and Orthotopic Transplant Tumor Model of Hepatocellar Carcinoma Cell Line with High Metastatic Potential in Nude Rat [J]. Laboratory Animal and Comparative Medicine, 2011, 31(1): 33-37. |
| [8] | SHEN Yan1,PENG Xiu-hua1,XU Chun-hua1,ZHOU Wen-jiang1,2. Establishment and Biological Characteristics of Orthotopic Transplantion Tumor Model of Hepatocellar Carcinoma Cell line in Nude Rat [J]. Laboratory Animal and Comparative Medicine, 2010, 30(6): 428-431. |
| [9] | YU Da-1, YU Hai-1, CHEN Li-Rong-2, CHEN Pei-Hui-2, WANG Hai-Jun-2. A New Orthotopic Transplantation Technique for Establishing Human Colon Cancer SW1116 Model in Nude Mice [J]. Laboratory Animal and Comparative Medicine, 2000, 20(2): 97-100. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||