
Laboratory Animal and Comparative Medicine ›› 2024, Vol. 44 ›› Issue (2): 180-191.DOI: 10.12300/j.issn.1674-5817.2023.123
• Animal Models of Human Diseases • Previous Articles Next Articles
Fangqi BAO1( ), Haiye TU2, Mingsun FANG3, Qian ZHANG3, Minli CHEN3(
), Haiye TU2, Mingsun FANG3, Qian ZHANG3, Minli CHEN3( )(
)( )
)
Received:2023-09-01
Revised:2023-11-24
Online:2024-04-25
Published:2024-04-25
Contact:
Minli CHEN
CLC Number:
Fangqi BAO,Haiye TU,Mingsun FANG,et al. Advances in Research on Pathological and Molecular Mechanism of Hyperuricemic Nephropathy Based on Animal Models[J]. Laboratory Animal and Comparative Medicine, 2024, 44(2): 180-191. DOI: 10.12300/j.issn.1674-5817.2023.123.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.slarc.org.cn/dwyx/EN/10.12300/j.issn.1674-5817.2023.123
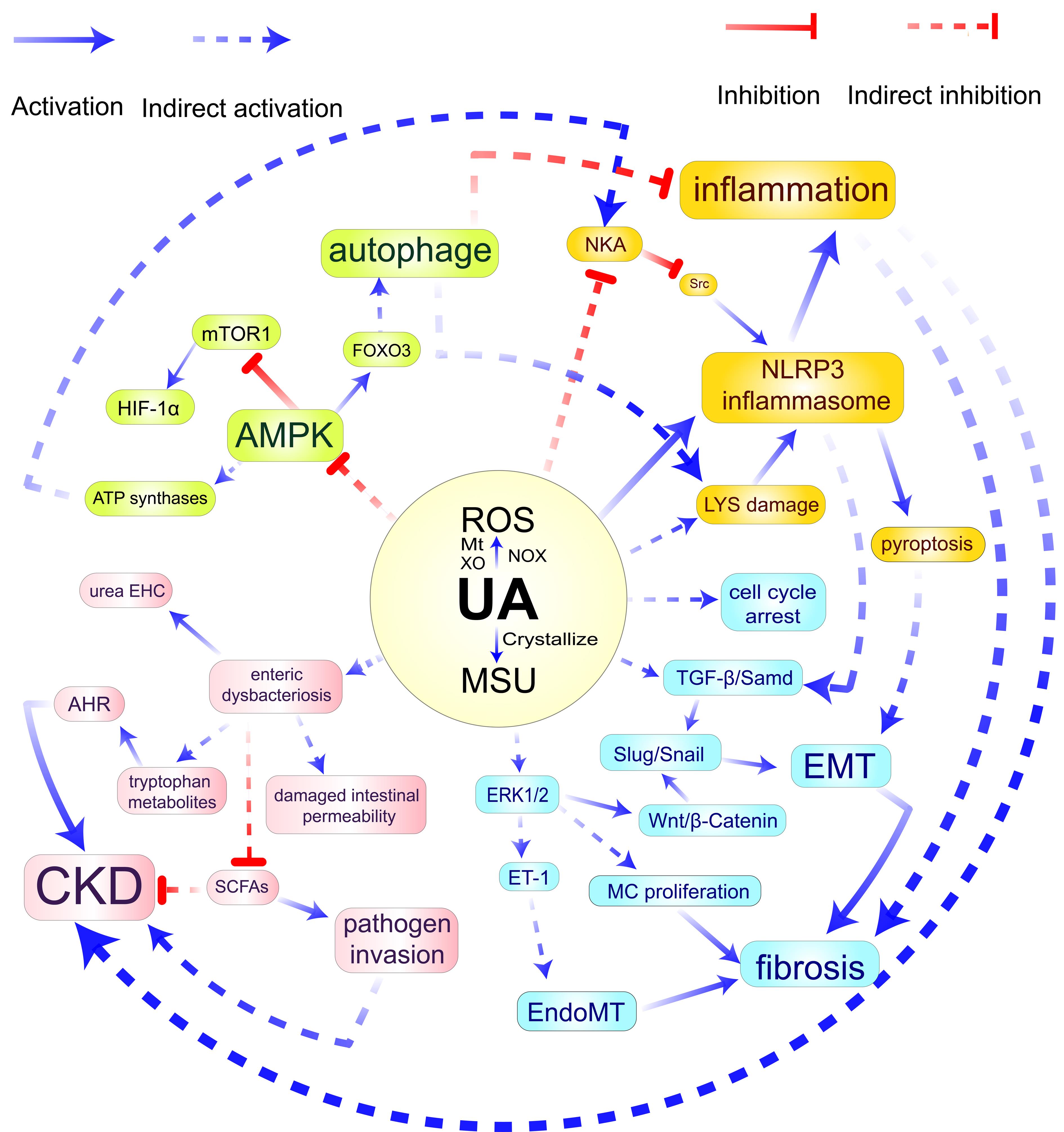
Figure 1 Pathological molecular mechanism of hyperuricemic nephropathyNote:HUA, Hyperuricemia; NOX, Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase; Mt, Mitochondria; XO, Xanthine oxidase; MSU, Monosodium urate; LYS, Lysosome; EMT, Epithelial-mesenchymal transition; MC, Mesangial cells; EndoMT, Endothelial-to-mesenchymal transition; SCFAs, Short-chain fatty acids; AHR, Aryl Hydrocarbon receptor; ET-1, Endothelin-1; NKA, Sodium-potassium ATPase; urea EHC, Urea enterohepatic circulation; ROS, Reactive oxygen species; CKD, Chronic kidney disease.
| 1 | RIDI R EL, TALLIMA H. Physiological functions and pathogenic potential of uric acid: a review[J]. J Adv Res, 2017, 8(5):487-493. DOI: 10.1016/j.jare.2017.03.003 . |
| 2 | DEMIRAY A, AFSAR B, COVIC A, et al. The role of uric acid in the acute myocardial infarction: a narrative review[J]. Angiology, 2022, 73(1):9-17. DOI: 10.1177/00033197211012546 . |
| 3 | ZOPPINI G, TARGHER G, CHONCHOL M, et al. Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function[J]. Diabetes Care, 2012, 35(1):99-104. DOI: 10.2337/dc11-1346 . |
| 4 | BADVE S V, PASCOE E M, TIKU A, et al. Effects of allopurinol on the progression of chronic kidney disease[J]. N Engl J Med, 2020, 382(26):2504-2513. DOI: 10.1056/NEJMoa1915833 . |
| 5 | DORIA A, GALECKI A T, SPINO C, et al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes[J]. N Engl J Med, 2020, 382(26):2493-2503. DOI: 10.1056/NEJMoa1916624 . |
| 6 | BELLOMO G, VENANZI S, VERDURA C, et al. Association of uric acid with change in kidney function in healthy normotensive individuals[J]. Am J Kidney Dis, 2010, 56(2):264-272. DOI: 10.1053/j.ajkd.2010.01.019 . |
| 7 | GOLDBERG A, GARCIA-ARROYO F, SASAI F, et al. Mini review: reappraisal of uric acid in chronic kidney disease[J]. Am J Nephrol, 2021, 52(10-11):837-844. DOI: 10.1159/000519491 . |
| 8 | SATO Y, FEIG D I, STACK A G, et al. The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD[J]. Nat Rev Nephrol, 2019, 15(12):767-775. DOI: 10.1038/s41581-019-0174-z . |
| 9 | LU J, DALBETH N, YIN H Y, et al. Mouse models for human hyperuricaemia: a critical review[J]. Nat Rev Rheumatol, 2019, 15(7):413-426. DOI: 10.1038/s41584-019-0222-x . |
| 10 | YANAI H, ADACHI H, HAKOSHIMA M, et al. Molecular biological and clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease[J]. Int J Mol Sci, 2021, 22(17):9221. DOI: 10.3390/ijms22179221 . |
| 11 | MACÍAS N, GOICOECHEA M, DE VINUESA M S, et al. Urate reduction and renal preservation: what is the evidence?[J]. Curr Rheumatol Rep, 2013, 15(12):386. DOI: 10.1007/s11926-013-0386-3 . |
| 12 | RAFEY M A, LIPKOWITZ M S, LEAL-PINTO E, et al. Uric acid transport[J]. Curr Opin Nephrol Hypertens, 2003, 12(5):511-516. DOI: 10.1097/00041552-200309000-00005 . |
| 13 | EJAZ A A, NAKAGAWA T, KANBAY M, et al. Hyperuricemia in kidney disease: a major risk factor for cardiovascular events, vascular calcification, and renal damage[J]. Semin Nephrol, 2020, 40(6):574-585. DOI: 10.1016/j.semnephrol.2020.12.004 . |
| 14 | MCCORMICK N, O'CONNOR M J, YOKOSE C, et al. Assessing the causal relationships between insulin resistance and hyperuricemia and gout using bidirectional Mendelian randomization[J]. Arthritis Rheumatol, 2021, 73(11):2096-2104. DOI: 10.1002/art.41779 . |
| 15 | RAMOS G K, GOLDFARB D S. Update on uric acid and the kidney[J]. Curr Rheumatol Rep, 2022, 24(5):132-138. DOI: 10.1007/s11926-022-01069-3 . |
| 16 | 张璐, 杨定位. 高尿酸血症肾病的诊治进展[J]. 中华临床医师杂志(电子版), 2019, 13(6):457-462. DOI: 10.3877/cma.j.issn.1674-0785.2019.06.011 . |
| ZHANG L, YANG D W. Progress in diagnosis and treatment of hyperuricemic nephropathy[J]. Chin J Clin Electron Ed, 2019, 13(6):457-462. DOI: 10.3877/cma.j.issn.1674-0785.2019.06.011 . | |
| 17 | 卢忠英, 郁建平, 朱梦琪, 等. 不同组合造模剂诱导大鼠高尿酸血症模型的比较研究[J]. 山地农业生物学报, 2014, 33(5):40-42, 67. DOI: 10.15958/j.cnki.sdnyswxb.2014.05.009 . |
| LU Z Y, YU J P, ZHU M Q, et al. Comparative study of different combinations of modeling agent-induced rat model of hyperuricemia[J]. J Mt Agric Biol, 2014, 33(5):40-42, 67. DOI: 10.15958/j.cnki.sdnyswxb.2014.05.009 . | |
| 18 | DEBOSCH B J, KLUTH O, FUJIWARA H, et al. Early-onset metabolic syndrome in mice lacking the intestinal uric acid transporter SLC2A9[J]. Nat Commun, 2014, 5:4642. DOI: 10.1038/ncomms5642 . |
| 19 | ZHU L R, DONG Y F, NA S, et al. Saponins extracted from Dioscorea collettii rhizomes regulate the expression of urate transporters in chronic hyperuricemia rats[J]. Biomed Pharmacother, 2017, 93:88-94. DOI: 10.1016/j.biopha.2017.06.022 . |
| 20 | SALEM C BEN, SLIM R, FATHALLAH N, et al. Drug-induced hyperuricaemia and gout[J]. Rheumatology, 2017, 56(5):679-688. DOI: 10.1093/rheumatology/kew293 . |
| 21 | HONG F, ZHENG A J, XU P F, et al. High-protein diet induces hyperuricemia in a new animal model for studying human gout[J]. Int J Mol Sci, 2020, 21(6):2147. DOI: 10.3390/ijms21062147 . |
| 22 | SAUTIN Y Y, NAKAGAWA T, ZHARIKOV S, et al. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress[J]. Am J Physiol Cell Physiol, 2007, 293(2): C584-C596. DOI: 10.1152/ajpcell.00600.2006 . |
| 23 | KIMURA Y, TSUKUI D, KONO H. Uric acid in inflammation and the pathogenesis of atherosclerosis[J]. Int J Mol Sci, 2021, 22(22):12394. DOI: 10.3390/ijms222212394 . |
| 24 | WAHEED Y, YANG F, SUN D. Role of asymptomatic hyperuricemia in the progression of chronic kidney disease and cardiovascular disease[J]. Korean J Intern Med, 2021, 36(6):1281-1293. DOI: 10.3904/kjim.2020.340 . |
| 25 | GHERGHINA M E, PERIDE I, TIGLIS M, et al. Uric acid and oxidative stress-relationship with cardiovascular, metabolic, and renal impairment[J]. Int J Mol Sci, 2022, 23(6):3188. DOI: 10.3390/ijms23063188 . |
| 26 | ELEFTHERIADIS T, PISSAS G, ANTONIADI G, et al. Allopurinol protects human glomerular endothelial cells from high glucose-induced reactive oxygen species generation, p53 overexpression and endothelial dysfunction[J]. Int Urol Nephrol, 2018, 50(1):179-186. DOI: 10.1007/s11255-017-1733-5 . |
| 27 | CHAO H H, LIU J C, LIN J W, et al. Uric acid stimulates endothelin-1 gene expression associated with NADPH oxidase in human aortic smooth muscle cells[J]. Acta Pharmacol Sin, 2008, 29(11):1301-1312. DOI: 10.1111/j.1745-7254.2008.00877.x . |
| 28 | ZHUANG Y B, FENG Q C, DING G X, et al. Activation of ERK1/2 by NADPH oxidase-originated reactive oxygen species mediates uric acid-induced mesangial cell proliferation[J]. Am J Physiol Renal Physiol, 2014, 307(4): F396-F406. DOI: 10.1152/ajprenal.00565.2013 . |
| 29 | CRISTÓBAL-GARCÍA M, GARCÍA-ARROYO F E, TAPIA E, et al. Renal oxidative stress induced by long-term hyperuricemia alters mitochondrial function and maintains systemic hypertension[J]. Oxid Med Cell Longev, 2015, 2015:535686. DOI: 10.1155/2015/535686 . |
| 30 | HONG Q, QI K, FENG Z, et al. Hyperuricemia induces endothelial dysfunction via mitochondrial Na+/Ca2+ exchanger-mediated mitochondrial calcium overload[J]. Cell Calcium, 2012, 51(5):402-410. DOI: 10.1016/j.ceca.2012.01.003 . |
| 31 | SU Y, HU L T, WANG Y N, et al. The Rho kinase signaling pathway participates in tubular mitochondrial oxidative injury and apoptosis in uric acid nephropathy[J]. J Int Med Res, 2021, 49(6):3000605211021752. DOI: 10.1177/03000605211021752 . |
| 32 | YANG L J, CHANG B C, GUO Y L, et al. The role of oxidative stress-mediated apoptosis in the pathogenesis of uric acid nephropathy[J]. Ren Fail, 2019, 41(1):616-622. DOI: 10.1080/0886022X.2019.1633350 . |
| 33 | BRAGA T T, FORNI M F, CORREA-COSTA M, et al. Soluble uric acid activates the NLRP3 inflammasome[J]. Sci Rep, 2017, 7:39884. DOI: 10.1038/srep39884 . |
| 34 | KOMORI H, YAMADA K, TAMAI I. Hyperuricemia enhances intracellular urate accumulation via down-regulation of cell-surface BCRP/ABCG2 expression in vascular endothelial cells[J]. Biochim Biophys Acta Biomembr, 2018, 1860(5):973-980. DOI: 10.1016/j.bbamem.2018.01.006 . |
| 35 | BROVOLD H, LUND T, SVISTOUNOV D, et al. Crystallized but not soluble uric acid elicits pro-inflammatory response in short-term whole blood cultures from healthy men[J]. Sci Rep, 2019, 9:10513. DOI: 10.1038/s41598-019-46935-w . |
| 36 | SU H Y, YANG C, LIANG D, et al. Research advances in the mechanisms of hyperuricemia-induced renal injury[J]. Biomed Res Int, 2020, 2020:5817348. DOI: 10.1155/2020/5817348 . |
| 37 | YE Y, ZHANG Y, WANG B, et al. CXCR1/CXCR2 antagonist G31P inhibits nephritis in a mouse model of uric acid nephropathy[J]. Biomed Pharmacother, 2018, 107:1142-1150. DOI: 10.1016/j.biopha.2018.07.077 . |
| 38 | CHOE J Y, CHOI C H, PARK K Y, et al. High-mobility group box 1 is responsible for monosodium urate crystal-induced inflammation in human U937 macrophages[J]. Biochem Biophys Res Commun, 2018, 503(4):3248-3255. DOI: 10.1016/j.bbrc.2018.08.139 . |
| 39 | VÄLIMÄKI E, MIETTINEN J J, LIETZÉN N, et al. Monosodium urate activates Src/Pyk2/PI3 kinase and cathepsin dependent unconventional protein secretion from human primary macrophages[J]. Mol Cell Proteomics, 2013, 12(3):749-763. DOI: 10.1074/mcp.M112.024661 . |
| 40 | WANG M, LIN X, YANG X M, et al. Research progress on related mechanisms of uric acid activating NLRP3 inflammasome in chronic kidney disease[J]. Ren Fail, 2022, 44(1):615-624. DOI: 10.1080/0886022X.2022.2036620 . |
| 41 | ISAKA Y, TAKABATAKE Y, TAKAHASHI A, et al. Hyperuricemia-induced inflammasome and kidney diseases[J]. Nephrol Dial Transplant, 2016, 31(6):890-896. DOI: 10.1093/ndt/gfv024 . |
| 42 | WANG G H, ZUO T, LI R. The mechanism of Arhalofenate in alleviating hyperuricemia-activating PPARγ thereby reducing caspase-1 activity[J]. Drug Dev Res, 2020, 81(7):859-866. DOI: 10.1002/ddr.21699 . |
| 43 | ELEFTHERIADIS T, PISSAS G, SOUNIDAKI M, et al. Urate crystals directly activate the T-cell receptor complex and induce T-cell proliferation[J]. Biomed Rep, 2017, 7(4):365-369. DOI: 10.3892/br.2017.960 . |
| 44 | KOKA R M, HUANG E, LIESKE J C. Adhesion of uric acid crystals to the surface of renal epithelial cells[J]. Am J Physiol Renal Physiol, 2000, 278(6): F989-F998. DOI: 10.1152/ajprenal.2000.278.6.F989 . |
| 45 | JOOSTEN L A B, CRIŞAN T O, BJORNSTAD P, et al. Asymptomatic hyperuricaemia: a silent activator of the innate immune system[J]. Nat Rev Rheumatol, 2020, 16(2):75-86. DOI: 10.1038/s41584-019-0334-3 . |
| 46 | ZHOU R B, TARDIVEL A, THORENS B, et al. Thioredoxin-interacting protein links oxidative stress to inflammasome activation[J]. Nat Immunol, 2010, 11(2):136-140. DOI: 10.1038/ni.1831 . |
| 47 | MISAWA T, TAKAHAMA M, KOZAKI T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome[J]. Nat Immunol, 2013, 14(5):454-460. DOI: 10.1038/ni.2550 . |
| 48 | ELLIOTT E I, MILLER A N, BANOTH B, et al. Cutting edge: mitochondrial assembly of the NLRP3 inflammasome complex is initiated at priming[J]. J Immunol, 2018, 200(9):3047-3052. DOI: 10.4049/jimmunol.1701723 . |
| 49 | XIAO J, ZHANG X L, FU C S, et al. Soluble uric acid increases NALP3 inflammasome and interleukin-1β expression in human primary renal proximal tubule epithelial cells through the Toll-like receptor 4-mediated pathway[J]. Int J Mol Med, 2015, 35(5):1347-1354. DOI: 10.3892/ijmm.2015.2148 . |
| 50 | CRIȘAN T O, CLEOPHAS M C, OOSTING M, et al. Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra[J]. Ann Rheum Dis, 2016, 75(4):755-762. DOI: 10.1136/annrheumdis-2014-206564 . |
| 51 | KIMURA Y, YANAGIDA T, ONDA A, et al. Soluble uric acid promotes atherosclerosis via AMPK (AMP-activated protein kinase)-mediated inflammation[J]. Arterioscler Thromb Vasc Biol, 2020, 40(3):570-582. DOI: 10.1161/ATVBAHA.119.313224 . |
| 52 | DE FRANCESCO E M, MAGGIOLINI M, MUSTI A M. Crosstalk between Notch, HIF-1α and GPER in breast cancer EMT[J]. Int J Mol Sci, 2018, 19(7):2011. DOI: 10.3390/ijms19072011 . |
| 53 | YANG Q M, FU C S, ZHANG X L, et al. Adiponectin protects against uric acid-induced renal tubular epithelial inflammatory responses via the AdipoR1/AMPK signaling pathway[J]. Int J Mol Med, 2019, 43(3):1542-1552. DOI: 10.3892/ijmm.2019.4072 . |
| 54 | YIN W, ZHOU Q L, OUYANG S X, et al. Uric acid regulates NLRP3/IL-1β signaling pathway and further induces vascular endothelial cells injury in early CKD through ROS activation and K+ efflux[J]. BMC Nephrol, 2019, 20(1):319. DOI: 10.1186/s12882-019-1506-8 . |
| 55 | XIAO J, ZHANG X L, FU C S, et al. Impaired Na+-K+-ATPase signaling in renal proximal tubule contributes to hyperuricemia-induced renal tubular injury[J]. Exp Mol Med, 2018, 50(3): e452. DOI: 10.1038/emm.2017.287 . |
| 56 | XIAO J, ZHU S B, GUAN H C, et al. AMPK alleviates high uric acid-induced Na+-K+-ATPase signaling impairment and cell injury in renal tubules[J]. Exp Mol Med, 2019, 51(5):1-14. DOI: 10.1038/s12276-019-0254-y . |
| 57 | MA Q Y, IMMLER R, PRUENSTER M, et al. Soluble uric acid inhibits β2 integrin-mediated neutrophil recruitment in innate immunity[J]. Blood, 2022, 139(23):3402-3417. DOI: 10.1182/blood.2021011234 . |
| 58 | SELLMAYR M, HERNANDEZ PETZSCHE M R, MA Q Y, et al. Only hyperuricemia with crystalluria, but not asymptomatic hyperuricemia, drives progression of chronic kidney disease[J]. J Am Soc Nephrol, 2020, 31(12):2773-2792. DOI: 10.1681/ASN.2020040523 . |
| 59 | ALBERTS B M, BARBER J S, SACRE S M, et al. Precipitation of soluble uric acid is necessary for in vitro activation of the NLRP3 inflammasome in primary human monocytes[J]. J Rheumatol, 2019, 46(9):1141-1150. DOI: 10.3899/jrheum.180855 . |
| 60 | MA Q Y, HONARPISHEH M, LI C Y, et al. Soluble uric acid is an intrinsic negative regulator of monocyte activation in monosodium urate crystal-induced tissue inflammation[J]. J Immunol, 2020, 205(3):789-800. DOI: 10.4049/jimmunol. 2000319 . |
| 61 | CHOI A M K, RYTER S W, LEVINE B. Autophagy in human health and disease[J]. N Engl J Med, 2013, 368(7):651-662. DOI: 10.1056/NEJMra1205406 . |
| 62 | SHI C S, SHENDEROV K, HUANG N N, et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction[J]. Nat Immunol, 2012, 13(3):255-263. DOI: 10.1038/ni.2215 . |
| 63 | BAO J F, SHI Y F, TAO M, et al. Pharmacological inhibition of autophagy by 3-MA attenuates hyperuricemic nephropathy[J]. Clin Sci, 2018, 132(21):2299-2322. DOI: 10.1042/CS20180563 . |
| 64 | HU J C, WU H, WANG D C, et al. Weicao capsule ameliorates renal injury through increasing autophagy and NLRP3 degradation in UAN rats[J]. Int J Biochem Cell Biol, 2018, 96:1-8. DOI: 10.1016/j.biocel.2018.01.001 . |
| 65 | HERZIG S, SHAW R J. AMPK: guardian of metabolism and mitochondrial homeostasis[J]. Nat Rev Mol Cell Biol, 2018, 19(2):121-135. DOI: 10.1038/nrm.2017.95 . |
| 66 | HU Y, SHI Y F, CHEN H, et al. Blockade of autophagy prevents the progression of hyperuricemic nephropathy through inhibiting NLRP3 inflammasome-mediated pyroptosis[J]. Front Immunol, 2022, 13:858494. DOI: 10.3389/fimmu. 2022. 858494 . |
| 67 | MAEJIMA I, TAKAHASHI A, OMORI H, et al. Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury[J]. EMBO J, 2013, 32(17):2336-2347. DOI: 10.1038/emboj.2013.171 . |
| 68 | COELHO S C, BERILLO O, CAILLON A, et al. Three-month endothelial human endothelin-1 overexpression causes blood pressure elevation and vascular and kidney injury[J]. Hypertension, 2018, 71(1):208-216. DOI: 10.1161/HYPERTENSIONAHA.117.09925 . |
| 69 | YANG X L, GU J, LV H C, et al. Uric acid induced inflammatory responses in endothelial cells via up-regulating(pro)renin receptor[J]. Biomedecine Pharmacother, 2019, 109:1163-1170. DOI: 10.1016/j.biopha.2018.10.129 . |
| 70 | REYES-MARTINEZ C, NGUYEN Q M, KASSAN M, et al. (pro)renin receptor-dependent induction of profibrotic factors is mediated by COX-2/EP4/NOX-4/smad pathway in collecting duct cells[J]. Front Pharmacol, 2019, 10:803. DOI: 10.3389/fphar.2019.00803 . |
| 71 | KO J, KANG H J, KIM D A, et al. Uric acid induced the phenotype transition of vascular endothelial cells via induction of oxidative stress and glycocalyx shedding[J]. FASEB J, 2019, 33(12):13334-13345. DOI: 10.1096/fj.201901148R . |
| 72 | ALBERTONI G, MAQUIGUSSA E, PESSOA E, et al. Soluble uric acid increases intracellular calcium through an angiotensin II-dependent mechanism in immortalized human mesangial cells[J]. Exp Biol Med, 2010, 235(7):825-832. DOI: 10.1258/ebm.2010.010007 . |
| 73 | RYU E S, KIM M J, SHIN H S, et al. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease[J]. Am J Physiol Renal Physiol, 2013, 304(5): F471-F480. DOI: 10.1152/ajprenal. 00560. 2012 . |
| 74 | LOVISA S, ZEISBERG M, KALLURI R. Partial epithelial-to-mesenchymal transition and other new mechanisms of kidney fibrosis[J]. Trends Endocrinol Metab, 2016, 27(10):681-695. DOI: 10.1016/j.tem.2016.06.004 . |
| 75 | REN Q, TAO S B, GUO F, et al. Natural flavonol fisetin attenuated hyperuricemic nephropathy via inhibiting IL-6/JAK2/STAT3 and TGF-β/SMAD3 signaling[J]. Phytomedicine, 2021, 87:153552. DOI: 10.1016/j.phymed.2021.153552 . |
| 76 | WANG W J, WANG X Y, CHUN J, et al. Inflammasome-independent NLRP3 augments TGF-β signaling in kidney epithelium[J]. J Immunol, 2013, 190(3):1239-1249. DOI: 10.4049/jimmunol.1201959 . |
| 77 | ROMERO C A, REMOR A, LATINI A, et al. Uric acid activates NRLP3 inflammasome in an in-vivo model of epithelial to mesenchymal transition in the kidney[J]. J Mol Histol, 2017, 48(3):209-218. DOI: 10.1007/s10735-017-9720-9 . |
| 78 | TAO M, SHI Y F, TANG L X, et al. Blockade of ERK1/2 by U0126 alleviates uric acid-induced EMT and tubular cell injury in rats with hyperuricemic nephropathy[J]. Am J Physiol Renal Physiol, 2019, 316(4): F660-F673. DOI: 10.1152/ajprenal. 00480.2018 . |
| 79 | ZHA D Q, WU S Q, GAO P, et al. Telmisartan attenuates uric acid-induced epithelial-mesenchymal transition in renal tubular cells[J]. Biomed Res Int, 2019, 2019:3851718. DOI: 10.1155/2019/3851718 . |
| 80 | SCHUNK S J, FLOEGE J, FLISER D, et al. WNT-β-catenin signalling―a versatile player in kidney injury and repair[J]. Nat Rev Nephrol, 2021, 17(3):172-184. DOI: 10.1038/s41581-020-00343-w . |
| 81 | ZHAO L, LI C Y, ZHOU B, et al. Crucial role of serum response factor in renal tubular epithelial cell epithelial-mesenchymal transition in hyperuricemic nephropathy[J]. Aging, 2019, 11(22):10597-10609. DOI: 10.18632/aging.102479 . |
| 82 | SHI Y F, XU L Q, TAO M, et al. Blockade of enhancer of zeste homolog 2 alleviates renal injury associated with hyperuricemia[J]. Am J Physiol Renal Physiol, 2019, 316(3): F488-F505. DOI: 10.1152/ajprenal.00234.2018 . |
| 83 | ALMEIDA A, MITCHELL A L, BOLAND M, et al. A new genomic blueprint of the human gut microbiota[J]. Nature, 2019, 568(7753):499-504. DOI: 10.1038/s41586-019-0965-1 . |
| 84 | STAVROPOULOU E, KANTARTZI K, TSIGALOU C, et al. Focus on the gut-kidney axis in health and disease[J]. Front Med, 2021, 7:620102. DOI: 10.3389/fmed.2020.620102 . |
| 85 | WEI J, ZHANG Y Q, DALBETH N, et al. Association between gut microbiota and elevated serum urate in two independent cohorts[J]. Arthritis Rheumatol, 2022, 74(4):682-691. DOI: 10.1002/art.42009 . |
| 86 | WANG J, CHEN Y, ZHONG H, et al. The gut microbiota as a target to control hyperuricemia pathogenesis: potential mechanisms and therapeutic strategies[J]. Crit Rev Food Sci Nutr, 2022, 62(14):3979-3989. DOI: 10.1080/10408398. 2021. 1874287 . |
| 87 | NIEUWDORP M, GILIJAMSE P W, PAI N, et al. Role of the microbiome in energy regulation and metabolism[J]. Gastroenterology, 2014, 146(6):1525-1533. DOI: 10.1053/j.gastro.2014.02.008 . |
| 88 | HE Y, FU L H, LI Y P, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity[J]. Cell Metab, 2021, 33(5):988-1000.e7. DOI: 10.1016/j.cmet.2021.03.002 . |
| 89 | PAN L B, HAN P, MA S R, et al. Abnormal metabolism of gut microbiota reveals the possible molecular mechanism of nephropathy induced by hyperuricemia[J]. Acta Pharm Sin B, 2020, 10(2):249-261. DOI: 10.1016/j.apsb.2019.10.007 . |
| 90 | XU D X, LV Q L, WANG X F, et al. Hyperuricemia is associated with impaired intestinal permeability in mice[J]. Am J Physiol Gastrointest Liver Physiol, 2019, 317(4): G484-G492. DOI: 10.1152/ajpgi.00151.2019 . |
| 91 | ZHAO H Y, CHEN X Y, ZHANG L, et al. Lacticaseibacillus rhamnosus Fmb14 prevents purine induced hyperuricemia and alleviate renal fibrosis through gut-kidney axis[J]. Pharmacol Res, 2022, 182:106350. DOI: 10.1016/j.phrs. 2022.106350 . |
| 92 | LIU X, LV Q L, REN H Y, et al. The altered gut microbiota of high-purine-induced hyperuricemia rats and its correlation with hyperuricemia[J]. PeerJ, 2020, 8: e8664. DOI: 10.7717/peerj.8664 . |
| 93 | NUKI G. An appraisal of the 2012 American college of rheumatology guidelines for the management of gout[J]. Curr Opin Rheumatol, 2014, 26(2):152-161. DOI: 10.1097/BOR. 0000000000000034 . |
| 94 | WHITE W B, SAAG K G, BECKER M A, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout[J]. N Engl J Med, 2018, 378(13):1200-1210. DOI: 10.1056/NEJMoa1710895 . |
| 95 | LIU P, WANG C, WANG Y, et al. Zishen Qingre Tongluo formula improves renal fatty acid oxidation and alleviated fibrosis via the regulation of the TGF- β1/Smad3 signaling pathway in hyperuricemic nephrology rats[J]. Biomed Res Int, 2021, 2021:2793823. DOI: 10.1155/2021/2793823 . |
| 96 | LI X Q, CHEN Y H, GAO X X, et al. Antihyperuricemic effect of green Alga Ulva lactuca ulvan through regulating urate transporters[J]. J Agric Food Chem, 2021, 69(38):11225-11235. DOI: 10.1021/acs.jafc.1c03607 . |
| 97 | WU D, CHEN R H, LI Q H, et al. Tea (Camellia sinensis) ameliorates hyperuricemia via uric acid metabolic pathways and gut microbiota[J]. Nutrients, 2022, 14(13):2666. DOI: 10.3390/nu14132666 . |
| 98 | WEN X H, LOU Y, SONG S Y, et al. Qu-Zhuo-Tong-Bi Decoction alleviates gouty arthritis by regulating butyrate-producing bacteria in mice[J]. Front Pharmacol, 2021, 11:610556. DOI: 10.3389/fphar.2020.610556 . |
| [1] | LIU Yayi, JIA Yunfeng, ZUO Yiming, ZHANG Junping, LÜ Shichao. Progress and Evaluation of Animal Model of Heart Qi-Yin Deficiency Syndrome [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 411-421. |
| [2] | ZHAO Xin, WANG Chenxi, SHI Wenqing, LOU Yuefen. Advances in the Application of Zebrafish in the Research of Inflammatory Bowel Disease Mechanisms and Drug Development [J]. Laboratory Animal and Comparative Medicine, 2025, 45(4): 422-431. |
| [3] | PAN Yicong, JIANG Wenhong, HU Ming, QIN Xiao. Optimization of Surgical Procedure and Efficacy Evaluation of Aortic Calcification Model in Rats with Chronic Kidney Disease [J]. Laboratory Animal and Comparative Medicine, 2025, 45(3): 279-289. |
| [4] | WANG Biying, LU Jiashuo, ZAN Guiying, CHEN Ruosong, CHAI Jingrui, LIU Jinggen, WANG Yujun. Establishment Methods and Application Progress of Rodent Models for Drug Addiction [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 158-166. |
| [5] | CHEN Yuhan, CHEN Jinling, LI Xin, OU Yanhua, WANG Si, CHEN Jingyi, WANG Xingyi, YUAN Jiali, DUAN Yuanyuan, YANG Zhongshan, NIU Haitao. Analysis of Animal Models of Myasthenia Gravis Based on Its Clinical Characteristics in Chinese and Western Medicine [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 176-186. |
| [6] | LIAN Hui, JIANG Yanling, LIU Jia, ZHANG Yuli, XIE Wei, XUE Xiaoou, LI Jian. Construction and Evaluation of a Rat Model of Abnormal Uterine Bleeding [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 130-146. |
| [7] | LUO Shixiong, ZHANG Sai, CHEN Hui. Research Progress in Establishment and Evaluation of Common Asthma Animal Models [J]. Laboratory Animal and Comparative Medicine, 2025, 45(2): 167-175. |
| [8] | FEI Bin, GUO Wenke, GUO Jianping. Research Progress on Animal Models for Hernia Diseases and New Hernia Repair Materials [J]. Laboratory Animal and Comparative Medicine, 2025, 45(1): 55-66. |
| [9] | YANG Jiahao, DING Chunlei, QIAN Fenghua, SUN Qi, JIANG Xusheng, CHEN Wen, SHEN Mengwen. Research Progress on Animal Models of Sepsis-Related Organ Injury [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 636-644. |
| [10] | SUN Xiaorong, SU Dan, GUI Wenjuan, CHEN Yue. Establishment and Evaluation of a Moderate-to-Severe Knee Osteoarthritis Model in Rats Induced by Surgery [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 597-604. |
| [11] | TIAN Fang, PAN Bin, SHI Jiayi, XU Yanyi, LI Weihua. Advances in Development of PM2.5-Exposed Animal Models and Their Application in Reproductive Toxicity Research [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 626-635. |
| [12] | ZHAO Xiaona, WANG Peng, YE Maoqing, QU Xinkai. Establishment of a New Hyperglycemic Obesity Cardiac Dysfunction Mouse Model with Triacsin C [J]. Laboratory Animal and Comparative Medicine, 2024, 44(6): 605-612. |
| [13] | TU Yingxin, JI Yilan, WANG Fei, YANG Dongming, WANG Dongdong, SUN Zhixin, DAI Yuexin, WANG Yanji, Guanghan KAN, WU Bin, ZHAO Deming, YANG Lifeng. Evaluation of Simulated Weightlessness Model of Hindlimb Unloading Miniature Pigs and Their Tissue Damage [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 475-486. |
| [14] | HUANG Dongyan, WU Jianhui. Establishment Methods and Application Evaluation of Animal Models in Reproductive Toxicology Research [J]. Laboratory Animal and Comparative Medicine, 2024, 44(5): 550-559. |
| [15] | ZHENG Yiqing, DENG Yasheng, FAN Yanping, LIANG Tianwei, HUANG Hui, LIU Yonghui, NI Zhaobing, LIN Jiang. Application Analysis of Animal Models for Pelvic Inflammatory Disease Based on Data Mining [J]. Laboratory Animal and Comparative Medicine, 2024, 44(4): 405-418. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||